Published online Jul 26, 2014. doi: 10.4330/wjc.v6.i7.675
Revised: May 28, 2014
Accepted: June 27, 2014
Published online: July 26, 2014
Processing time: 141 Days and 3.1 Hours
AIM: To evaluate the referrals with suspected arrhythmogenic right ventricular cardiomyopathy (ARVC) and compare cardiac MR (cMR) findings against clinical diagnosis.
METHODS: A retrospective analysis of 114 (age range 16 to 83, males 55% and females 45%) patients referred for cMR with a suspected diagnosis of ARVC between May 2006 and February 2010 was performed after obtaining institutional approval for service evaluation. Reasons for referral including clinical symptoms and family history of sudden death, electrocardiogram and echo abnormalities, cMR findings, final clinical diagnosis and information about clinical management were obtained. The results of cMR were classified as major, minor, non-specific or negative depending on both functional and tissue characterisation and the cMR results were compared against the final clinical diagnosis.
RESULTS: The most common reasons for referral included arrhythmias (30%) and a family history of sudden death (20%). Of the total cohort of 114 patients: 4 patients (4%) had major cMR findings for ARVC, 13 patients (11%) had minor cMR findings, 2 patients had non-specific cMR findings relating to the right ventricle and 95 patients had a negative cMR. Of the 4 patients who had major cMR findings, 3 (75%) had a positive clinical diagnosis. In contrast, of the 13 patients who had minor cMR findings, only 2 (15%) had a positive clinical diagnosis. Out of the 95 negative patients, clinical details were available for 81 patients and none of them had ARVC. Excluding the 14 patients with no clinical data and final diagnosis, the sensitivity of the test was 100%, specificity 87%, positive predictive value 29% and the negative predictive value 100%.
CONCLUSION: CMR is a useful tool for ARVC evaluation because of the high negative predictive value as the outcome has a significant impact on the clinical decision-making.
Core tip: This study was designed to evaluate the referrals with suspected Arrhythmogenic right ventricular cardiomyopathy (ARVC) and compare the findings of cardiac magnetic resonance imaging (cMR) against clinical diagnosis. Currently the diagnosis depends upon a combination of variety of factors including imaging findings. We evaluated all the referrals in our institution over a 4-year period and found a high sensitivity and specificity of cMR for ARVC diagnosis. We have concluded that cMR is a very useful tool for ARVC evaluation because of the very high negative predictive value as the outcome has a significant impact on the clinical decision-making.
- Citation: Chellamuthu S, Smith AM, Thomas SM, Hill C, Brown PWG, Al-Mohammad A. Is cardiac MRI an effective test for arrhythmogenic right ventricular cardiomyopathy diagnosis? World J Cardiol 2014; 6(7): 675-681
- URL: https://www.wjgnet.com/1949-8462/full/v6/i7/675.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i7.675
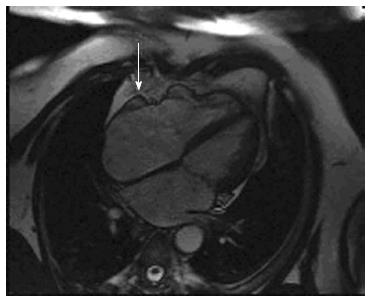
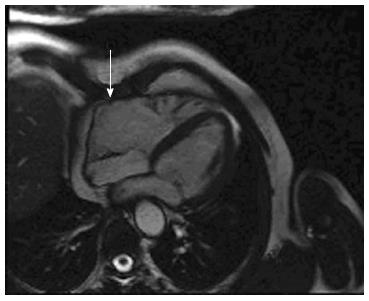
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a genetic form of cardiomyopathy characterized by fibro-fatty replacement primarily of right ventricular muscle. ARVC is inherited predominantly as an autosomal dominant pattern. There are also recessive forms described caused by mutations in the plakoglobin and desmoplakin (e.g., Naxos Disease, Carvajal Syndrome) which are associated with a cutaneous phenotype[1-3]. Although the name describes a “right” ventricular process, it is now recognized that left ventricular involvement is much more common and acknowledged at an earlier stage than before[4-6]. In the early stage of the disease, structural changes may be absent or subtle and are due to myocardial injury, inflammation and repair[7] and are confined to a localized region of the right ventricle (RV), typically the RV outflow tract, pulmonary infundibulum or the RV apex, which form a “triangle of dysplasia[8] (Figure 1, see arrows). These areas are eventually replaced by fibrous and fatty tissue[7,9] resulting in aneurysm formation, which is commonly seen in the basal inferior wall below the tricuspid valve[10]. Ventricular aneurysms at these sites can be considered pathognomonic of ARVC[7] (Figure 2, see arrows). These changes contribute to the electrical instability, which triggers ventricular tachycardia and sudden cardiac death (SCD)[11,12].
Clinical manifestations of ARVC are variable. The most common symptoms are palpitations and syncope, due to the occurrence of ventricular tachycardia. Occasionally, SCD may be the first event. ARVC is a relatively common cause of unexpected SCD in the young, especially in athletes[13,14]. Progressive structural involvement results in failure of the right or left ventricle depending on which one is predominantly affected and eventually to biventricular heart failure[15-17]. Following the diagnosis of ARVC or the occurrence of SCD due to suspected ARVC; evaluation of family members is frequently initiated.
Histological confirmation is required for the definitive diagnosis of ARVC; however myocardial biopsy may not necessarily be sensitive due to the segmental nature of the disease[18]. An International Task Force (Table 1) was developed in 1994, which proposed major and minor criteria for the diagnosis of ARVC[19]. The diagnostic criteria depends on a variety of factors including functional and structural abnormalities on imaging such as cMR or echocardiography, tissue characterisation (i.e., endomyocardial biopsy), repolarization and depolarisation abnormalities in the electrocardiogram (ECG), arrhythmias, family history and genetic analysis[20]. These criteria although were highly specific, lacked the sensitivity for early disease[21,22]. In 2010, revised criteria were proposed to include quantitative parameters particularly for the imaging studies (Table 2) to improve diagnostic sensitivity whilst maintaining specificity[5].
| 1 Global and/or regional dysfunction and structural alterations (detected by echocardiography, angiography, magnetic resonance imaging, or radionuclide scintigraphy) |
| Major: Severe dilatation and reduction of right ventricular ejection fraction with no (or only mild) left ventricular impairment. Localized right ventricular aneurysms (akinetic or dyskinetic areas with diastolic bulging). Severe segmental dilatation of the right ventricle |
| Minor: Mild global right ventricular dilatation and/or ejection fraction reduction with normal left ventricle Mild segmental dilatation of the right ventricle Regional right ventricular hypokinesia |
| 2 Tissue characterization of wall |
| Major: Fibro-fatty replacement of myocardium on endomyocardial biopsy |
| 3 Repolarisation Abnormalities |
| Minor: Inverted T waves in right precordial leads (V2 and V3) in people aged > 12 yr, in absence of right bundle branch block |
| 4 Depolarization/conduction abnormalities |
| Major: Epsilon waves or localized prolongation (> 110 ms) of the QRS complex in right precordial leads (V1-V3) |
| Minor: Late potentials (signal-averaged ECG) |
| 5 Arrhythmias |
| Minor: Left bundle branch block type ventricular tachycardia (sustained and non-sustained) by ECG, Holter or exercise testing. Frequent ventricular extra-systoles (> 1000/24 h) by Holter |
| 6 Family history |
| Major: Familial disease confirmed at necropsy or surgery |
| Minor: Family history of premature sudden death (< 35 yr) due to suspected right ventricular dysplasia. Familial history (clinical diagnosis based on present criteria) |
| Major |
| By 2D echo: |
| Regional RV akinesia, dyskinesia or aneurysm and one of the following (end diastole): |
| 1 Parasternal long axis view RVOT (PLAX) ≥ 32 mm (corrected for body size (PLAX/BSA) ≥ 19 mm/m2) |
| 2 Parasternal short axis view RVOT (PSAX) ≥ 36 mm (corrected for body size (PSAX/BSA) ≥ 21 mm/m2) |
| 3 Or fractional area change (FAC) ≤ 33% |
| By MRI: |
| Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and one of the following: |
| 1 Right ventricular end diastolic volume (RVEDV/BSA) ≥ 110 mL/m2 (male) or ≥ 100 mL/m2 (female) |
| 2 Or RVEF ≤ 40% |
| By RV angiography: |
| Regional RV akinesia, dyskinesia or aneurysm |
| Minor |
| By 2D echo Regional RV akinesia or dyskinesia and one of the following (end diastole): |
| 1 Parasternal long axis view RVOT (PLAX) ≥ 29 - < 32 mm (corrected for body size (PLAX/BSA) ≥ 16 - < 19 mm/m2) |
| 2 Parasternal short axis view RVOT (PSAX) ≥ 32 - < 36 mm (corrected for body size(PSAX/BSA) ≥ 18 - < 21 mm/m2) |
| 3 Or FAC > 33% - ≤ 40% |
| By MRI Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and one of the following: |
| 1 Right ventricular end diastolic volume/BSA ≥ 100 - < 110 mL/m2 (male) or ≥ 90 - < 100 mL/m2 (female) |
| 2 RVEF > 40% - ≤ 45% |
CMR has an important role in the diagnosis of ARVC as it allows three-dimensional visualisation of the ventricles and is very useful in the assessment of functional and structural abnormalities[23]. Previous studies have demonstrated that cMR has high sensitivity and specificity for ARVC diagnosis[24,25] and also play an important role in the evaluation of ARVC even in patients who do not meet the Task Force Criteria[26]. Due to the high sensitivity and specificity, cMR has also been suggested as a routine method of examination if ARVC is clinically suspected[25]. Apart from excluding ARVC, cMR is also sometimes useful in finding ARVC mimics or other clinically significant findings[26,27].
The requests to rule out ARVC constitute a significant proportion of the total cMR referrals in our institution from the cardiologists. Although the previous studies have demonstrated the critical role of cMR in the diagnosis of ARVC, the impact of cMR outcome in an unselected population is not widely analysed. So this study was undertaken to find out what percentage of patients with a suspected diagnosis of ARVC referred for cMR had positive clinical results and whether the outcome helped in deciding further patients’ care.
All the patients who were referred for cMR with a suspected diagnosis of ARVC or with a family history of suspected or confirmed ARVC from May 2006 to February 2010 in our institution were included in this retrospective analysis (Table 3). A total of 121 patients were referred in this period. Seven patients were not scanned due to claustrophobia. Therefore, the study included 114 patients. Out of the 114 patients who underwent cMR, 63 patients were male (55%) and 51 were female (45%). The age range was from 16 to 83 years, however 82% of the patients were between 20 and 60 years of age. The majority of these patients (84%) were referred from the cardiologists in our teaching institution, and the rest came from the cardiologists from the five district general hospitals and one private hospital. The three most common reasons for referral were arrhythmias (30%), family history of sudden death (20%) and abnormal RV on echocardiography or on a previous cMR (19%). The other reasons for referral included history of palpitations and syncope, Supra-ventricular tachycardia, suspected Brugada syndrome, abnormal left ventricle (LV) and dilated cardiomyopathy on echo, frequent ectopics and cardiac arrest.
| Time period | May 2006-Feb 2010 |
| Total No. of patients referred | 121 |
| Total No. of patients scanned | 114 |
| Age range | 16 to 83 |
| 16-20 | 7 (6%) |
| 21-30 | 19 (17%) |
| 31-40 | 25 (22%) |
| 41-50 | 28 (24%) |
| 51-60 | 21 (18%) |
| 61-70 | 10 (9%) |
| 71-80 | 3 (3%) |
| 81-90 | 1 (1%) |
| Males | 63 (55%) |
| Females | 51 (45%) |
| Referrals | |
| Teaching Hospitals | 96 (84%) |
| District General Hospitals | 18 (16%) |
| Reasons for referral | |
| Arrhythmias | 34 (30%) |
| Family history of sudden death | 23 (20%) |
| Others | 57 (50%) |
| Others | |
| Abnormal RV in echo | 22 (19%) |
| Brugada syndrome | 8 (7%) |
| Syncope | 8 (7%) |
| Palpitations | 6 (5%) |
| Dilated cardiomyopathy in echo | 4 (3.5%) |
| Frequent ectopics | 4 (3.5%) |
| SVT | 3 (3%) |
| Abnormal LV | 1 (1%) |
| Cardiac arrest | 1 (1%) |
The institutional review board approved this retrospective study. Since this was a service evaluation, formal ethical approval was not required. However, the patients’ confidentiality was respected.
All the cardiac magnetic resonance imaging (MRI) scans were performed on a Siemens Avanto 1.5 Tesla magnetic resonance scanner using a body coil. The ARVC protocol included: (1) scout images; (2) dark blood Half-Fourier Acquisition Single-Shot Turbo Spin-Echo imaging in 3 planes-axial, coronal and sagittal; and (3) a series of balanced, steady state free precession (25 phase) cine images, with standard views in the following planes; Vertical long-axis (VLA) or 2-chamber; 4-chamber (FCH); Left ventricular outflow tract (LVOT) or 3 chamber; Right ventricular outflow tract; short axis stack at 10 mm intervals to allow quantification of ventricular volumes and function; Axial stack to assess for RV free wall regional wall motion abnormality; (4) gadolinium was administered at a rate of 0.1 mmol/kg and followed by early gadolinium inversion recovery images in the LVOT, FCH, VLA planes, with an inversion time (TI) of 440 ms; and then (5) late gadolinium inversion recovery images were obtained of the LVOT, FCH and VLA planes and as a short axis stack; repeated with a phase swap, with the TI set to produce optimal myocardial nulling. Right ventricular volumes were analysed from the short axis stack and compared with indexed normal values.
Patient details including age, sex, ECG and echo abnormalities, family history, MRI findings and information about clinical management were obtained. We analyzed the data for cMR findings, which fitted with the major and minor criteria for ARVC. The major MRI criteria for ARVC included severe global/segmental dilatation of the RV and global systolic dysfunction. The minor criteria included mild global/segmental dilatation of the RV, regional contraction abnormalities and global diastolic dysfunction according to the Task Force Criteria. The gadolinium enhancement if seen was also recorded. At least 2 radiologists and 1 cardiologist jointly reported the scans. The MRI findings were correlated with clinical outcome.
The overall sensitivity and specificity of cMR to diagnose ARVC and the positive and negative predictive values in three different groups (arrhythmias, family history and others) were calculated. Values were expressed in percentages.
CMR findings with abnormalities related to the right ventricle were classified as major, minor and non-specific according to whether they met major or minor cMR diagnostic criteria of ARVC. There were also some non-specific findings related to the RV such as mild dyskinesia, thinning of RV wall and mildly impaired RV function.
Of the 114 patients, 19 patients had RV related abnormalities (Table 4). Of these, 4 patients had major cMR criteria, 13 had minor criteria and 2 patients had non-specific features. Out of the 4 who had major criteria for ARVC, 3 were clinically proven to have ARVC of whom 2 had an implantable cardioverter defibrillator (ICD) fitted. The third patient, who also had family history of ARVC, died soon after the diagnosis. Only this patient (1 out of 4) showed extensive RV free wall late gadolinium enhancement. The fourth patient did not meet the full diagnostic criteria, but still had an ICD fitted for recurrent episodes of ventricular tachycardia (VT). Out of the 13 patients with minor criteria, 2 were clinically proven to have ARVC. None of these patients had late gadolinium enhancement. Eight patients with either minor or non-specific criteria had repeat MR scan, which suggested either no significant or mild change compared to the earlier scans. Out of the 95 patients who had negative cMR for ARVC, clinical data were available for 81 patients and none of them had a positive clinical diagnosis of ARVC. Of these patients, 63 patients had normal scans and 8 had dilated cardiomyopathy. Other significant diagnoses included left to right shunt (1), LV infarction (1) and RV infarction (1). The remaining patients had mild chamber or aortic root abnormality.
| RV abnormalities related to ARVC | 19 (17%) |
| Major | 4 (4%) |
| Minor | 13 (11%) |
| Non specific | 2 (2%) |
| Other diagnoses | 95 (83%) |
| Normal | 63 (55%) |
| Dilated cardiomyopathy | 8 (7%) |
| Left to right shunt | 1 (1%) |
| RV infarction | 1 (1%) |
| LV infarction | 1 (1%) |
| Other mild abnormalities | 21 (18%) |
| Clinically proven ARVC | 5 (4%) |
| cMR major | 3 |
| cMR minor | 2 |
| Non specific (out of 2) | 0 |
| Others (out of 95)1 | 0 |
| Family history of sudden death | 23 |
| Clinically proven ARVC | 1 |
| Minor and non specific criteria for ARVC (not clinically proven) | 3 |
| Normal | 15 |
| LV hypertrophy | 1 |
| LV dyssynchrony | 1 |
| LV infarct | 1 |
| Dilated cardiomyopathy | 1 |
There were 23 patients referred with a family history of sudden death. One had major cMR criteria, one had minor cMR criteria and 2 had non-specific findings related to the RV on cMR. Apart from the one patient who had major criteria, there was no other clinically proven ARVC in patients with family history. Out of the remaining 19 patients, 15 patients had normal scans, 1 had LV hypertrophy, 1 had LV dyssynchrony, 1 had an LV infarct with RV impairment and one had dilated cardiomyopathy.
In summary, of the total cohort of 114 patients, 17 % had scans showing abnormalities related to RV and 83% had scans not suggestive of ARVC. Four percent of the study population had clinical proven ARVC and in all of them, MR was positive for either major or minor criteria. Excluding the 14 patients with no clinical data and final diagnosis, the overall sensitivity of the test was 100%, specificity 87%, positive predictive value 29% and the negative predictive value 100%. If split by criteria, the positive predictive value for major criteria was 75% and minor criteria 15%. The positive predictive values for different patient groups (arrhythmias (30%), family history (20%), and others (50%) are given in Table 5. The negative cMR diagnoses were reassuring in terms of clinical management especially for the patients with family history of sudden death.
| Clinical history - No ofpatients | Major criteria present | Clinically proven ARVC | Positive predictive value | Minor criteria present | Clinically proven ARVC | Positive predictive value |
| Arrhythmia (30%) | 2 | 1 | 50% | 3 | 2 | 67% |
| Family history of SCD (20%) | 1 | 1 | 100% | 1 | 0 | 0% |
| Others (50%) | 1 | 1 | 100% | 9 | 0 | 0% |
This study has shown that in this cohort of patients 15% fulfill imaging criteria for ARVC, with a subsequent 75% of cases fulfilling Task Force criteria for the diagnosis of ARVC if they had a major CMR criterion. Conversely a negative CMR scan for ARVC on imaging criteria translated into no subsequent diagnosis of ARVC.
Referrals for cMR in our centre come from a variety of sources, including the Inherited Cardiac Conditions service and the Arrhythmia service, which are based regionally at our institution. In addition, referrals are received from general cardiology clinics in our institution and from the district general hospitals in our region. The scans are reported jointly by radiologists and cardiologists at multidisciplinary meetings to maximize the clinical relevance of the reports.
In our study 15% of the patients fulfilled imaging criteria for ARVC. Major criteria were found in 4% of the cases and minor criteria were found in 11%. Data from other centers report a detection rate of 3%-10%[26,27], for unselected cMR referrals for possible ARVC. Therefore our detection rate does not differ significantly from these. These detection rates may fall following implementation of the modified Task Force Criteria[5], with one study showing a significant drop in the number of positive scans[28].
Our study showed that none of the patients with CMR scans that were negative for ARVC on imaging criteria were subsequently diagnosed with ARVC. This is an important finding and is reassuring to the clinicians involved. This negative predictive valve of 100% compares to previous studies which have also shown the diagnostic accuracy of CMR in the diagnosis of ARVC[28]. While it may be perceived by some that many studies proved to be negative, we claim that these studies are particularly helpful to the clinician and the patient alike in excluding important pathology such as ARVC, particularly in patients presenting with arrhythmias or those with a family history of either ARVC or of sudden cardiac death. Interestingly we also found evidence of other pathologies in 11% of patients with scans negative for ARVC on imaging criteria, the majority of these were diagnosed as dilated cardiomyopathy. This is in keeping with other studies, which report an incidence of significant other etiologies being diagnosed in 4%-8% of the cases[26,27].
We have also shown that scans positive for ARVC on imaging criteria translate into a high percentage of patients formally fulfilling Task Force criteria for ARVC (75% of cases with a major imaging criteria, 15% with a minor criteria) and that no scans negative for ARVC on imaging criteria were subsequently diagnosed with ARVC. These reflect a reassuring performance by our clinically effective service where scans are reviewed in a joint cardiology and radiology multidisciplinary team meeting. In conclusion, CMR is a useful tool for excluding ARVC, because of a high negative predictive value and is especially helpful in patients with family history of sudden death. A positive scan correlates well with clinical findings. The technique is cost effective as the positive or negative outcomes have significant impact on the clinical decision-making.
Limitations of this paper are that the number of patients studied were small, with no control group and this precluded more detailed statistical analysis, such as hazard ratio’s. Further work in this area should include a larger study population.
Arrhythmogenic right ventricular cardiomyopathy (ARVC), an inherited disorder is a relatively common cause of sudden death especially in young athletes. The diagnostic criteria is however not simple and depends on fulfilling modified Task Force Criteria which involves a lot of diagnostic work up including multimodality imaging. Cardiac magnetic resonance imaging (cMR) is one such useful tool and also the one frequently requested to evaluate for ARVC. Although the previous studies have demonstrated the critical role of cMR in the diagnosis of ARVC, the impact of cMR outcome in an unselected population is not widely analysed.
The definitive diagnosis requires endomyocardial biopsy, which is an invasive procedure, however even the biopsy may still not be sensitive due to the patchy nature of the disease. Cardiac MR has emerged over the years as a very useful non-invasive modality of choice and the research hotspot is to find out whether it can serve as a one-stop shop for the evaluation of ARVC.
Data from other centers report a detection rate of 3%-10%, for unselected CMR referrals for possible ARVC. This study results show 4% of the referrals had positive clinical diagnosis and none of the patients with negative CMR scans were subsequently diagnosed with ARVC. This is an important finding and is reassuring to the clinicians involved. Although significant proportion of studies proved to be negative, the authors claim that these studies are particularly helpful to the clinician and the patient alike in excluding important pathology such as ARVC, particularly in patients presenting with arrhythmias or those with a family history of either ARVC or of sudden cardiac death.
The study results show that cardiac MR is a useful tool for excluding ARVC, because of a high negative predictive value and the positive scan also correlates well with clinical findings, which makes the study cost effective as both the outcomes have significant impact on the clinical decision-making.
“Arrhythmogenic cardiomyopathy” - “cardiomyopathy” refers to disease of the heart muscle which when affected by inflammation with subsequent fibrosis and fat infiltration can cause “arrhythmogenic” potential which triggers the heart muscle to produce very high heart rates such as ventricular tachycardia and fibrillation due to electrical instability which can result in sudden death.
The manuscript is well written and highly organized. The readability is excellent. In this retrospective analysis of 114 patients referred for CMR because of arrhythmias or family history of sudden death, the results of CMR were classified depending on both functional and tissue characterisation and the clinical information were used. The assessment and judgment of the images of CMR was performed jointly by radiologist and cardiologist. This study shows that CMR has an important role in the diagnosis of ARVC as it allows 3-D visualization of the ventricles and CMR is sometimes useful in finding other disorders for patient’s symptoms.
P- Reviewer: Ramsay M, Satoh H, Said SAM, Salemi VMC, Tobita K S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Protonotarios N, Tsatsopoulou A, Anastasakis A, Sevdalis E, McKoy G, Stratos K, Gatzoulis K, Tentolouris K, Spiliopoulou C, Panagiotakos D. Genotype-phenotype assessment in autosomal recessive arrhythmogenic right ventricular cardiomyopathy (Naxos disease) caused by a deletion in plakoglobin. J Am Coll Cardiol. 2001;38:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Carvajal-Huerta L. Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy. J Am Acad Dermatol. 1998;39:418-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 160] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Protonotarios N, Tsatsopoulou A. Naxos disease and Carvajal syndrome: cardiocutaneous disorders that highlight the pathogenesis and broaden the spectrum of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Pathol. 2004;13:185-194. [PubMed] |
| 4. | Tavora F, Zhang M, Franco M, Oliveira JB, Li L, Fowler D, Zhao Z, Cresswell N, Burke A. Distribution of biventricular disease in arrhythmogenic cardiomyopathy: an autopsy study. Hum Pathol. 2012;43:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533-1541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1626] [Cited by in RCA: 1436] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 6. | Saguner AM, Brunckhorst C, Duru F. Arrhythmogenic ventricular cardiomyopathy: A paradigm shift from right to biventricular disease. World J Cardiol. 2014;6:154-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 7. | Thiene G, Basso C, Calabrese F, Angelini A, Valente M. Pathology and pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Herz. 2000;25:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1065] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 9. | Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 562] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 10. | Thiene G, Basso C, Danieli G, Rampazzo A, Corrado D, Nava A. Arrhythmogenic right ventricular cardiomyopathy a still underrecognized clinic entity. Trends Cardiovasc Med. 1997;7:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Lemery R, Brugada P, Janssen J, Cheriex E, Dugernier T, Wellens HJ. Nonischemic sustained ventricular tachycardia: clinical outcome in 12 patients with arrhythmogenic right ventricular dysplasia. J Am Coll Cardiol. 1989;14:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Nava A, Canciani B, Daliento L, Miraglia G, Buja G, Fasoli G, Martini B, Scognamiglio R, Thiene G. Juvenile sudden death and effort ventricular tachycardias in a family with right ventricular cardiomyopathy. Int J Cardiol. 1988;21:111-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1161] [Cited by in RCA: 1061] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 14. | Corrado D, Thiene G, Nava A, Rossi L, Pennelli N. Sudden death in young competitive athletes: clinicopathologic correlations in 22 cases. Am J Med. 1990;89:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 321] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Kullo IJ, Edwards WD, Seward JB. Right ventricular dysplasia: the Mayo Clinic experience. Mayo Clin Proc. 1995;70:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Pinamonti B, Di Lenarda A, Sinagra G, Silvestri F, Bussani R, Camerini F. Long-term evolution of right ventricular dysplasia-cardiomyopathy. The Heart Muscle Disease Study Group. Am Heart J. 1995;129:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Corrado D, Fontaine G, Marcus FI, McKenna WJ, Nava A, Thiene G, Wichter T. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: need for an international registry. Study Group on Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy of the Working Groups on Myocardial and Pericardial Disease and Arrhythmias of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the World Heart Federation. Circulation. 2000;101:E101-E106. [PubMed] |
| 18. | Angelini A, Basso C, Nava A, Thiene G. Endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy. Am Heart J. 1996;132:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 85] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1046] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 20. | Quarta G, Sado DM, Moon JC. Cardiomyopathies: focus on cardiovascular magnetic resonance. Br J Radiol. 2011;84 Spec No 3:S296-S305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Towbin JA. Arrhythmogenic right ventricular cardiomyopathy: a paradigm of overlapping disorders. Ann Noninvasive Electrocardiol. 2008;13:325-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Hamid MS, Norman M, Quraishi A, Firoozi S, Thaman R, Gimeno JR, Sachdev B, Rowland E, Elliott PM, McKenna WJ. Prospective evaluation of relatives for familial arrhythmogenic right ventricular cardiomyopathy/dysplasia reveals a need to broaden diagnostic criteria. J Am Coll Cardiol. 2002;40:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 188] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Kayser HW, van der Wall EE, Sivananthan MU, Plein S, Bloomer TN, de Roos A. Diagnosis of arrhythmogenic right ventricular dysplasia: a review. Radiographics. 2002;22:639-48; discussion 649-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Tandri H, Macedo R, Calkins H, Marcus F, Cannom D, Scheinman M, Daubert J, Estes M, Wilber D, Talajic M. Role of magnetic resonance imaging in arrhythmogenic right ventricular dysplasia: insights from the North American arrhythmogenic right ventricular dysplasia (ARVD/C) study. Am Heart J. 2008;155:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Xiaojing H, Jiannong Z, Weibo X. The utility of magnetic resonance imaging in the evaluation of arrhythmogenic right ventricular cardiomyopathy. J Radiol. 2009;90:717-723. [PubMed] |
| 26. | Looi KL, Edwards C, Hart H, Christiansen JP. Utility of cardiac magnetic resonance in the evaluation of unselected patients with possible arrhythmogenic right ventricular cardiomyopathy. Clin Med Insights Cardiol. 2012;6:153-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Quarta G, Husain SI, Flett AS, Sado DM, Chao CY, Tomé Esteban MT, McKenna WJ, Pantazis A, Moon JC. Arrhythmogenic right ventricular cardiomyopathy mimics: role of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Vermes E, Strohm O, Otmani A, Childs H, Duff H, Friedrich MG. Impact of the revision of arrhythmogenic right ventricular cardiomyopathy/dysplasia task force criteria on its prevalence by CMR criteria. JACC Cardiovasc Imaging. 2011;4:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |