INTRODUCTION
PPARs are ligand-activated transcription factors of the nuclear receptor superfamily, and they comprise four members encoded by distinct genes (α, β, γ and δ). The PPARs undergo transactivation or transrepression under distinct mechanisms that lead to the induction or repression of target gene expression[1]. PPARs bind to sequence-specific target elements in the promoter region of target genes following heterodimerization with the retinoid receptor, and in doing so, they control the majority of steps in cellular fatty acid uptake, utilization, oxidation, and storage pathways; cell growth and migration; oxidative stress; and inflammation in the cardiovascular system[1,2]. Each PPAR is primarily located in a distinct set of tissues, is stimulated by different ligands, and has different effects[2]. Certain new effects of PPARs on hypertension have been identified in recent studies, and the present mini-review focuses on the literature related to the effects that PPARs and their agonists exert in this area. Each member of the PPAR family possesses distinct functions that are determined by their ligand affinity, expression, and activity, which are dependent on the metabolic pathway and the type of tissue.
PPAR STRUCTURE
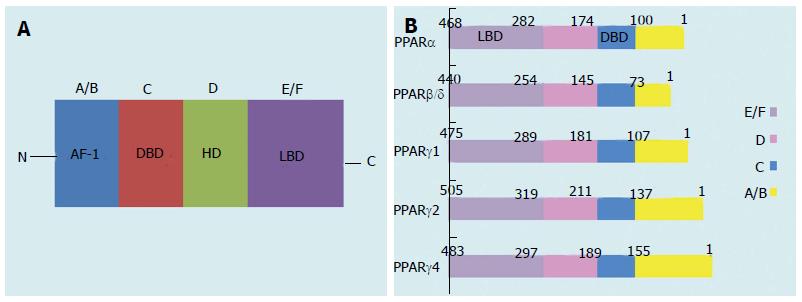
PPARs are orphan nuclear receptors belonging to the steroid, retinoid, and thyroid hormone receptor superfamily of ligand-activated transcription factors[3,4]. Three distinct receptor types have been cloned and characterized: PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3)[5]. Like other nuclear receptors, these PPAR isoforms have five or six structural regions within four functional domains, termed A/B, C, D, and E/F (Figure 1A)[5]. The variable NH2-terminal end, which is a ligand-independent transactivation domain (the A/B domain), contains activation function (AF)-1, which is a target of kinase phosphorylation[5]. The 70-amino-acid-long PPAR DNA-binding domain (the C domain) contains two highly conserved zinc finger motifs and promotes the binding of receptors to a DNA sequence in the promoter region of target genes, which is known as the peroxisome proliferator response element (PPRE)[5]. The hinge region (the D domain) acts as a docking site for cofactors. The C-terminal or ligand-binding domain (the E/F domain) is responsible for ligand specificity and the activation of PPAR binding to the PPRE, which increases target gene expression. The E/F domain uses cofactors for the transactivation via the ligand-dependent trans-AF-2[5]. When activated by endogenous or synthetic ligands, the PPARs, like other nuclear hormone receptors, heterodimerize with the 9-cis-retinoic acid receptor (retinoid × receptor)[5]. The PPAR-retinoid × receptor heterodimer undergoes conformational changes, binds to the PPRE in the promoter region of the target gene, and alters coactivator/corepressor dynamics to modulate the transcription machinery, which in turn affects the initiation of transcription (via upregulation or downregulation) and the abundance of messenger RNA (mRNA) in the target genes[6,7]. PPARs are also drug targets; currently, PPARα agonists (fibrates) are in clinical use for treating dyslipidemia, and PPARγ agonists (thiazolidinediones (TZDs)) are being used to treat type 2 diabetes mellitus (T2DM)[8].
Figure 1 Schematic structure of peroxisome proliferator-activated receptor protein isoforms.
A/B, C, D, and E/F indicate the N-terminal A/B domain containing a ligand-independent AF-1, the DBD, the hinge region, and the C-terminal LBD containing AF-2, respectively. AF-1 is responsible for phosphorylation, while AF-2 promotes the recruitment of co-activators for gene transcription. PPAR: Peroxisome proliferator-activated receptor; AF-1: Activation function-1; DBD: DNA-binding domain; HD: Hinge domain; LBD: Ligand-binding domain. Figure adapted from reference[8].
PPAR EXPRESSION
The PPAR family possesses distinct functions that are determined by their ligand affinity, expression, and activity, which are dependent on the metabolic pathway and the type of tissue[1]. The characteristics of each PPAR isotype are described below.
PPARα was the first PPAR isotype to be cloned, and its name comes from its activation by peroxisome proliferator chemicals[9,10]. Its expression is greatest in tissues with a high fatty acid oxidation rates, such as heart, liver and skeletal muscle, and functions as a major regulator of fatty acid homeostasis[10-13]. PPARα expression is also significant in the adipose, adrenal and kidney tissue (particularly brown adipose tissue), and the majority of cell types, including endothelial, smooth muscle, and macrophages, in the vasculature[12-14].
PPARβ/δ (PPARδ) is expressed at relatively high levels in liver, kidney, cardiac and skeletal muscle, adipose tissue, brain, colon, and vasculature[14-17]. Unlike PPARα and PPARγ, PPARδ does not seem to be the target of available drugs[8]. The unavailability of PPARδ-targeted drugs may be due to its wide ranging expression. The physiological function of PPARδ is much less studied and understood[8].
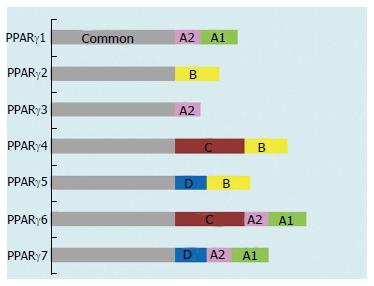
PPARγ is highly expressed in adipose tissue and plays an indispensable role in the regulation of adipocyte differentiation, lipid storage, and glucose metabolism and in the transcriptional regulation of a number of genes involved in these metabolic processes[13,18-20]. Some key target genes of PPARγ include the fat-specific ap2 gene, LPL, fatty acid transport, fatty acid-binding protein, FAT/CD36, acyl-CoA synthase, GLUT4, glucokinase, phosphoenolpyruvate carboxykinase, uncoupling proteins 1, 2, and 3 and LXRα[18,19,21]. PPARγ also regulates genes involved in insulin signaling and the expression of proinflammatory cytokines such as tumor necrosis factor (TNF)-α[20,21]. It also has significant anti-inflammatory effects[18,19,21] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3246744/ - R49. Most importantly, PPARγ is a well-recognized cellular target for the antidiabetic thiazolidinediones, which sensitize cells to insulin and improve insulin sensitivity and action[22-24]. To date, seven mRNA transcripts generated through different forms of initiation and the alternative splicing of five exons at the 5’-terminal region (A1, A2, B, C and D) have been identified (Figure 2)[24-26]. Each mRNA transcript is different, based on the combination of five exons. They have been designated PPARγ1, -γ2, -γ3, -γ4, -γ5, -γ6, and -γ7. PPARγ1, -γ3, -γ5, and -γ7 mRNA transcripts translate to the identical PPARγ1 protein. PPARγ2 mRNA yields PPARγ2 protein, while PPARγ4 and -γ6 mRNA transcripts produce identical PPARγ4 protein (Figure 1B)[25-27]. The PPARγ1 mRNA isoform is expressed in a range of tissues: cardiac and skeletal muscle; pancreatic β-cells; the spleen, intestines, kidneys, and adrenal gland; vascular cells such as endothelial cells (ECs) and smooth muscle cells; and monocytes/macrophages[26,28,29]. The expression of PPARγ2 mRNA is primarily restricted to adipose tissue, whereas PPARγ3 mRNA is abundant in macrophages, the large intestine (colon), and adipocytes[24,26,30]. High levels of PPARγ4, -γ5, -γ6, and -γ7 mRNA transcripts are expressed in macrophages, while PPARγ6 and -γ7 mRNAs are also detected in adipose tissue[24,25,27,30].
Figure 2 Domain structure of the peroxisome proliferator-activated receptor γ isoforms.
PPAR: Peroxisome proliferator-activated receptor. Figure adapted from reference[8].
PPAR ACTIVATION AND REGULATORY ROLES
PPARs are found primarily within the nucleus without their ligand and localize to target gene promoters with either co-activator or co-repressor complexes[31]. To date, many ligands had been identified that activate and modulate PPAR functions[31]. Endogenous lipid metabolites from saturated or unsaturated fatty acids, for example, are able to bind to nuclear receptors and activate or repress gene expression[31]. Another group of PPAR ligands consists of lipid metabolites from essential fatty acids, such as arachidonic acid derived from lipoxygenase or cyclooxygenase activity[31]. In particular, the best-characterized endogenous ligands known to stimulate PPARα are the eicosanoids LTB4 and 8-hydroxyeicosatetraenoic acid (8(S)-HETE), while 15d-PGJ2 and 13-HODE activate PPARγ[31]. Other essential fatty acid metabolites, such as 15-HETE, have been suggested to activate PPARβ/δ[31].
The discovery of PPARs as a key regulator of metabolic pathways has provided significant insight into the mechanisms involved in this process[28,32]. PPARs act as nutritional sensors that regulate a variety of homeostatic functions, including metabolism, inflammation, and development[28,32,33]. PPARs are involved in many functions, particularly those having to do with the regulation of vascular tone, inflammation, and energy homeostasis. Therefore, they represent important targets for addressing hypertension, obesity, obesity-induced inflammation, and metabolic syndrome in general[1,32-34]. PPARs may influence the inflammatory response either directly through the transcriptional downregulation of proinflammatory genes via mechanisms involving transrepression or indirectly via their transcriptional effects on lipid metabolism[1,8]. Because of their pleiotropic effects, they are now known to be active in a number of disease conditions, and they represent potent therapeutic targets for a wide range of diseases[1,8,32,34]. PPAR agonists may be of benefit, either alone or in combination with other drugs that influence the inflammatory response, in treating hypertension, atherosclerosis, and metabolic derangements associated with obesity[34].
The endogenous ligands that bind to PPARα with the highest affinity are saturated/unsaturated fatty acids, leukotriene derivatives, and VLDL hydrolysis products[33]. Examples of synthetic ligands that bind PPARα are the fibrate class of hypolipidemic drugs, the experimental ligand Wy-14643 ([4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio] acetic acid) and some phthalate monoesters (monoethylhexyl phthalate), and herbicides (lactofen)[33]. PPARα is a major regulator of the mitochondrial and peroxisomal β-oxidation pathway, and as will be discussed below, it is suggested that these pathways are involved in the pathogenesis of various liver complications[33]. PPARα activation inhibits vascular smooth muscle proinflammatory responses, attenuating the development of atherosclerosis[15,35]. PPARα ligands negatively regulate interleukin (IL)-6 promoter activation, and chronic treatment with fenofibrate, a PPARα agonist, suppresses IL-6-induced atherosclerosis[36]. The absence of PPARα expression is suggested to prolonged the inflammatory response, and PPARα has anti-inflammatory properties[36]. Furthermore, the PPARα ligand, fenofibrate, may repress ICAM-1 and VCAM-1 expression in endothelial cells[36]. In addition, PPARα activation has been reported to inhibit NF-κB activation and inflammatory gene expression[35].
Activation of the nuclear hormone receptor PPARβ/δ is known to both improve insulin resistance and plasma high-density lipoprotein levels and to exhibit anti-inflammatory properties in the vessel wall through the inhibition of vascular cell adhesion molecule 1 and monocyte chemoattractant protein 1 expression[37].
Although PPARγ was first to be recognized as an anti-inflammatory agent, both PPARα and PPARδ are also known to have similar effects[34]. Inflammation is a significant aspect of the damage that hypertensive disease causes[34]. PPARs are now seen as important determinants of macrophage polarization[34]. Monocyte precursors of classically and alternatively activated macrophages are being identified as important participants in the progress of metabolic syndrome-related cardiovascular disease, including hypertension, hyperlipidemia, and obesity[8,38,39]. The activation of PPARβ/δ has been shown to increase lipid catabolism in the skeletal muscle, heart, and adipose tissue and to improve the serum lipid profile and insulin sensitivity[39,40]. Further, PPARβ/δ ligands stop weight gain and reduce macrophage-derived inflammation[40]. One new approach that may prevent or regress hypertension-induced vascular, renal, and, perhaps, brain changes is the activation of nuclear receptors, which not only have metabolic effects but also exert anti-inflammatory actions through PPARα and PPARγ[41]. PPARα and PPARγ are therapeutic targets for hypertriglyceridemia and insulin resistance, respectively[42,43].
Covalent modifications include phosphorylation, ubiquitylation, O-GlcNAcylation, and SUMOylation[44]. Covalent modifications of PPARγ are key regulatory mechanisms that control both PPARγ protein stability and transcriptional activity[39,44]. PPARγ functions as a master switch in controlling adipocyte differentiation and development, and its activation has an important role in glucose metabolism by enhancing insulin sensitization[39,45]. PPARγ is a primary target for TZD-structured insulin sensitizers such as pioglitazone and rosiglitazone, which are used in the treatment of T2DM[39,45]. Additionally, PPARγ activation inhibits adhesion cascades and detrimental vascular inflammatory events[39,45]. Furthermore, although the primary action of select ARBs, which partially activate PPARγ, is to lower blood pressure, they may also be effective in treating insulin resistance and dyslipidemia absent the toxicity associated with full PPARγ agonists[39,46].
PPARγ activation is known to have an influence on the events connected with the development and progression of atherosclerotic lesions[14,15,24,34,39,47]. PPARγ and its ligands may exert direct antiatherosclerotic action[14,15,24,48-50] Consistent with the anti-inflammatory properties of PPARγ and the TZDs, aortas showed decreased accumulation of macrophages in the lesions as well as attenuated expression of proatherogenic agents. Interestingly, these changes occurred independently of improvements in dyslipidemia, glycemic control, and hypertension, which supports the assumption of a direct vascular effect[8,50,51]. Moreover, PPARγ activation plays a distinct role in regulating the physiology and expression of endothelial nitric oxide synthase (eNOS) in the endothelium, resulting in enhanced generation of vascular nitric oxide[45]. PPARγ activation-mediated vascular anti-inflammatory and direct endothelial functional regulatory actions could therefore be beneficial in improving vascular function in patients with atherosclerosis and hypertension with or without DM[45]. Unfortunately, PPARγ agonists can exert long-term effects on certain patients, including increased body weight, fluid retention, and risk of heart failure[39]. This is unfortunate, as TZDs show consistent efficacy in the treatment of T2DM[39]. More recently, there has been increased concern about the association between TZD and bone loss[39]. The association with bone loss is an especially worrisome concern because fracture is usually when it is detected[39]. The biguanide metformin is currently the first-line medication in the treatment of T2DM due to increasing concerns about the safety of TZDs[39]. While the cardiac side effect profile of rosiglitazone-like PPARγ full agonists is unfortunate, the therapeutic potential of novel pharmacological agents targeting PPARγ submaximal cannot be excluded. Interestingly, newly synthesized partial agonists of PPARγ, such as balaglitazone, MBX-102, MK-0533, PAR-1622, PAM-1616, KR-62776, and SPPARγM5, have a reduced tendency to cause the adverse effects associated with full agonists of PPARγ or may be entirely devoid of such effects[45]. Therefore, with as much as 50% of patients with ischemic stroke and transient ischemic attack also having insulin resistance, drugs capable of addressing both hypertension and insulin resistance could be of great benefit in preventing stroke[46]. In summary, PPARγ is implicated both in the maintenance of vascular homeostasis and in the pathogenesis of a number of vascular conditions such as atherosclerosis, hypertension, and restenosis[28,29,39,52]. TZDs, which are PPARγ agonists, lower blood pressure and exert protective vascular effects through largely unknown mechanisms[39,52]. In contrast, loss-of-function dominant-negative mutations in human PPARγ cause insulin resistance and severe early onset hypertension[52].
EFFECTS OF PPARS ON BLOOD PRESSURE
PPARα ligands have been reported to decrease blood pressure in various models of hypertension[36]. Several mechanisms have been proposed for the antihypertensive effects of PPARα agonists such as the increased excretion of Na+ through reduced Na+-K+ ATPase activity in the proximal tubules, increased cytochrome P450 (CYP) 4A expression, and increased renal tubular 20-HETE production, which exerts a natriuretic effect[36,53-57]. A recent report has described a crosstalk between PPARα and IL-6 in the regulation of blood pressure[36,58]. Furthermore, another report demonstrates that PPARα activation attenuates angiotensin-II (Ang-II)-induced hypertension through the upregulation of CYP4A and CYP2J and the attenuation of plasma IL-6, renal MCP-1 and other inflammatory markers, and the renal expression of ICAM-1 and COX-2[36].
A PPARβ/δ agonist has been reported to induce progressive systolic arterial blood pressure and heart rate reduction, and to reduce mesenteric arterial remodeling, endothelial dysfunction, and aortic vasoconstriction in response to Ang-II[37]. These were accompanied by a significant increase in eNOS activity attributed to upregulated eNOS and downregulated caveolin 1 protein expression[37]. Moreover, the PPARβ/δ agonist also inhibited vascular superoxide production, downregulated p22phox and p47phox protein expression, decreased both basal and Ang-II-stimulated NADPH oxidase activity, inhibited extracellular-regulated kinase 1/2 activation, and reduced the expression of proinflammatory and proatherogenic genes, including IL-1β, IL-6, and intercellular adhesion molecule 1[37]. Further, the same study showed that PPARβ/δ activation, both in vitro and in vivo, increased the expression of RGS4 and RGS5, which are regulators of G protein-coupled signaling proteins; RGS4 and RGS4, in turn, negatively modulated the vascular actions of Ang-II[37]. PPARβ/δ activation also exerted antihypertensive effects, restored vascular structure and function, and reduced the oxidative, proinflammatory, and proatherogenic statuses[37]. Hence, PPARβ/δ was proposed as a new therapeutic target in hypertension[37].
It has been reported recently that independent of its blood glucose-lowering effects, PPARγ demonstrates pleiotropic beneficial effects on vasculature[59]. The effect may possibly be due to PPARγ-mediated inhibition of Ang-II type 1 receptor (AT1R) expression as well as Ang-II-mediated signaling pathways, which may result in suppression of the renin-angiotensin system (RAS) and lead to a lower blood pressure[59].
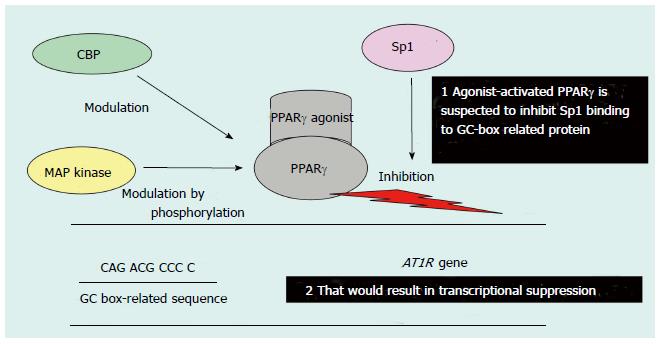
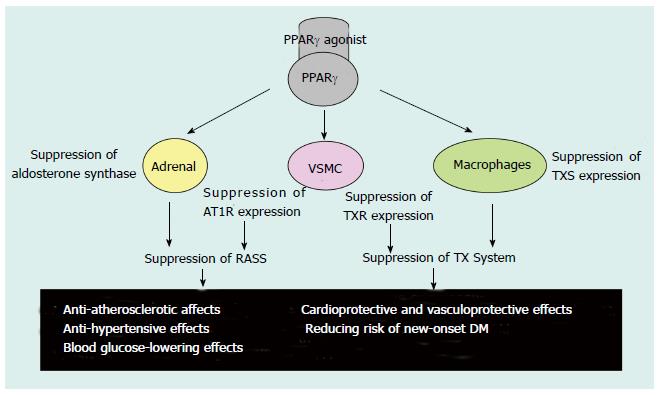
However, it has also been speculated that PPARγ-induced AT1R gene transcription suppression is mediated through the inhibition of Sp1 binding to DNA. This inhibition is due to the protein-protein interaction between ligand-activated PPARγ and Sp1; indeed, the PPARγ ligand-mediated suppression of AT1R expression has been demonstrated previously (Figure 3)[60-62]. Interestingly, transcription suppression was abrogated by the over-expression of the coactivator CERB-binding protein (CBP) and PPARγ phosphorylation by mitogen-activated protein (MAP) kinase, most likely because of the functional modification of PPARγ (Figure 3)[60]. Moreover, PPARγ ligands have been shown to suppress Ang-II-induced phosphatidylinositol 3-kinase and MAP kinase and to ameliorate AngII-mediated inflammatory responses by interfering with the Toll-like receptor 4-dependent signaling pathway[62,63]. Therefore, PPARγ not only downregulates AT1R expression but also inhibits Ang II-mediated signaling pathways, which may result in RAS suppression (Figure 4)[62-64]. On the other hand, transgenic mice expressing a dominant negative PPARγ P465L mutation are hypertensive, which is consistent with the phenotype of patients who have an equivalent PPARγ P467L mutation without affecting components of the RAS[59]. Thus, ligand-activated PPARγ may lower blood pressure through several different mechanisms in addition to inhibiting the RAS[59].
Figure 3 Possible mechanism of peroxisome proliferator-activated receptor γ-agonist-mediated transcriptional suppression of the Ang-II type 1 receptor gene promoter.
PPAR: Peroxisome proliferator-activated receptor; AT1R: Ang-II type 1 receptor; CBP: CERB-binding protein; MAP: Mitogen-activated protein. Figure adapted from reference[71].
Figure 4 Possible effects of Peroxisome proliferator-activated receptor γ agonists.
PPAR: Peroxisome proliferator-activated receptor; AT1R: Ang-II type 1 receptor; RAAS: Renin-angiotensin-aldosterone system; TX: Thromboxane; TXS: TX synthase; TXR: TX receptor; VSMC: Vascular smooth muscle cells; DM: Diabetes mellitus. Table adapted from reference[71].
In terms of blood pressure, the transient administration of ARBs may prevent the development of hypertension, and high doses of ARBs may regress mild hypertension[65]. Next-generation ARBs are becoming available that are intended not only to antagonize AT1R but also to block endothelin receptors, function as nitric oxide donors, inhibit neprilysin activity, increase natriuretic peptide levels, or stimulate PPARγ[66]. It has been shown that ARBs have benefits beyond their established cardioprotective and vasculoprotective effects, including lowering risk of new-onset diabetes and its associated cardiovascular effects[67]. Furthermore, it has also been found that the drug telmisartan can selectively activate PPARγ in targeting DM, and it therefore provides an approach to the prevention and treatment of cardiovascular complications in high-risk elderly patients suffering from hypertension and new-onset DM[67]. The beneficial metabolic effects of telmisartan have been attributed to its action as an Ang-II receptor blocker and as a partial PPARγ agonist, and it has also been found that telmisartan may have the strongest binding affinity to AT1R[43,68]. Treatment with telmisartan has been shown to significantly improve endothelial dysfunction and inhibit lipid accumulation in the liver[43]. It is possible that the favorable characteristics of telmisartan are due to its action as a partial PPARγ agonist, apart from its blood pressure-lowering effect as an Ang-II blocker, possibly earning it the name “metabosartan”[43]. These observations suggest that because of its unique PPARγ-modulating activity, telmisartan may be one of the most promising sartans for the treatment of cardiometabolic disorders[68].
EVIDENCE FROM ANIMAL AND HUMAN STUDIES
PPARγ activation is suggested to be beneficial in inflammatory diseases, not only in humans but also in rats and pigs[69]. The question is now whether PPARγ activation mitigates immunological stress such as mastitis in livestock. In livestock species in general, however, data on the use of synthetic PPAR agonists are limited[69]. Considering the high amino acid identities ranging from 95% to 98% for the PPAR proteins in all species, one may believe that bovine and porcine PPARs could also be targeted using the existing synthetic PPAR agonists[69]. However, because only a minor overlap between the Wy-regulated genes from mouse and human primary hepatocytes was found and because PPREs are not fundamentally conserved among species, activation of the PPARs does not necessarily activate the same array of genes in one species as in another[69]. Data from the literature makes it clear that further studies on the impact of PPAR ligands in livestock are necessary as such investigations may identify unconsidered health and sanitation benefits.
It has been shown that WY14643, a potent PPARα agonist, has cardioprotective and cardiodepressive effects when used to treat encephalomyocarditis virus-induced myocarditis in diabetic mice[38]. The cardioprotective effect may be due to its anti-inflammatory properties and its ability to increase cardiac adiponectin expression, whereas the reduced cardiac efficiency may be due to its enhancement of cardiac UCP3 mRNA expression[38]. In animals, the pharmacological or genetic elevation of plasma adiponectin relieves obesity-induced endothelial dysfunction and hypertension, and prevents atherosclerosis, myocardial infarction, and diabetic cardiomyopathy[70]. These therapeutic benefits of PPARγ agonists (TZDs) are mediated by the induction of adiponectin[70]. Adiponectin protects cardiovascular health through its vasodilator, anti-apoptotic, anti-inflammatory, and anti-oxidative activities in both cardiac and vascular cells[70].
PPARγ agonists are known to lower blood pressure in humans, possibly through the suppression of the RAS, by mechanisms including the inhibition of AT1R expression, Ang-II-mediated signaling pathways, and Ang-II-induced adrenal aldosterone synthesis/secretion[52,71]. PPARγ agonists also inhibit the progression of atherosclerosis in humans, possibly through a pathway involving suppression of the RAS and the thromboxane system, as well as the protection of endothelial function[71]. Moreover, PPARγ-agonist-mediated renal protection, particularly the reduction of albuminuria, has been reported in diabetic nephropathy, including animal models of the disease, and in nondiabetic renal dysfunction[71]. The renal protective activities may reflect, at least in part, the ability of PPARγ agonists to lower blood pressure, protect endothelial function, and cause vasodilation of the glomerular efferent arterioles[71]. In addition, it has recently been reported that PPARγ agonists have antineoplastic effects and that they can ameliorate polycystic kidney, polycystic liver, and cardiac defects through the β-catenin, c-Myc, CFTR, MCP-1, S6, ERK, and TGF-β signaling pathways in animal models of chronic kidney disease (CKD)[71]. The multiple therapeutic actions of PPARγ agonists leave no doubt that they will produce new approaches to lifestyle-related and other diseases[71].
However, negative (harmful) aspects of PPARs have also been reported. TZDs are insulin-sensitizing antidiabetes agents that act through PPARγ to cause a durable improvement in glycemic control in patients with T2DM[72,73]. These benefits must be weighed against the side effects of the drug, which include weight gain, fluid retention, atypical fractures, and possibly, bladder cancer[72,73]. Despite having similar effects on glycemic control, pioglitazone and rosiglitazone appear to have different effects on cardiovascular outcomes[72,73]. Rosiglitazone has been associated with an increased risk of myocardial infarction, and its use in the United States is restricted because of cardiovascular safety concerns[72,73]. PPAR-α/γ or -γ/δ dual agonists are now under development[74,75].
As the literature has been indicating, disorders of pregnancy, such as preeclampsia and gestational diabetes, are potential targets for treatment with PPAR ligands[76]. In clinical cases, including preeclampsia, gestational diabetes, and intrauterine growth restriction, aberrant regulation of components of the PPAR system parallels the dysregulation of metabolism, inflammation, and angiogenesis[76]. These actions are the result of the roles of the PPARs in regulating human trophoblast invasion and early placental development[76]. PPARs are involved in trophoblast invasion, placental development, parturition, and pregnancy-specific diseases, particularly preeclampsia and gestational diabetes[76]. The PPAR system’s involvement in pregnancy under physiologic and pathologic conditions has yet to be fully clarified due to a lack of knowledge about endogenous PPAR ligands[76]. Partially characterized inflammatory, angiogenic, and metabolic disturbances in pregnancy-related diseases suggest that these synthetic PPAR agonists may be of potential use in these conditions[76].
To date, several large clinical trials of hypertension using PPAR agonists have been conducted worldwide, including in Japan. The Losartan Intervention for Endpoint reduction in hypertension (LIFE) study compares the effects of losartan- (a PPARγ agonist) and atenolol- (a β blocker) based antihypertensive treatment on cardiovascular morbidity and mortality in a population of 9193 hypertensive patients with left ventricular hypertrophy (LVH)[77]. In the LIFE study, losartan-based treatment further reduced the primary composite end point (cardiovascular death, myocardial infarction, or stroke) by 13% [relative risk reduction (RRR) 0.87, 95%CI: 0.77-0.98, P = 0.021]. The further reduction in stroke with losartan (RRR 0.75, 95%CI: 0.63-0.89, P = 0.001) was the major contributing factor to the reduction in the primary end point[77].
The Study on Cognition and Prognosis in the Elderly (SCOPE) assessed the effect of candesartan (PPARγ agonist) on cardiovascular and cognitive outcomes in elderly patients (aged 70-89 years) with mild to moderate hypertension[78]. Patients were randomized to candesartan 8-16 mg daily (n = 2477) or placebo (n = 2460) and followed for an average of 3.7 years[78]. Other antihypertensive drugs were added if blood pressure remained greater than 160 mmHg systolic and/or 90 mmHg diastolic[78]. Due to extensive add-on therapy, particularly in patients randomized to placebo, the between-treatment difference in blood pressure was only 3.2/1.6 mmHg[78]. The main analysis showed, however, that non-fatal stroke was reduced by 28% (P = 0.04) in the candesartan group compared with the control group, and a non-significant 11% reduction in the primary endpoint of major cardiovascular events was seen (P = 0.19)[78]. In conclusion, the findings of SCOPE suggest that candesartan treatment reduces cardiovascular morbidity and mortality in old and very old patients with mild to moderate hypertension. Candesartan-based antihypertensive treatment may also have positive effects on cognitive function and quality of life[78].
The Valsartan (PPARγ agonist) Antihypertensive Long-term Use Evaluation (VALUE) trial was designed to evaluate the hypothesis that for the same blood-pressure control, valsartan would reduce cardiac morbidity and mortality more than amlodipine (a calcium channel blocker) in hypertensive patients at high cardiovascular risk[79]. Blood pressure was reduced by both treatments, but the effects of the amlodipine-based regimen were more pronounced, particularly in the early period (blood pressure 4.0/2.1 mmHg lower in the amlodipine group than the valsartan group after 1 mo; 1.5/1.3 mmHg after 1 year; P < 0.001 between groups)[79]. There was no difference between the treatment groups in the primary composite endpoint, which was the occurrence of cardiac disease[79].
The Trial of Preventing Hypertension (TROPHY) investigated whether pharmacological treatment of prehypertension prevents or postpones stage 1 hypertension[80]. Participants with repeated blood pressure measurements of 130-139 and/or 85-89 mmHg were randomly assigned to 2 years of candesartan or placebo, followed by 2 years of placebo for all[80]. The 4-year incidence of hypertension was significantly (P < 0.01) lower than that previously reported in the placebo (-11.3%) and candesartan (-11.0%) groups[80]. During the first 2 years, hypertension developed in 162 placebo and 53 candesartan participants (RRR 68%, P < 0.001)[80]. After 4 years, hypertension occurred in 197 placebo and 165 candesartan participants (RRR 18%, P < 0.009)[80]. The new definition resulted in a lower incidence of hypertension, but the outcomes were remarkably similar with both definitions and confirmed our original findings[80].
In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial and the Telmisartan Randomized AssessmeNt Study in ACE-I iNtolerant Subjects with Cardiovascular Disease, researchers assessed the cardioprotective and antidiabetic effects of telmisartan[67]. The collective data suggest that telmisartan is a promising drug for controlling hypertension and reducing vascular risk in high-risk elderly patients with new-onset diabetes[52]. Furthermore, several clinical studies have demonstrated the blood pressure-lowering effect of TZDs as PPARγ ligands[81]. The recent PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive Study), which included 5238 T2DM enrollees, also demonstrated a significant decrease in systolic blood pressure (3 mmHg) following treatment with pioglitazone (a TZD)[82].
Disappointingly, the results from the Fenofibrate Intervention and Event Lowering in Diabetes trial failed to show a reduction in risk for the primary end-point (coronary heart disease death and nonfatal myocardial infarction) of coronary events with fenofibrate therapy[83]. There are many explanations for these results, including the use of a low cardiovascular risk diabetic population; however, more investigation is clearly needed to understand the clinical relevance of fibrates for treating CVD[83].
In a sub-analysis of the Candesartan Antihypertensive Survival Evaluation in Japan trial, researchers examined the relationship between the achieved blood pressure and cardiovascular events in hypertensive patients with T2DM, CKD, or LVH at baseline[84]. A higher achieved blood pressure was associated with an increased risk of cardiovascular events in hypertensive patients with complications (T2DM, CKD, or LVH)[84]. In patients with LVH, who achieved a systolic/diastolic blood pressure (SBP/DBP) < 130/75-79 mmHg, the risk of cardiovascular events was reduced to the same level as in those without LVH, an SBP/DBP < 130/75-79 mmHg[84]. However, the risks of cardiovascular events in patients with DM or CKD, who achieved an SBP/DBP < 130/75-79 mmHg, were still significantly higher than in those without DM or CKD[84].
CONCLUSION
Although a decade or more has passed since the pleiotropic effects of PPARγ were first reported, numerous studies on its novel effects continue to appear each month. In addition to the effects on blood pressure, atherosclerosis, and kidney dysfunction described above, anti-cancer effects of PPARγ ligands have recently been reported[59]. The usefulness and effectiveness of PPARγ ligands in the treatment of lifestyle-related diseases will be increasingly appreciated[59,85].
Further refinement of experimental strategies, group-specific chemical modification of potential compounds, and the development of specific and reliable translational models and biomarkers to better understand their safety and efficacy should all be of great assistance in the future clinical development of novel types of PPAR agonists[8]. Moreover, future efforts to further delineate the physiology, pharmacology, and molecular functions of the PPARs may identify additional novel targets that can also be exploited in the development of superior, efficacious, and tissue-/PPAR isotype-specific agonists for the treatment of hypertension[8].
There are clearly many uncertainties about the use of PPAR agonists in the treatment of cardiovascular disease. They have highly complex biologic effects resulting from the activation or suppression of dozens of genes, and the biologic effects of the protein targets for most of these genes remain largely unknown. Moreover, they possess different properties for different species[2]. Further efforts to completely investigate the effects of the PPARs and their agonists and the mechanisms by which they improve lifestyle-related diseases are required, including high blood pressure, in both human and animal models[2]. Additionally, the adverse effects of PPARγ agonists on cardiac function and water retention and the mechanisms responsible for these effects should be clarified in detail, particularly in humans[2]. Finally, the combination of PPARs with reagents or with other cardiovascular drugs such as diuretics and ARBs should be studied[2].
ACKNOWLEDGMENTS
I would like to express my deep gratitude to Professor Tsugiyasu Kanda, my supervisors, for enthusiastic encouragement and useful critiques of this work. I would also like to thank Dr. Emiri Muranaka, for her advice and assistance in keeping my progress on schedule. Finally, I wish to thank my parents for their support and encouragement throughout my study.