Published online Aug 26, 2014. doi: 10.4330/wjc.v6.i8.764
Revised: April 29, 2014
Accepted: May 28, 2014
Published online: August 26, 2014
Processing time: 217 Days and 1.3 Hours
Hypertrophic cardiomyopathy (HCM) is the most common cause of sudden cardiac death (SCD) in the young, particularly among athletes. Identifying high risk individuals is very important for SCD prevention. The purpose of this review is to stress that noninvasive diagnostic testing is important for risk assessment. Extreme left ventricular hypertrophy and documented ventricular tachycardia and fibrillation increase the risk of SCD. Fragmented QRS and T wave inversion in multiple leads are more common in high risk patients. Cardiac magnetic resonance imaging provides complete visualization of the left ventricular chamber, allowing precise localization of the distribution of hypertrophy and measurement of wall thickness and cardiac mass. Moreover, with late gadolinium enhancement, patchy myocardial fibrosis within the area of hypertrophy can be detected, which is also helpful in risk stratification. Genetic testing is encouraged in all cases, especially in those with a family history of HCM and SCD.
Core tip: Hypertrophic cardiomyopathy (HCM) is the most common cause of sudden cardiac death (SCD) in the young, particularly among athletes. Noninvasive diagnostic testing is important for risk assessment. Extreme left ventricular hypertrophy, documented ventricular tachycardia and fibrillation increase the risk of SCD. Fragmented QRS complex and T wave inversion in multiple leads are more common in high risk patients. Cardiac magnetic resonance imaging with late gadolinium enhancement, patchy myocardial fibrosis within the area of hypertrophy can be detected, which is also helpful in risk stratification. Genetic testing is encouraged in all cases, especially in those with family history of HCM and SCD.
- Citation: Zhang L, Mmagu O, Liu L, Li D, Fan Y, Baranchuk A, Kowey PR. Hypertrophic cardiomyopathy: Can the noninvasive diagnostic testing identify high risk patients? World J Cardiol 2014; 6(8): 764-770
- URL: https://www.wjgnet.com/1949-8462/full/v6/i8/764.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i8.764
Hypertrophic cardiomyopathy (HCM) is a common autosomal dominant cardiac disease, affecting 1 in 500 people[1]. Cardiomyocyte hypertrophy, disarray, fibrosis and ventricular wall thickening are the pathological hallmarks of HCM. Although the majority of affected individuals present with mild symptoms or are asymptomatic, HCM is the most common identifiable cause of premature sudden cardiac death (SCD) in the young, especially the young athlete[1]. Since SCD can be the first manifestation in concealed cases and some symptomatic patients do bear a high risk of SCD, timely diagnosis and risk stratification for appropriate therapy and SCD prevention such as prophylactic implantable cardioverter-defibrillator (ICD) therapy are very important. The common risk factors associated with SCD are family history of HCM-related SCD, left ventricular wall thickness ≥ 30 mm, documented ventricular tachyarrhythmia such as frequent and/or prolonged bursts of non-sustained ventricular tachycardia (VT) and ventricular fibrillation (VF), as well as abnormal blood pressure response to exercise[2].
The diagnosis of HCM is based on echocardiography and/or cardiac magnetic resonance images (CMRI), wherein a non-dilated, hypertrophied left ventricle is found in the absence of any other systemic or cardiac event that explains the specific pathology, mostly arterial hypertension[3]. Since the outcome varies among affected individuals, the purpose of this review is to elaborate the usefulness of noninvasive diagnostic testing for identifying high risk patients with HCM.
The electrocardiogram (ECG), the most basic test in cardiovascular disease management, is abnormal in the vast majority of patients diagnosed with HCM. In general, if patients meet the ECG criteria for left ventricular hypertrophy (LVH), the absence of an apparent cause should raise the suspicion of HCM[4]. The history and physical examination may be negative, and the signs of LVH on an ECG may occur earlier than the increase in the thickness of left ventricular wall detected by echocardiography[4,5]. Making a correct diagnosis in a timely manner is essential in SCD prevention.
Presence of abnormal Q waves such as deep Q waves in multiple leads is common in patients with HCM[6]. Abnormal Q waves may appear prior to the increased QRS amplitude[7]. A deep Q wave is considered if the amplitude ≥ 3 mm or 1/4 of the R wave. In HCM deep Q waves are usually seen in more than two contiguous leads[8]. There are differences in terms of the significance of deep Q waves between the young and adults[6]. Presence of deep Q waves in children has yielded a higher specificity and sensitivity than adults in the diagnosis of HCM[6]. The mechanisms of deep Q waves in HCM are: (1) the electrical inactivation due to myocardial fibrosis; and (2) the altered direction of resultant initial QRS vector due to increased electrical forces of disproportionate hypertrophy of the basal septal and/or ventricular free wall, unopposed by apical forces[9]. Presence of deep Q waves in multiple leads is thought to be associated with an increased incidence of SCD[9].
Increased QRS amplitude is the most common ECG abnormality in HCM. It has been reported that increased QRS amplitude in the limb leads increases the likelihood of SCD in both children and adults with HCM[10]. Increased QRS duration (QRSD) is seen in septal and concentric HCM patients[11]. Ostman-Smith et al[10] measured QRS amplitude and duration in HCM subjects with and without cardiac arrest and SCD. They found that increased QRS amplitude-duration product is a better indicator of high risk HCM patients. Among the high risk patients that have undergone ICD therapy, there is a positive correlation between increased QRSD and defibrillation thresholds[12].
It is known that fragmented QRS complex (fQRS) in multiple ECG leads is associated with myocardial scarring or fibrosis in ischemic and non-ischemic cardiomyopathies. In the latter, patchy fibrosis are located in mid-myocardium or sub-epicardium, and predominantly in the perivalvular areas. Femenía et al[13] reported a female patient with recurrent syncope diagnosed with HCM at age 9 and had an ICD placed at age 16 after aborted SCD due to VF. Her ECG at age 16 showed fQRS in 12 leads. During a 2-year follow-up, this patient presented with sustained VT requiring anti-tachycardia pacing and ICD shocks[13]. In a large sample study, they found that fQRS located in the lateral area increases the likelihood of ICD therapy[14]. Therefore they postulated fQRS should be incorporated in multivariate models for SCD prediction, along with more classical risk factors[13,14].
ST segment elevation in HCM is viewed as a marker of disease progression[15]. Furuki et al[15] found a close correlation between convex ST elevation and left ventricular enlargement and wall motion abnormalities with a specificity of 85% and a sensitivity of 62%, respectively.
Ostman-Smith et al[10] found that HCM patients with high risk for SCD had negative T waves in the limb and precordial leads. Moreover, negative T waves in the precordial leads has a positive correlation with the extent of LVH in HCM[7]. On echocardiography, the maximum wall thickness was 19.2 ± 5.2 mm with negative T waves compared to 13.5 ± 5.1 mm without negative T waves[7]. Microvolt-T wave alternans (TWA), a surrogate for unstable ventricular repolarization properties, have been associated with an increased likelihood of VT/VF[16]. Momiyama et al[16] also demonstrated that among 7/17 HCM patients classified as high-risk individuals, only two of them did not show TWA.
Documented VT and/or VF are direct risk factors for SCD[17]. In HCM, VF may occur without the preceding VT. A study by Cha et al[18] revealed that sinus tachycardia or atrial fibrillation were the most common rhythms that initiated sustained VT followed by ICD discharges in high risk patients. Sustained VT is common in symptomatic individuals[19]. Medeiros et al[20] noted that the arrhythmias with the highest prevalence according to their ICD storage recordings were sustained VT and VF.
Recurrent or repetitive non-sustained VT (>10 beats) is considered a risk for SCD in HCM[21]. On ambulatory ECG monitoring, non-sustained VT occurs in about 25% of HCM patients[22]. Gimeno et al[22] showed that exercise-induced non-sustained VT was associated with a 3.73 fold rise in SCD. 2D speckle tracking has also been used as an important tool to predict non-sustained VT in HCM patients[23]. According to one study, the results obtained by 2D speckle tracking are similar to the results obtained by ambulatory Holter ECG[23]. There have also been reports of the incidence of non-sustained VT by provocative maneuvers such as Valsalva[24]. Increased vagal tone has been considered a potential mechanism for the occurrence of non-sustained VT[24,25]. The current recommendation is consideration of ICD placement, even if non-sustained VT is the only risk factor[21].
Echocardiography is an integral diagnostic modality for HCM because it is highly reproducible and cost effective. LVH, the most important phenotypic characteristic of HCM, can be easily revealed by echocardiography. The extent of left ventricular wall thickness is associated with an increased risk of SCD. Maximum left ventricular wall thickness ≥ 30 mm is termed extreme left ventricular hypertrophy, and is an independent predictor of SCD in the young[26-28]. Spirito et al[26] observed 480 cases of HCM consecutively for an average follow-up of 6.5 years. Patients were divided into five groups according to the maximum left ventricular wall thickness: ≤ 15 mm, 16-19 mm, 20-24 mm, 25-29 mm, and ≥ 30 mm, respectively. They found that the 20-year cumulative risk for SCD was up to 40% in the group with left ventricular wall thickness ≥ 30 mm. Patients with extreme left ventricular hypertrophy were mostly young, with only mild symptoms or with no symptoms at all. Neither did they have any evidence of left ventricular outflow tract obstruction. Thus the authors suggest that young patients with extreme left ventricular hypertrophy (≥ 30 mm), should consider prophylactic implantation of ICD regardless of the presence or absence of other risk factors. Elliott et al[27] found that in HCM patients with left ventricular wall thickness, the relative risk (RR) increased by 1.31 (95%CI: 1.03-1.66) for each additional 5 mm. In HCM microvascular dysfunction, cardiomyocyte hypertrophy and disarray can lead to myocardial ischemia and fibrosis[29]. The latter is a substrate for reentrant tachyarrhythmia and SCD[30]. HCM patients with extreme left ventricular hypertrophy indeed bear a higher risk of SCD and more frequent ICD discharges. Nevertheless, it does not necessarily mean that patients with left ventricular wall thickness < 30 mm are considered low risk. In the later stages of the disease when the left ventricular ejection fraction (LVEF) may be below 50% with left ventricular wall thinning, apical aneurysm and ventricular chamber dilatation[31], the risks of SCD and all-cause mortality increase[31,32].
Although some of the newer systems are safe for ICD patients, MRI in general is hazardous to patients with implanted devices. As an alternative, Shiozaki et al[33] examined the value of delayed enhancement multidetector computed tomography (MDCT). They showed that myocardial fibrosis was found in 96.4% of patients with ICD using MDCT[33]. However, it must be noted that ICD cables caused artifacts and may have overrepresented the findings of myocardial fibrosis in these patients[33].
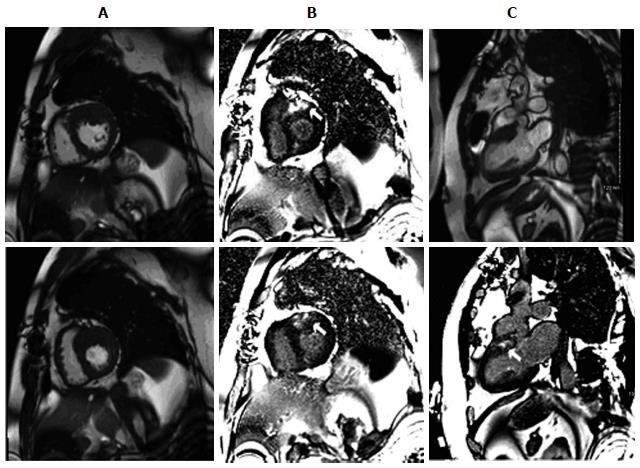
Although echocardiography plays a central role in the assessment of HCM, it is sometimes limited by poor acoustic windows, incomplete visualization of the left ventricular wall, and inaccurate evaluation of left ventricular mass[34]. With excellent spatial resolution and border definition, CMRI provides complete visualization of the left ventricular chamber, allowing precise localization of the distribution of hypertrophy and measurement of wall thickness and cardiac mass (Figure 1). CMRI is superior to echocardiography for the detection of apical and focal basal anteroseptal variants and in recognizing noncontiguous areas of HCM[35]. CMR cine imaging provides evaluation of cardiac morphological information including systolic anterior motion of the anterior mitral leaflet with dynamic outflow tract obstruction, mitral regurgitation, apical aneurysms, and papillary muscle abnormalities. CMR stress perfusion imaging can identify areas of microvascular dysfunction or mismatch between left ventricular mass and coronary flow.
Based on CMRI findings in patients with HCM, distribution and extent of LVH is variable including asymmetrical septal, apical, localized, or concentric hypertrophy, but these usually are not extensive. Basal anterior left ventricular free wall and the contiguous anterior ventricular septum are the most commonly hypertrophied segments[34]. LVH can be focal (1-2 segments), intermediate (3-7 segments), or diffuse (> 8 segments). The number of hypertrophied segments is greater in patients with left ventricular outflow tract obstruction than without and was associated with an advanced New York Heart Association functional class. Left ventricular wall thickness was greater in segments with late gadolinium enhancement (LGE) than without. Segmental left ventricular hypertrophy largely confined to the anterolateral free wall, posterior septum, or apex were underestimated or undetected by echocardiography. These observations support an emerging role for CMR in the contemporary evaluation of patients with HCM.
Moreover, LGE plays a critical role in risk stratifying HCM patients (Figure 1). Myocardial fibrosis is present in up to 80% of patients with HCM, with a characteristic patchy pattern of LGE generally occurring in areas of hypertrophy[34]. In addition, the extent of fibrosis has been shown to correlate positively with regional hypertrophy and inversely with regional contraction. Consistently, in another clinical study[36] with 243 consecutive HCM patients, the presence of scar was an independent predictor of death, with an odds ratio of 5.47 for all-cause mortality and of 8.01 for cardiac mortality. Similarly, the risk of unplanned heart failure admissions, deterioration to NYHA functional class III or IV, or heart failure-related death has been shown to be statistically greater in those with fibrosis[37]. In a study with 424 HCM patients[38], LGE-positive patients were more likely to have episodes of non-sustained VT, more episodes of non-sustained VT per patient, and a higher frequency of ventricular extrasystoles per 24 h, with all cases of SCD and appropriate ICD discharges occurring in LGE-positive patients. More recently, a meta-analysis[39] of four studies evaluating 1063 HCM patients over an average follow-up of 3.1 years demonstrated that there are significant relationships between LGE and cardiovascular mortality, heart failure death, and all-cause mortality in HCM patients. Additionally, LGE and SCD/aborted SCD displayed a trend toward significance. The assessment of LGE in HCM patients by CMRI has the potential to provide important information to improve risk stratification in clinical practice.
Since the pathogenic missense mutation in the β-myosin heavy chain gene (MYH7 R403Q) was revealed two decades ago, > 1400 mutations have been identified in putative HCM-susceptibility genes. The most common genetic subtype is sarcomeric-HCM, caused by mutations in genes encoding proteins in the myofilaments of the cardiac sarcomere[40]. Among patients with positive genetic tests, MYBPC3 (myosin-binding protein C) and MYH7 are, by far, the two most common HCM-associated genes with an estimated prevalence of 25%-35% for each gene, while other genes including troponin T, troponin I, α-tropomyosin, and α-actin each account for a small proportion of patients (1% to 5%)[41]. Collectively, the known causal genes account for about two-thirds of all HCM cases while one-third of the causal genes for HCM are yet to be identified[42].
Genetic testing for HCM has been commercially available for almost a decade. However, the low mutation detection rate and cost have hindered uptake[43]. Currently, genetic testing has been recommended for any patients with an established clinical diagnosis of HCM and for family members following the identification of the HCM-causative mutation in the index case[44]. Multivariate analysis advocates this recommendation by identifying female gender, increased left-ventricular wall thickness, family history of hypertrophic cardiomyopathy, and family history of SCD as being associated with greatest chance of identifying a gene mutation[43].
Including genetic testing in the diagnostic strategy is also more likely to be cost effective than clinical tests alone when considering family screening and prevention of SCD[45,46]. The results of genetic testing identifies mutation carriers who will benefit from regular clinical investigation or early discussion of ICD. On the other hand, the result of genetic testing also identifies relatives without the causal mutation, who can be released without the need for long-term follow-up[47].
With rapid developments in genetic testing technology, a whole exome or a panel of HCM-related genes can now be tested by the next generation sequencing simultaneously, which provides an opportunity to detect multiple mutations in the same or different genes that are responsible for HCM. Emerging evidence documents that patients with HCM who carry more than one independent disease-causing gene mutation may be at a greater risk for severe disease expression and adverse outcome[48-51], especially in the absence of other conventional risk factors[52]. These observations support the emerging hypothesis that double (or compound) mutations detected by genetic testing may confer a gene dosage effect in HCM, thereby predisposing patients to adverse disease consequence[52]. It is observed that multiple mutation carriers are more likely to have suffered an out-of-hospital cardiac arrest or SCD[43]. For those patients who test positive for two or three mutations, frequent follow-up or early intervention may be required. Therefore, the integration of genetic testing into the current testing paradigm is likely to improve the general management of affected families.
P- Reviewer: Fett JD S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2218] [Cited by in RCA: 2128] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 2. | Sherrid MV, Cotiga D, Hart D, Ehlert F, Haas TS, Shen WK, Link MS, Estes NA, Epstein AE, Semsarian C. Relation of 12-lead electrocardiogram patterns to implanted defibrillator-terminated ventricular tachyarrhythmias in hypertrophic cardiomyopathy. Am J Cardiol. 2009;104:1722-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1394] [Cited by in RCA: 1421] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 4. | Savage DD, Seides SF, Clark CE, Henry WL, Maron BJ, Robinson FC, Epstein SE. Electrocardiographic findings in patients with obstructive and nonobstructive hypertrophic cardiomyopathy. Circulation. 1978;58:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 127] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Pérez-Riera AR, de Lucca AA, Barbosa-Barros R, Yanowitz FG, de Cano SF, Cano MN, Palandri-Chagas AC. Value of electro-vectorcardiogram in hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2013;18:311-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Konno T, Shimizu M, Ino H, Yamaguchi M, Terai H, Uchiyama K, Oe K, Mabuchi T, Kaneda T, Mabuchi H. Diagnostic value of abnormal Q waves for identification of preclinical carriers of hypertrophic cardiomyopathy based on a molecular genetic diagnosis. Eur Heart J. 2004;25:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Konno T, Fujino N, Hayashi K, Uchiyama K, Masuta E, Katoh H, Sakamoto Y, Tsubokawa T, Ino H, Yamagishi M. Differences in the diagnostic value of various criteria of negative T waves for hypertrophic cardiomyopathy based on a molecular genetic diagnosis. Clin Sci (Lond). 2007;112:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Dumont CA, Monserrat L, Soler R, Rodríguez E, Fernandez X, Peteiro J, Bouzas A, Bouzas B, Castro-Beiras A. Interpretation of electrocardiographic abnormalities in hypertrophic cardiomyopathy with cardiac magnetic resonance. Eur Heart J. 2006;27:1725-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Koga Y, Yamaga A, Hiyamuta K, Ikeda H, Toshima H. Mechanisms of abnormal Q waves in hypertrophic cardiomyopathy assessed by intracoronary electrocardiography. J Cardiovasc Electrophysiol. 2004;15:1402-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Ostman-Smith I, Wisten A, Nylander E, Bratt EL, Granelli Ad, Oulhaj A, Ljungström E. Electrocardiographic amplitudes: a new risk factor for sudden death in hypertrophic cardiomyopathy. Eur Heart J. 2010;31:439-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Song BG, Yang HS, Hwang HK, Kang GH, Park YH, Chun WJ, Oh JH. Correlation of electrocardiographic changes and myocardial fibrosis in patients with hypertrophic cardiomyopathy detected by cardiac magnetic resonance imaging. Clin Cardiol. 2013;36:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Nagai T, Kurita T, Satomi K, Noda T, Okamura H, Shimizu W, Suyama K, Aihara N, Kobayashi J, Kamakura S. QRS prolongation is associated with high defibrillation thresholds during cardioverter-defibrillator implantations in patients with hypertrophic cardiomyopathy. Circ J. 2009;73:1028-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Femenía F, Arce M, Arrieta M, Baranchuk A. Surface fragmented QRS in a patient with hypertrophic cardiomyopathy and malignant arrhythmias: Is there an association? J Cardiovasc Dis Res. 2012;3:32-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Femenía F, Arce M, Van Grieken J, Trucco E, Mont L, Abello M, Merino JL, Rivero-Ayerza M, Gorenek B, Rodriguez C. Fragmented QRS as a predictor of arrhythmic events in patients with hypertrophic obstructive cardiomyopathy. J Interv Card Electrophysiol. 2013;38:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Furuki M, Kawai H, Onishi T, Hirata K. Value of convex-type ST-segment elevation and abnormal Q waves for electrocardiographic-based identification of left ventricular remodeling in hypertrophic cardiomyopathy. Kobe J Med Sci. 2009;55:E16-E29. [PubMed] |
| 16. | Momiyama Y, Hartikainen J, Nagayoshi H, Albrecht P, Kautzner J, Saumarez RC, McKenna WJ, Camm AJ. Exercise-induced T-wave alternans as a marker of high risk in patients with hypertrophic cardiomyopathy. Jpn Circ J. 1997;61:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Elliott PM, Sharma S, Varnava A, Poloniecki J, Rowland E, McKenna WJ. Survival after cardiac arrest or sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1999;33:1596-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Cha YM, Gersh BJ, Maron BJ, Boriani G, Spirito P, Hodge DO, Weivoda PL, Trusty JM, Friedman PA, Hammill SC. Electrophysiologic manifestations of ventricular tachyarrhythmias provoking appropriate defibrillator interventions in high-risk patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2007;18:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Gao XJ, Kang LM, Zhang J, Dou KF, Yuan JS, Yang YJ. Mid-ventricular obstructive hypertrophic cardiomyopathy with apical aneurysm and sustained ventricular tachycardia: a case report and literature review. Chin Med J (Engl). 2011;124:1754-1757. [PubMed] |
| 20. | Medeiros Pde T, Martinelli Filho M, Arteaga E, Costa R, Siqueira S, Mady C, Piegas LS, Ramires JA. Hypertrophic cardiomyopathy: the importance of arrhythmic events in patients at risk for sudden cardiac death. Arq Bras Cardiol. 2006;87:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Maron BJ, Spirito P. Implantable defibrillators and prevention of sudden death in hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2008;19:1118-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Gimeno JR, Tomé-Esteban M, Lofiego C, Hurtado J, Pantazis A, Mist B, Lambiase P, McKenna WJ, Elliott PM. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur Heart J. 2009;30:2599-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Correia E, Rodrigues B, Santos LF, Moreira D, Gama P, Cabral C, Santos O. Longitudinal left ventricular strain in hypertrophic cardiomyopathy: correlation with nonsustained ventricular tachycardia. Echocardiography. 2011;28:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Khan MU, Khouzam RN, Khalid H, Baqir R, Moten M. Nonsustained ventricular tachycardia induced by valsalva manoeuvre in a patient with nonobstructive hypertrophic cardiomyopathy. Can J Cardiol. 2013;29:1741.e5-1741.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 26. | Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 771] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 27. | Elliott PM, Gimeno Blanes JR, Mahon NG, Poloniecki JD, McKenna WJ. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Anastasakis A, Theopistou A, Rigopoulos A, Kotsiopoulou C, Georgopoulos S, Fragakis K, Sevdalis E, Stefanadis C. Sudden cardiac death: investigation of the classical risk factors in a community-based hypertrophic cardiomyopathy cohort. Hellenic J Cardiol. 2013;54:281-288. [PubMed] |
| 29. | Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007;115:2418-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 30. | Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation. 2010;121:445-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 473] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 32. | Maron MS, Finley JJ, Bos JM, Hauser TH, Manning WJ, Haas TS, Lesser JR, Udelson JE, Ackerman MJ, Maron BJ. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation. 2008;118:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 383] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 33. | Shiozaki AA, Senra T, Arteaga E, Pita CG, Martinelli Filho M, Avila LF, Parga Filho JR, Mady C, Rochitte CE. Myocardial fibrosis in patients with hypertrophic cardiomyopathy and high risk for sudden death. Arq Bras Cardiol. 2010;94:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J Am Coll Cardiol. 1995;26:1699-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 422] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 35. | Valente AM, Lakdawala NK, Powell AJ, Evans SP, Cirino AL, Orav EJ, MacRae CA, Colan SD, Ho CY. Comparison of echocardiographic and cardiac magnetic resonance imaging in hypertrophic cardiomyopathy sarcomere mutation carriers without left ventricular hypertrophy. Circ Cardiovasc Genet. 2013;6:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 456] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 37. | Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, Nassenstein K, Schlosser T, Sabin GV, Sechtem U. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 445] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 38. | Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 39. | Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 40. | Tester DJ, Ackerman MJ. Genetic testing for potentially lethal, highly treatable inherited cardiomyopathies/channelopathies in clinical practice. Circulation. 2011;123:1021-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 41. | Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60:705-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 518] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 42. | Marian AJ. Hypertrophic cardiomyopathy: from genetics to treatment. Eur J Clin Invest. 2010;40:360-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Ingles J, Sarina T, Yeates L, Hunt L, Macciocca I, McCormack L, Winship I, McGaughran J, Atherton J, Semsarian C. Clinical predictors of genetic testing outcomes in hypertrophic cardiomyopathy. Genet Med. 2013;15:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8:1308-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 760] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 45. | Wordsworth S, Leal J, Blair E, Legood R, Thomson K, Seller A, Taylor J, Watkins H. DNA testing for hypertrophic cardiomyopathy: a cost-effectiveness model. Eur Heart J. 2010;31:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Ingles J, McGaughran J, Scuffham PA, Atherton J, Semsarian C. A cost-effectiveness model of genetic testing for the evaluation of families with hypertrophic cardiomyopathy. Heart. 2012;98:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Charron P. Genetic analysis for predictive screening in hypertrophic cardiomyopathy. Heart. 2012;98:603-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Kelly M, Semsarian C. Multiple mutations in genetic cardiovascular disease: a marker of disease severity? Circ Cardiovasc Genet. 2009;2:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Ingles J, Doolan A, Chiu C, Seidman J, Seidman C, Semsarian C. Compound and double mutations in patients with hypertrophic cardiomyopathy: implications for genetic testing and counselling. J Med Genet. 2005;42:e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 50. | Tsoutsman T, Kelly M, Ng DC, Tan JE, Tu E, Lam L, Bogoyevitch MA, Seidman CE, Seidman JG, Semsarian C. Severe heart failure and early mortality in a double-mutation mouse model of familial hypertrophic cardiomyopathy. Circulation. 2008;117:1820-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Girolami F, Ho CY, Semsarian C, Baldi M, Will ML, Baldini K, Torricelli F, Yeates L, Cecchi F, Ackerman MJ. Clinical features and outcome of hypertrophic cardiomyopathy associated with triple sarcomere protein gene mutations. J Am Coll Cardiol. 2010;55:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 52. | Maron BJ, Maron MS, Semsarian C. Double or compound sarcomere mutations in hypertrophic cardiomyopathy: a potential link to sudden death in the absence of conventional risk factors. Heart Rhythm. 2012;9:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |