Published online Jun 26, 2014. doi: 10.4330/wjc.v6.i6.507
Revised: April 5, 2014
Accepted: May 8, 2014
Published online: June 26, 2014
Processing time: 198 Days and 12.9 Hours
AIM: To undertake a review of the evidence that nifedipine GITS and lercanidipine are therapeutically equivalent in the management of essential hypertension.
METHODS: A systematic review of the published literature was prompted by the findings of two meta-analyses which indicated that there was a lower incidence of peripheral (ankle) oedema with lercanidipine. However, neither meta-analysis gave detailed attention to comparative antihypertensive efficacy or cardiovascular protection. Accordingly, a systematic, detailed and critical review was undertaken of individual published papers. The review started with those studies incorporated into the 2 meta-analyses and then all other salient and directly relevant papers identified through the following search criteria: all randomized controlled trials in which the therapeutic profile and antihypertensive effects of lercanidipine were directly compared with those of nifedipine GITS (in hypertensive patients). The search strategy was focused on the reports of clinical trials of lercanidipine vs nifedipine GITS, which were identified through a systematic search of PubMed (from 1966 to October 2012), Embase (from 1980 to October 2012) and the Cochrane library (from 1 October 2008 to end October 2013). The search combined terms related to lercanidipine vs nifedipine GITS (including MeSH search using calcium antagonists, calcium channel blockers and dihydropyridines).
RESULTS: With regard to blood pressure (BP) control and the consistency of BP control throughout 24-h, there is limited published evidence. However, two studies using 24 h ambulatory blood pressure monitoring clearly identified the dose-dependency of BP lowering with lercanidipine and its variably sustained 24-h efficacy. In contrast, there is evidence of a consistent antihypertensive effect throughout 24 h with nifedipine GITS. The incidence of the most common “side effect”, i.e., peripheral (ankle) oedema can be estimated as follows. For every 100 patients treated with lercanidipine, 2.5 will report oedema compared to 6 patients treated with nifedipine GITS. However, 98 or 99 patients will continue treatment with nifedipine GITS, compared with 99.5 patients on lercanidipine. Finally, with regard to outcome studies of cardiovascular (CV) morbidity and mortality, there is definitive outcome evidence for nifedipine GITS but there is no evidence that treatment with lercanidipine leads to reductions in CV morbidity and mortality.
CONCLUSION: There is no evidence in terms of long-term BP control and CV protection to justify the contention that lercanidipine is therapeutically equivalent to nifedipine GITS.
Core tip: Even in this time of “evidence-based medicine”, there is a widespread presumption of “class effects” in therapeutic practice including that for antihypertensive drug treatments. Thus, guidelines tend to recommend treatment not with specific agents but with groups or classes such as “calcium channel blockers” on the presumption of the therapeutic equivalence or inter-changeability of different agents. This literature review focuses attention on the apparent therapeutic advantage of lercanidipine over nifedipine GITS on the basis of a lower incidence of the adverse effect of peripheral (ankle) oedema. Overall, however, the balance of evidence of efficacy favours nifedipine GITS.
- Citation: Elliott HL, Meredith PA. Thrapeutic equivalence in the treatment of hypertension: Can lercanidipine and nifedipine GITS be considered to be interchangeable? World J Cardiol 2014; 6(6): 507-513
- URL: https://www.wjgnet.com/1949-8462/full/v6/i6/507.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i6.507
Hypertension treatment guidelines, particularly those in Europe, recommend a long-acting dihydropyridine calcium channel blocker (CCB) in the routine management of patients with hypertension, either as first line monotherapy or as a suitable combination partner for all other types of antihypertensive drug[1,2]. In general, however, the guidelines do not nominate individual agents and there is an overall presumption of a “class” effect, i.e., there is a presumption of therapeutic equivalence amongst all dihydropyridine CCBs licensed for once daily administration. The picture is further complicated by the mechanism harnessed to attain the suitability for once daily administration[3]. There have been three alternative approaches: (1) An intrinsic, extended elimination half life, as with amlodipine; (2) An “apparent” prolongation of half life via a sophisticated, modified release formulation, as with nifedipine GITS (Gastro-Intestinal Therapeutic System); and (3) An increased duration of action via increased membrane-binding characteristics (attributed to a high membrane partition coefficient) despite a relatively short elimination half life, as with lercanidipine and lacidipine.
Since direct, comparative outcome studies within a drug class are rare, therapeutic equivalence is usually assumed through an amalgamation of different types of evidence: for example, members of the same chemical family with similar pharmacological characteristics; comparisons of published papers which separately evaluate the drugs in question; comparative studies of the drugs, usually in parallel group designs, for surrogate end-points and adverse drug reactions (ADRs).
With regard to ADRs, peripheral (ankle) oedema is a well-recognised, dose-dependent “side effect” associated with chronic treatment with long-acting dihydropyridine CCBs such as nifedipine GITS, lercanidipine and amlodipine. There remains some debate, however, about the relative incidence of peripheral oedema with each of these individual agents and, in particular, the claims of a lesser incidence with lercanidipine[4-11]. There also is considerable doubt as to whether or not the balance between antihypertensive efficacy and tolerability is superior with lercanidipine.
The fundamental remit of this paper is a critical review of the published information relating to the comparisons of two dihydropyridine CCBs, nifedipine (in its GITS formulation: Gastro-Intestinal Therapeutic System) and lercanidipine. Such information has been derived from a limited number of published direct, head-to-head comparisons and a small number of additional publications from which relevant comparative information can be derived.
The question of therapeutic equivalence and the inter-changeability of the two drugs have been addressed under three sub-headings: (1) The fundamental pharmacological response: in this case, blood pressure reduction; (2) The profile of adverse drug reactions: in this case, peripheral oedema; and (3) The long term treatment benefits: in this case, cardiovascular protection.
This review was conducted in three phases: Phase 1-The starting point was a meta-analysis published in August 2009 as a systematic review of randomised, controlled, comparative clinical trials published during all years through to August 2008[6]; Phase 2-The second stage was a critical review of a second, updated meta- analysis published in 2011[7]; and Phase 3-The third component was a systematic, detailed and critical review of individual papers. First, those studies incorporated into the 2 meta-analyses. Then, in addition, all other salient and directly relevant papers identified through the following search criteria: all randomized controlled trials in which the therapeutic profile and antihypertensive effects of lercanidipine were directly compared with those of nifedipine GITS (in hypertensive patients). The search strategy was focused on the reports of clinical trials of lercanidipine vs nifedipine GITS, which were identified through a systematic search of PubMed (from 1966 to October 2012), Embase (from 1980 to October 2012) and the Cochrane library (from 1 October 2008 to end October 2013). The search combined terms related to lercanidipine vs nifedipine GITS (including MeSH search using calcium antagonists, calcium channel blockers and dihydropyridines).
The reference lists of original reports and meta-analyses of studies involving dihydropyridine calcium antagonists (retrieved through the electronic searches) were also scrutinised to identify studies that might not have been included in the computerized databases.
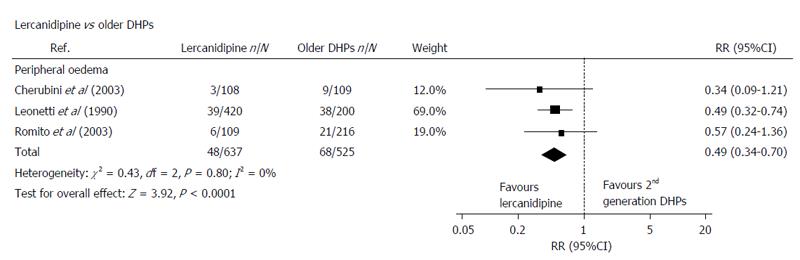
For the purposes of this meta-analysis, lercanidipine was compared with a group of so-called “first generation dihydropyridine CCBs” (including nifedipine GITS and amlodipine)[6]. The overall conclusion was that there was no significant difference between lercanidipine and these other competitor, “first generation CCBs” in terms of antihypertensive efficacy but there was a significant difference in favour of lercanidipine with respect to the incidence of, and withdrawal rates for, peripheral oedema (Figure 1).
Although three studies involving nifedipine GITS were cited in this paper, only 2 were included in the meta-analysis[8-10]. It is important to note that within the statistical terms of the analysis itself, there were no significant differences between Nifedipine GITS and lercanidipine for the withdrawal rates for these 2 individual studies[8,9] (Figure 1).
The 3 studies directly involving nifedipine GITS and lercanidipine are reviewed in greater detail below.
The second meta-analysis was more rigorous and more comprehensive but was essentially a repeat of the first analysis insofar as no new studies involving nifedipine GITS had been added[7]. However, overall, it was a larger and more robust analysis by an independent group using stricter criteria.
In essence, the result was the same as for the first meta-analysis even although only 3 studies were incorporated for the comparison of lercanidipine and “older DHPs” (the same 2 studies with nifedipine GITS and a study involving amlodipine).
The conclusion was that, relative to “older DHPs”, lercanidipine was associated with a reduced incidence of oedema: however, this component of the analysis was heavily influenced/weighted (78%) by the results of a study involving lercanidipine and amlodipine[11]. Once again, within the structure of the meta-analysis, the same 2 individual studies with nifedipine GITS showed no statistically significant difference between nifedipine GITS and lercanidipine as far as the incidence of peripheral oedema was concerned (Figure 2)[8,9].
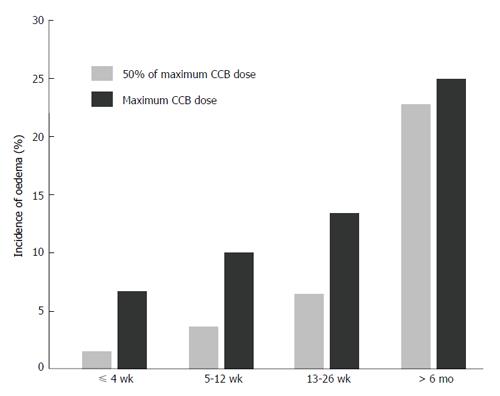
Additional features of clinical relevance and of practical importance in this second meta-analysis were as follows (Figure 3): (1) confirmation that the incidence of peripheral oedema is dose-dependent; (2) identification that the development of peripheral oedema is time-dependent, up to 6 mo treatment; and (3) awareness that the reduced incidence of peripheral oedema with “lipophilic DHPs” relative to “older DHPs” is not a unique feature of lercanidipine because lacidipine and manidipine were components of these analyses.
The comprehensive search of the literature databases revealed, in addition to the 3 studies cited in the first meta-analysis, a further 5 studies that met the pre-defined search criteria for the exploration of pertinent treatment issues.
Comparative studies: Romito et al[9] reported a double blind, parallel group study of a total of 250 patients which compared lercanidipine (10 and 20 mg), felodipine (10 and 20 mg) and nifedipine GITS (30 and 60 mg) across an 8 wk treatment period. No significant differences in antihypertensive efficacy were reported.
The incidence of ADRs was significantly lower with both lercanidipine and nifedipine GITS: in particular, there were lower rates for ankle oedema with lercanidipine (5.5%) and nifedipine GITS (6.6%), relative to felodipine (13%). The incidence of ankle oedema was not significantly different for lercanidipine and nifedipine GITS.
Ankle oedema led to the withdrawal of 1 patient receiving lercanidipine (n = 109) compared to 4 patients receiving nifedipine GITS (n = 106) and 5 patients on felodipine (n = 110).These differences were not statistically significant.
Cherubini et al[8] reported a double blind, randomised, parallel group study over 24 wk in elderly hypertensive patients comparing lacidipine (2 and 4 mg), lercanidipine (5 and 10 mg) and nifedipine GITS (30 and 60 mg).
The BP responder and normalisation rates were remarkably high with all 3 treatments, approximating to 100% in the case of nifedipine GITS, but not significantly different with the other 2 treatments. The incidence of oedema was lowest at 2.8% in the lercanidipine group (n = 96) compared to 7.5% with lacidipine (n = 99) and 10.1% with nifedipine GITS (n = 97) but this was not statistically significant. There were 2 withdrawals in the nifedipine group on account of oedema (out of 109 patients) and no withdrawals in the lercanidipine group.
There must be some concerns about the sensitivity of the BP methodology in this study because the BP responses were remarkably and unexpectedly high with all 3 drugs, especially considering that the doses of lacidipine and lercanidipine were relatively modest. With particular respect to lercanidipine, the consensus (in the published literature) is that 10 and 20 mg lercanidipine are the equivalent doses vs 30 and 60 mg nifedipine GITS. In fact, the 10/20 mg vs 30/60 mg comparability is specifically noted in the paper by Romito et al[9].
Fogari et al[10] designed specifically to assess indices of ankle volume and sub-cutaneous tissue pressure in patients randomly assigned in a double blind manner to 12 wk treatment with either lercanidipine (10 and 20 mg) or nifedipine GITS (30 and 60 mg).
There were no patient reports for peripheral oedema (hence the study was not incorporated into either of the published meta-analyses) and there were no patient withdrawals from either treatment group. There were statistically significant differences in ankle volume indices indicating a greater degree of ankle oedema with nifedipine GITS.
In summary, using a relatively sophisticated research methodology, this study demonstrated that nifedipine GITS had a greater propensity for the development of peripheral (ankle) oedema relative to lercanidipine. However, there were no clinical reports of any difference in incidence or withdrawal rates.
Other studies: Ambrosioni et al[12] reported the findings of 2 small studies exploring the antihypertensive efficacy of lercanidipine in doses of 5, 10 and 20 mg once daily in a total of 44 patients. Multiple 24-h ambulatory BP recordings were obtained and the following are the principal conclusions.
There was no statistically significant BP reduction with 5 mg lercanidipine but both single and multiple doses of 10 and 20 mg lercanidipine significantly reduced BP across 24 h. However, the BP reduction was not consistent across 24 h as assessed by the trough peak (TP) ratio. The TP ratios for the single dose study were not reported but, from one of the figures in the published paper, it can be estimated at about 39% with 10 mg and 44% with 20 mg: the corresponding values for multiple dosing were stated to be greater than 60%.
Omboni et al[13] reported a clinical study of more than 200 patients essentially confirmed the above findings: “At a dose of 10 mg lercanidipine had a significant and durable antihypertensive effect over 24 h but at 5 mg the effect was less consistent and did not last 24 h”. For 10 mg lercanidipine the TP ratio was reported at above 60% and from one of the figures in the published paper it appears to fall into the range 60%-75%.
Borghi et al[14] reported an open label, sequential treatment study lasting for a total of 8 wk and reliant upon a BP measurement obtained at a single time point (not defined in relation to drug administration).
A total of 125 patients were entered into the study because they were known to be experiencing “calcium antagonist-specific ADRs”, including peripheral oedema, whilst on treatment with amlodipine, felodipine, nifedipine GITS or nitrendipine. Patients were switched to lercanidipine and assessed after 4 wk treatment and then re-assigned to their original CCB and assessed again after a further 4 wk treatment.
In brief, no BP difference was detected (142/87 on lercanidipine and 141/87 mmHg on the other CCBs). Peripheral oedema was reported by 52% of patients after 4 wk of lercanidipine and by 87% of patients returned to their original CCB. The study did not report a direct comparison for the 28 patients treated with nifedipine GITS.
Once again, the BP methodology had little or no discriminatory power and the principal conclusion reflects a comparison of lercanidipine against a group of “calcium antagonists”, with amlodipine accounting for more than half of the patients.
Barrios et al[15] reported an observational study, conventional clinic BP control was significantly better (but of borderline clinical significance) at 144.4/83.3 in 233 patients receiving lercanidipine 20 mg daily, compared to 145.0/84.5 mmHg in 104 patients receiving either amlodipine 10 mg or nifedipine GITS 60 mg daily. However, and in addition to other methodological concerns, there was no direct comparison involving nifedipine GITS, nor any data on the number of patients receiving GITS. Furthermore, approximately 50% of the patient population were receiving concomitant antihypertensive drugs, with approximately 30% receiving 2 additional antihypertensive drugs.
Any interpretation of the available published literature is potentially compromised by issues relating to dosage equivalence, methodology, study reliability, statistical power, investigator bias, funding/sponsorship etc. Nevertheless, the following is presented as an objective summary of the available evidence evaluating whether or not lercanidipine and nifedipine GITS can be considered to be therapeutically equivalent for the management of hypertension.
The initial report of antihypertensive equivalence reflected the achieved BPs at 24 h post-dose in the 3 comparative studies cited in the original meta-analysis[6], i.e., there were no statistically significant differences. However, this interpretation not only reflected a rather insensitive measure of overall BP control but also raised concerns because: (1) one of these studies was not designed as a BP comparison; and (2) a second study had clear methodological flaws because it assigned a responder rate of 86% for 5mg lercanidipine, a dose which 2 other studies found to be inadequately effective. Thus, there is a very “thin” evidence base for direct antihypertensive equivalence if reliance is placed upon conventional clinic BP measurements.
There obviously are other clinical reports which assessed the antihypertensive efficacy of lercanidipine but these were not direct, head-to-head comparisons. Overall, whilst equivalence (with nifedipine GITS) was inferred on the basis of results which were similar, these results cannot be directly compared in statistical terms because they were generated by different research groups, in different patient populations, using different methodologies, etc. There also are publications in which deductions are made in spite of confounding factors and the TOLERANCE study is an illustrative example[15]. As discussed above, there was no direct comparison of lercanidipine and nifedipine GITS in this study, nor any data on the number of patients receiving GITS, and approximately 50% of the patient population were receiving at least one other antihypertensive drug.
In the absence of any other evidence, it might have proved difficult to challenge the conclusion of antihypertensive equivalence (which is actually based on one single, relatively robust, direct comparison!) but there were 2 studies employing 24 h ambulatory BP monitoring which explored the antihypertensive efficacy of lercanidipine in greater detail[12,13]. Both studies identified the dose-dependency of the 24 h BP lowering effects with lercanidipine which, overall, displayed poorly sustained 24-h efficacy. Additionally these 2 papers incorporated measurements of trough: TP as part of their assessments of antihypertensive efficacy throughout 24 h. Whilst TP ratio as an index of antihypertensive efficacy is not without its limitations[16], values of respectively 39% and 44% (estimated) following single 10 and 20 mg doses of lercanidipine and of “greater than 60%” for both doses during steady state treatment are not particularly high. In contrast, the published data with nifedipine GITS (by the same group, using the same methodology as for one of the lercanidipine studies) demonstrated that the drug elicits a consistent antihypertensive effect that is independent of dose and is characterised by a TP ratio approximating to 100%[17].
In summary, it may be reasonably concluded that BP control throughout 24-h is more consistent and better sustained with nifedipine GITS than with lercanidipine during chronic treatment.
Despite the paucity of direct comparative studies, there is a reasonable volume of evidence to indicate that this “side effect” is less likely to occur with lercanidipine than with nifedipine GITS.
In the absence of definitive statistics, a reasonable approximation of the practical consequences of this differentiating characteristic is derived from the second meta-analysis which incorporated data from 99469 patients. In brief, for every 100 patients treated with lercanidipine, 2.5 will report peripheral oedema compared to 6 patients treated with nifedipine GITS: correspondingly, 0.5 patients will withdraw from lercanidipine treatment compared to 1.1 treated with nifedipine GITS. The corollary of this is that there will be 98 or 99 patients continuing treatment with nifedipine GITS, compared with 99.5 patients treated with lercanidipine.
As a footnote, however, there is the suggestion that this potentially advantageous feature is not unique to lercanidipine insofar as it may also be a feature of treatment with lacidipine and manidipine.
For nifedipine GITS there is definitive evidence of benefit in the treatment of hypertension and stable coronary artery disease[18,19]. As there are no clinical outcome studies, there is no evidence that treatment with lercanidipine leads to reductions in cardiovascular (CV) morbidity and mortality.
In summary, the available evidence confirm the claims that lercanidipine has a lesser incidence of peripheral (ankle) oedema, relative to treatment with nifedipine GITS. Whilst this may be factually accurate, it is a trivial difference in terms of clinical practice, particularly with respect to patient withdrawals, whereby 98 patients (out of 100) will continue treatment with nifedipine GITS. Set against this minor advantage and albeit with incomplete evidence, the antihypertensive efficacy of nifedipine GITS appears to be superior, particularly in respect of sustained 24-h BP control.
In conclusion, the ultimate aim of antihypertensive drug treatment (with CCBs and other classes of drugs) is sustained, long term BP control leading to a significant reduction in CV morbidity and mortality. There is no compelling evidence with lercanidipine to undermine the proven ability of nifedipine GITS to reduce death and CV events on the basis of an occasional, inconvenient but essentially innocuous adverse effect. Thus, there is no justification for assuming therapeutic equivalence between lercanidipine and nifedipine GITS and no grounds for considering that they are interchangeable if CV protection is the ultimate goal.
Drugs in the same “class” are often considered to be therapeutically equivalent and, therefore, inter-changeable. This review scrutinises the published literature to compare two antihypertensive drugs (lercanidipine and nifedipine GITS) and to assess whether or not there is good evidence that these drugs are therapeutically similar.
Several new guidelines from international organisations have been recently published to advise on the treatment of hypertension but, as a general rule, no guidance is given on the choice of individual drugs. This critical review is intended for the prescribing clinician to allow him or her to make an informed evidence-based assessment which, in this case, addresses the comparison of two calcium channel blocking drugs.
The practical conclusion is that lercanidipine and nifedipine GITS cannot be considered to be inter-changeable because, although it appears less likely to cause peripheral (ankle) oedema, there is no direct evidence that lercanidipine can provide cardiovascular protection. In contrast, there is clear evidence of consistent 24-h blood pressure control and long-term cardiovascular protection with nifedipine GITS.
This is an interesting paper. The authors have reviewed the role of lercanidipine in hypertension and compared it to nifedipine GITS in an extensive manner.
P- Reviewers: Balamuthusamy S, Rossi GP, Wan Y S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
| 1. | Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3425] [Cited by in RCA: 3338] [Article Influence: 303.5] [Reference Citation Analysis (0)] |
| 2. | NHS National Institute for Health and Clinical Excellence. Hypertension: Clinical management of primary hypertension in adults, CG127. 2006; Available from: http://guidance.nice.org.uk/CG127/Guidance/pdf/English. |
| 3. | Meredith PA, Elliott HL. Dihydropyridine calcium channel blockers: basic pharmacological similarities but fundamental therapeutic differences. J Hypertens. 2004;22:1641-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | McClellan KJ, Jarvis B. Lercanidipine: a review of its use in hypertension. Drugs. 2000;60:1123-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Croom KF, Wellington K. Modified-release nifedipine: a review of the use of modified-release formulations in the treatment of hypertension and angina pectoris. Drugs. 2006;66:497-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Makarounas-Kirchmann K, Glover-Koudounas S, Ferrari P. Results of a meta-analysis comparing the tolerability of lercanidipine and other dihydropyridine calcium channel blockers. Clin Ther. 2009;31:1652-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Makani H, Bangalore S, Romero J, Htyte N, Berrios RS, Makwana H, Messerli FH. Peripheral edema associated with calcium channel blockers: incidence and withdrawal rate--a meta-analysis of randomized trials. J Hypertens. 2011;29:1270-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Cherubini A, Fabris F, Ferrari E, Cucinotta D, Antonelli Incalzi R, Senin U. Comparative effects of lercanidipine, lacidipine, and nifedipine gastrointestinal therapeutic system on blood pressure and heart rate in elderly hypertensive patients: the ELderly and LErcanidipine (ELLE) study. Arch Gerontol Geriatr. 2003;37:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Romito R, Pansini MI, Perticone F, Antonelli G, Pitzalis M, Rizzon P. Comparative effect of lercanidipine, felodipine, and nifedipine GITS on blood pressure and heart rate in patients with mild to moderate arterial hypertension: the Lercanidipine in Adults (LEAD) Study. J Clin Hypertens (Greenwich). 2003;5:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Fogari R, Malamani GD, Zoppi A, Preti P, Vanasia A, Fogari E, Mugellini A. Comparative effect of lercanidipine and nifedipine gastrointestinal therapeutic system on ankle volume and subcutaneous interstitial pressure in hypertensive patients: a double-blind, randomized, parallel-group study. Curr Ther Res Clin Exp. 2000;61:850-862. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Leonetti G, Magnani B, Pessina AC, Rappelli A, Trimarco B, Zanchetti A. Tolerability of long-term treatment with lercanidipine versus amlodipine and lacidipine in elderly hypertensives. Am J Hypertens. 2002;15:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Ambrosioni E, Circo A. Activity of Lercanidipine Administered in Single and Repeated Doses Once Daily as Monitored over 24 Hours in Patients with Mild to Moderate Essential Hypertension. J Cardiovasc Pharmacol. 1997;29:S16-S20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Omboni S, Zanchetti A. Antihypertensive efficacy of lercanidipine at 2.5, 5 and 10 mg in mild to moderate essential hypertensives assessed by clinic and ambulatory blood pressure measurements. Multicenter Study Investigators. J Hypertens. 1998;16:1831-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Borghi C, Prandin MG, Dormi A, Ambrosioni E. Improved tolerability of the dihydropyridine calcium-channel antagonist lercanidipine: the lercanidipine challenge trial. Blood Press Suppl. 2003;1:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Barrios V, Escobar C, de la Figuera M, Llisterri JL, Honorato J, Segura J, Calderón A. Tolerability of high doses of lercanidipine versus high doses of other dihydropyridines in daily clinical practice: the TOLERANCE Study. Cardiovasc Ther. 2008;26:2-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Elliott HL, Meredith PA. Analysis of trough: peak ratio and the assessment of anti-hypertensive drug action. J Hum Hypertens. 1995;9:423-427. [PubMed] |
| 17. | Zanchetti A. Trough and peak effects of a single daily dose of nifedipine gastrointestinal therapeutic system (GITS) as assessed by ambulatory blood pressure monitoring. Italian Nifedipine GITS Study Group. J Hypertens Suppl. 1994;12:S23-S27. [PubMed] |
| 18. | Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, Ruilope LM. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet. 2000;356:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 766] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 19. | Poole-Wilson PA, Lubsen J, Kirwan BA, van Dalen FJ, Wagener G, Danchin N, Just H, Fox KA, Pocock SJ, Clayton TC. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet. 2004;364:849-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 345] [Article Influence: 16.4] [Reference Citation Analysis (0)] |