Published online Apr 26, 2014. doi: 10.4330/wjc.v6.i4.196
Revised: November 6, 2013
Accepted: March 13, 2014
Published online: April 26, 2014
Processing time: 221 Days and 3.8 Hours
AIM: To assess the current diagnostic and therapeutic management and the clinical implications of congenital single coronary artery (SCA) in adults.
METHODS: We identified 15 patients with a SCA detected from four Dutch angiography centers in the period between 2010 and 2013. Symptomatic patients who underwent routine diagnostic coronary angiography (CAG) for suspected coronary artery disease and who incidentally were found to have isolated SCA were analyzed.
RESULTS: Fifteen (7 females) with a mean age of 58.5 ± 13.78 years (range 43-86) had a SCA. Conventional CAG demonstrated congenital isolated SCA originating as a single ostium from the right sinus of Valsalva in 6 patients and originating from the left in 9 patients. Minimal to moderate coronary atherosclerotic changes were found in 4, and severe stenotic lesions in another 4 patients. Seven patients were free of coronary atherosclerosis. Runs of non-sustained ventricular tachycardia were documented in 2 patients, one of whom demonstrated transmural ischemic changes on presentation. Myocardial perfusion scintigraphic evidence of transmural myocardial ischemia was found in 1 patient due to kinking and squeezing of the SCA with an interarterial course between the aorta and pulmonary artery. Multi-slice computed tomography (MSCT) was helpful to delineate the course of the anomalous artery relative to the aorta and pulmonary artery. Percutaneous coronary intervention was successfully performed in 3 patients. Eight patients were managed medically. Arterial bypass graft was performed in 4 patients with the squeezed SCA.
CONCLUSION: SCA may be associated with transient transmural myocardial ischemia and aborted sudden death in the absence of coronary atherosclerosis. The availability and sophistication of MSCT facilitates the delineation of the course of a SCA. We present a Dutch case series and review of the literature.
Core tip: A Dutch case series of 15 adult patients with congenital isolated single coronary artery (SCA) are presented. Conventional coronary angiography demonstrated congenital isolated SCA originating as a single ostium from the right sinus of Valsalva in 6 patients and originating from the left in 9 patients. SCA may be associated with symptomatic transient transmural myocardial ischemia, non-sustained ventricular tachycardia, and aborted sudden death in the absence or presence of coronary atherosclerosis. The availability of multi-slice computed tomography (MSCT) and cardiovascular magnetic resonance imaging facilitates the delineation of the course of the anomalous vessel. MSCT was helpful to delineate the course of the anomalous artery relative to the aorta and pulmonary artery. Percutaneous coronary intervention was successfully performed in 3 patients. Eight patients were managed medically. Arterial bypass graft was performed in 4 patients with the squeezed SCA. The literature addressing SCA is reviewed.
- Citation: Said SA, de Voogt WG, Bulut S, Han J, Polak P, Nijhuis RL, op den Akker JW, Slootweg A. Coronary artery disease in congenital single coronary artery in adults: A Dutch case series. World J Cardiol 2014; 6(4): 196-204
- URL: https://www.wjgnet.com/1949-8462/full/v6/i4/196.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i4.196
A single coronary artery (SCA) is defined as a single aortic orifice or origin providing for all of the coronary blood perfusion of the entire myocardium[1-3]. In 1967, Halperin et al[4] reported the first ante mortem angiographic diagnosis of SCA arising from the left sinus of Valsalva (LSV). SCA is a rare congenital anomaly and occurs as an incidental finding in approximately 0.066% of the coronary angiography (CAG) population[5]. SCA has been reported in association with and without atherosclerotic changes[6,7] or in association with coronary artery fistulas[8,9], bicuspid aortic valves, and with hypertrophic cardiomyopathy[1,7,10,11].
An equal distribution is found between SCA originating from the right sinus of Valsalva (RSV) and the LSV[2,12]. Exact delineation of the course of the abnormal coronaries relative to the aorta and pulmonary artery is of major importance as myocardial ischemia during exertion can be caused by kinking or squeezing of the branches of the anomalous SCA between the aorta and pulmonary artery. CAG is the first diagnostic tool in the detection of a SCA. Once abnormal coronary arteries are suspected, multi-slice computed tomography (MSCT) and cardiac magnetic resonance (CMR) imaging[13] scans are excellent tools for non-invasive determination of the course of the abnormal coronaries relative to the aorta and pulmonary artery[14]. Determination of the course of incidentally found congenital coronary anomalies during routine CAG without the direct availability of CMR or MSCT scanning is challenging.
We discuss the clinical presentation and angiographic findings of 15 adult symptomatic patients with congenital isolated SCA incidentally found during routine CAG.
Between 2010 and 2013, 15 adult patients with a mean age of 58.5 ± 13.78 years (range 43-86) were diagnosed with a SCA during CAG in 4 Dutch angiography centers (Hospital Group Twente, Almelo; St. Lucas Andreas Hospital, Amsterdam; St. Anna Hospital, Geldrop; Hospital Group Twente, Hengelo; and Gerle Hospital, Zutphen). Indications for CAG were angina pectoris, dyspnea, and syncope.
The angiograms were reviewed by at least two experienced cardiologists who reached a consensus on the origin and course of the SCA. The angiographic variations and the course of the anomalous artery were defined according to the classification of Lipton et al[1]. The definition of SCA was adopted from Angelini et al[15] and defined as an isolated coronary artery arising from the sinus of Valsalva through a single ostium and with no evidence of a second ostium, thus being responsible for supplying blood to the entire myocardial tissue, regardless of its distribution.
Significant atherosclerosis was defined as luminal narrowing of ≥ 75% detected in a main branch of the epicardial coronary arteries. Patients were categorized as having significant single, double, or triple vessel disease when a significant lesion was found in one or more coronary artery branches arising from the SCA and supplying the right coronary artery (RCA), circumflex (Cx), or left anterior descending coronary artery regions. A 12-lead ECG was performed in all patients.
An exercise tolerance test (ETT) was performed in 10 patients, myocardial perfusion test [methoxy-isobutyl-isonitrile (MIBI) scan] in five patients, and 13ammonia-adenosine positron emission tomography (positron emission tomography-computed tomography) scan in one patient. MSCT was performed in 6 patients using a retrospective ECG-gated procedure (128-slice, Philips Medical Systems, Best, The Netherlands).
Patients comprised 8 males and 7 females, aged between 43 and 86 years (mean 58.5 ± 13.78). Effort angina pectoris was found in 6 patients, 4 had dyspnea on exertion, 4 complained of atypical chest pain, fainting and pre-syncope, 1 had recurrent syncopal attacks, and 2 presented with acute coronary syndrome. Patients’ characteristics are presented in Table 1. Between 2010 and 2013, 8917 coronary angiograms were performed in the 4 Dutch angiography centers all together, with an incidence of 15/8917 (0.017%).
| Case/gender/age | Clinical presentation | Rest ECG | Risk factors | ETT | MIBI scan | CAD | Management | CAG 1classification | MSCT |
| 1/F/45 | AP, DOE | SR | - | Inconclusive | NA | None | CMM | R-IIP | NA |
| 2/M/56 | DOE | SR | + | Positive | NA | Intermediate lesion | CMM | R-I | Overestimation of the Cx-lesion |
| RD | Ischemia IL | FFR 0.93 | |||||||
| 3/F/60 | AP | SR | - | NA | NA | Mild | CMM | R-III | NA |
| LBBB | |||||||||
| 4/M/86 | ACS | SR | + | Negative | NA | Significant | PCI | L-I | NA |
| 5/M/63 | Effort AP | SR | + | Positive | NA | Significant | PCI | L-IIA | NA |
| Negative T | |||||||||
| Inferior leads | |||||||||
| 6/F/43 | ACP, fainting and pre- syncope | SR | + | Inconclusive | Positive 13N-adenosine PET-CT: normal | None | CABG | L-IIB | Course: between aorta and pulmonary artery |
| 7/M/48 | AP, syncope | SR | + | Negative | Negative | None | CMM | L-I | NA |
| NSVT (5 beats) | Ergonovine test: | ||||||||
| No spasm | |||||||||
| 8/F/53 | DOE, palpitation | SR | + | NA | Positive | Intermediate lesion | CMM | R-IIA | NA |
| RD | |||||||||
| NSVT (20 beats) | |||||||||
| 9/M/46 | AP, palpitation | SR | - | NA | NA | None | CMM | R-IIA | NA |
| 10/M/63 | AP | SR | + | Positive | NA | Significant | CABG | L-IIB | Course: between aorta and pulmonary artery |
| 11/F/83 | NSTEMI | SR | + | NA | NA | Significant | PCI | R-III | NA |
| 12/F/47 | ACP | SR | + | Negative | NA | None | CMM | L-IIA | NA |
| 13/F/53 | CP syncope | SR | - | Negative | Negative | None | CABG | L-IIB | Course: between aorta and pulmonary artery |
| 14/M/72 | DOE | SR | + | NA | NA | Intermediate lesion | CABG | L-IIB | Course: between aorta and pulmonary artery |
| 15/M/41 | ACP | SR | + | Negative | Negative | None | CMM | L-IIA | Benign course |
| LBBB |
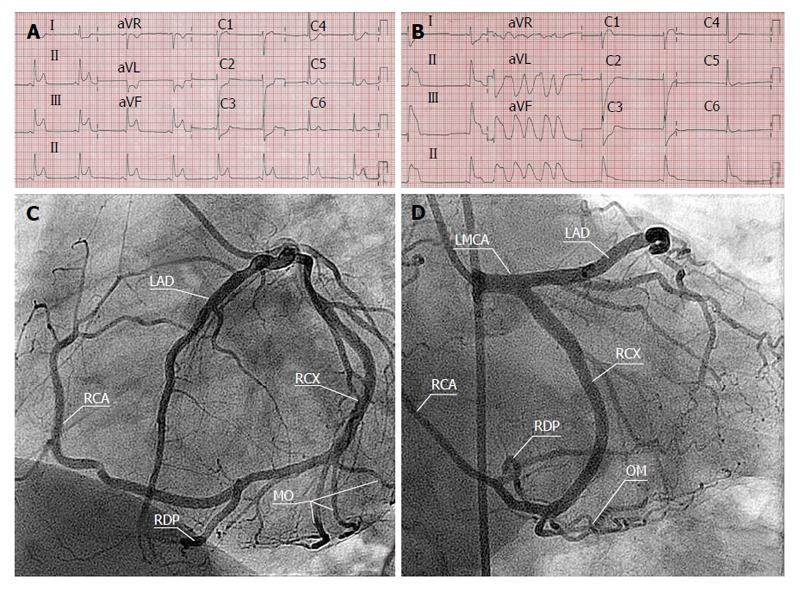
On the resting ECG, all patients were in sinus rhythm and had normal PR intervals. Two patients (patients 3 and 15) had a complete left bundle branch block. One patient (patient 5) had inverted T-waves in the inferior leads. In another patient (patient 8), ECG evidence of an old infero postero lateral infarction was shown. Short runs of non-sustained ventricular tachycardia (NSVT), varying from 5 to 20 beats/min with a frequency of 176/min and duration of 1800 ms, were documented in 2 patients (patients 7 and 8) (Figure 1).
ETT was inconclusive in 2 patients (1 and 6). Diagnostic CAG showed no significant coronary artery lesions in either patient. In patient number 6, the PET scan was positive due to kinking and squeezing of the SCA with a course between the aorta and pulmonary artery. This patient underwent coronary artery bypass grafting (CABG) whereby a mammary arterial graft was anastomosed to the RCA. In 3 patients (2, 5 and 10), the ETT was positive for myocardial ischemia. Of the 3 patients with positive ETT, 1 had significant CAD and underwent percutaneous coronary intervention (PCI). The other 2 patients demonstrated an intermediate lesion distally located in the coronary arterial tree and were managed medically. The ETT was negative in 5 patients (4, 7, 12, 13 and 15). Despite a negative ETT, patient number 4 showed a significant coronary lesion on CAG and underwent PCI. Patient number 7 had no significant coronary artery lesions and the ergonovine test was negative. Patient number 8 had a positive MIBI scan and CAG showed an intermediate lesion, which was managed medically. MSCT scan of 5 patients (6, 10, 13, 14 and 15) demonstrated an interarterial course and they underwent CABG, whereby a mammary arterial graft was anastomosed to the RCA in 4 and the fifth showed a benign course.
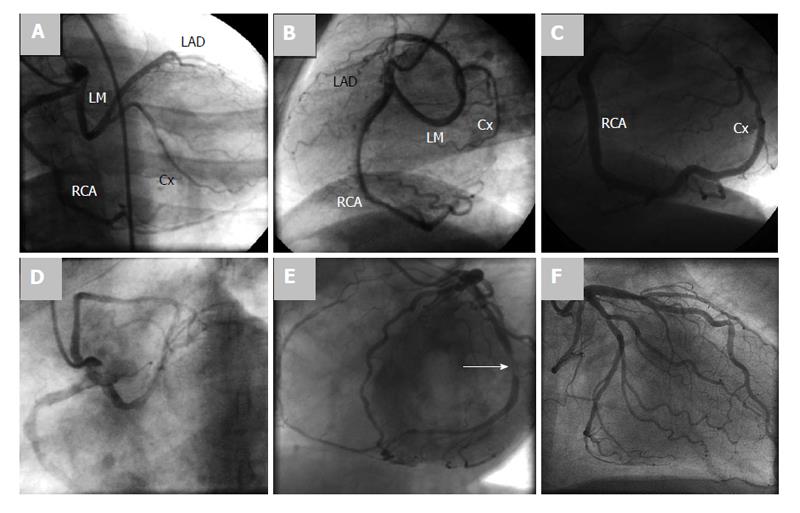
Conventional CAG demonstrated a SCA originating as a single ostium from the RSV in 6 patients and SCA originating as a single ostium from the LSV in 9 patients (Figures 2 and 3).
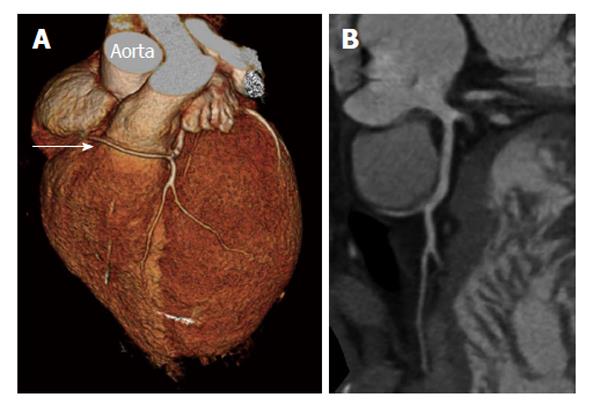
MSCT was performed in 6 patients (2, 6, 10, 13, 14 and 15). In patient 2, MSCT confirmed the diagnosis of SCA but gave an overestimation of the severity of the coronary lesion in the Cx trajectory, which was not significant (0.93) by fractional flow reserve measurement (Figure 4). In 4 patients (6, 10, 13 and 14), SCA was also proven by MSCT depicting clearly the course of the SCA running between the aorta and the pulmonary artery (Figures 5, 6). MSCT in patient 15 demonstrated a benign course of the SCA (Figure 7).
The coronary arterial circulation may rarely be supplied by a SCA arising from either the right, left or posterior sinus of Valsalva[16]. The course of the SCA can be highly variable. In the last century, different classification systems for SCA based on necropsy findings and angiographic variants were suggested in the fifties by Smith[3] (3 types), in the seventies by Lipton et al[1], in the eighties by Roberts[17], and finally through the nineties by Shirani et al[2] and Roberts et al[18].
Recently, a clinically useful classification scheme has been published, using either subgroups based on the site of origin and course of the anomalous coronary artery or descriptive anatomic terminology. In 2005, Rigatelli et al[19,20] based his classification on clinical significance of the anomaly and launched a global practical classification of four categories (class A: benign; class B: relevant due to fixed myocardial ischemia; class C: severe, involved in sudden cardiac death (SCD); and class D: critical due to worsened clinical picture). The clinical significance and management of the various types of SCA are different as shown in Table 2.
| Class | Subtypes | Clinical significance | Current series |
| A | E.g., ectopic origin of Cx from RSV1 | Benign natural history, asymptomatic careful follow-up with conservative medical management or percutaneous intervention | Patients: none1 |
| B | Ectopic origin of Cx from the RCA | Relevant, related to myocardial ischemia | Patients: 1, 2, 3, 7, 8, 9, 12, 15 |
| R-I, R-II, R-III anterior/posterior course2 | Careful follow-up with conservative medical management or percutaneous intervention | ||
| C | L-I, L-II, L-III anterior/posterior course2 | Severe, potentially related to sudden cardiac death | |
| R-I, R-II, R-III between/interseptal course2 | Requires surgical treatment | Patients: 6, 10, 13, 14 | |
| D | L-I, L-II, L-III between/interseptal course2 | Critical, class B or C with superimposed coronary artery atherosclerotic disease | Patients: 4, 5, 11 |
| B or C subgroups with concomitant coronary atherosclerosis | Requires urgent percutaneous management or surgical treatment |
Cheitlin et al[21] expressed the pathological significance of a SCA or of both coronary arteries originating from the RSV when the anomalous artery that supplies the left coronary distribution passes leftward with an inter-arterial course between the aorta and pulmonary trunk, rendering it prone to compression and kinking on physical exercise. This variant is considered malignant since it is associated with SCD in adolescents and young adults, especially on the athletic arena. It has been found that anomalous origin of the left coronary artery from the right aortic sinus is consistently related to sudden death in more than half of the cases (53%)[18].
On the other hand, when a SCA originating from the LSV or both coronary arteries arising from separate ostia located in the LSV with the RCA passing inter-arterially (between the aorta and pulmonary trunk, e.g., subtype Lipton L-IIB) is less deleterious even though compression can occur but SCD is a rare event. Four of our 15 patients (patients 6, 10, 13 and 14), having the abovementioned subtype, underwent successful arterial bypass grafting to the RCA.
In a necropsy series, SCA was found in 18% of subjects. Fifty percent arose from the RSV and 50% originated from the LSV. Sudden death was twofold more frequently associated with the SCA arising from the RSV (18%) compared with those from the LSV (9%)[12].
Coronary artery anomalies are associated with life threatening symptoms and may cause SCD during or after strenuous exercise. The most common congenital coronary artery anomalies causing SCD involve an anomalous origin of either the right or left coronary artery arising from the left or the RSV, respectively[22]. SCD is common (82%) when the anomalous LCA has an inter-arterial course passing between the aorta and main pulmonary artery[12]. Moreover, SCD may rarely occur after surgical repair[23]. The incidence is very low and estimated at 0.024% to 0.098% in the general population[1,5,9,19,24]. The incidence of all coronary artery anomalies in the necropsy series is approximately 0.23% and varying from 0.3% to 13% in the angiographic series[1,9,25,26]. Recently, the incidence of SCA, using dual-source computer tomography angiography, was estimated at 0.05% in the Chinese adult population[27]. SCA may be associated with longevity and has been reported in an octogenarian[28].
Diagnostic modalities: The correct diagnosis of a SCA and its course is not always easily made based on conventional CAG only. Precise delineation of anatomical and functional characteristics requires further complementary diagnostic modalities such as MSCT or CMR[29-31].
Conventional CAG: Isolated SCA may be incidentally detected on routine CAG[32], as was the case in our current series. Even with multiple projections and different angiographic views and the use of a pulmonary artery catheter, the identification of the origin and proximal course of the vessel can be difficult[13]. Serota et al[33] proposed an angiographic technique (the dot-and-eye method) for rapid identification of the course of SCA but even with this method, identification remains difficult.
MSCT CAG: MSCT has been very useful in the diagnosis and identification of the origin and course of SCA[28,32]. Although the radiation dose using new algorithms is decreasing, this rapidly developing non-invasive technique still has the disadvantage of radiation exposure. However, the spatial resolution (0.4-0.6 mm3) is higher than CMR and the temporal resolution of 64-slice double source MSCT is around 83 ms[30,31,34-37]. In 5 of our patients (patients 6, 10, 13, 14 and 15) of the current series, 128-slice MSCT confirmed the diagnosis of a SCA with clear demonstration of the inter-arterial course of the RCA originating from the LSV in four (Figure 6) and a benign course of the RCA from LSV in one (Figure 7).
Cardiovascular MR imaging: This technique has the advantage of not using ionizing radiation and has no need for the use of iodinated nephrotoxic ionic or non-ionic contrast agents. Image acquisition occurs with fairly good spatial and temporal resolution, but acquisition and imaging time is long, which makes routine use difficult and time consuming. Cardiovascular magnetic resonance proved to be useful in determining the anatomy and functional significance of SCA[38]. Both the MSCT and the CMR imaging techniques have the additional advantage of 3-D reconstruction of the areas of the coronaries relative to the aorta and pulmonary artery. This makes a definitive diagnosis of squeezed aberrant coronary arteries between the great vessels feasible[13].
The detection of atherosclerotic coronary artery disease (CAD) in the presence of coronary anomalies is of practical importance, especially when a decision between PCI and CABG has to be made. For diagnostic and therapeutic reasons, the knowledge of possible variations of the coronary anatomy, their different origin, and their course is of pivotal importance. Symptomatic patients with associated significant CAD may be treated with routine interventions such as PCI or CABG[6,39]. Angiographic recognition of coronary artery anomalies prior to surgery is of great importance. During operation, surgical complications may occur if an unrecognized anomalous vessel is excluded from perfusion during cardiopulmonary bypass or if the surgeon inadvertently damages an artery with an anomalous pathway.
Because of the reported high mortality, the occurrence of “symptomatic or asymptomatic” squeezing of SCA, regardless of the degree of atherosclerosis or site of origin, justifies arterial grafting, as was shown in 4 of our series (patients 6, 10, 13 and 14).
Significant atherosclerotic CAD[37] in association with coronary artery anomalies has been reported in 26%-60% of cases[1,2,40-42]. Rigatelli et al[43] suggested that benign coronary artery anomalies are not associated with or involved in the development of premature atherosclerotic CAD. Indeed the high percentage of coronary artery stenosis could be biased by the indication to perform CAG as SCA is mainly found during this diagnostic procedure. Only 4 of our 15 (27%) patients (patients 4, 5, 10 and 11) had significant CAD and 3 of them required percutaneous intervention. When the SCA does not course between the aorta and pulmonary artery, it is not vulnerable to acute angulations or kinking of the coronaries. SCA may be associated with longevity and patients in the 7th and 8th decade of life have been reported[3,12,13,44-47], as was the case in 2 octogenarians from our current series (patients 4 and 11).
Although a SCA is often a benign congenital anomaly, in which sudden death is a rare complication, different diagnostic modalities should be used to exclude an inter-arterial course between the aorta and pulmonary artery to detect patients at risk for serious complications.
Congenital coronary artery anomalies, detected at necropsy, associated with sudden death and without antecedent signs have been recognized in calves[48]. SCA is not limited to the human race, it has also been reported in other mammals such as horses[49], syrian hamsters[50] and minipigs[51].
As was shown in our patient’s population, SCA can be associated with longevity. It has been documented up till the 8th decade of life. In the adult population, SCA-isolated or in association with acquired atherosclerotic changes-may cause severe sequelae. In some cases without CAD, the course of the SCA may be malignant.
SCA may be associated with symptomatic transient transmural myocardial ischemia, NSVT, and aborted sudden death in the absence or presence of coronary atherosclerosis. The availability of MSCT and CMR facilitates the delineation of the course of the anomalous vessel. The accurate delineation of the course of the anomalous vessel is of great importance even in patients without CAD and in cases of surgical intervention where anatomic details of the course of the vessel are of importance.
The authors appreciate the dedicated assistance of the personnel of the catheterization laboratories of Hospital Group Twente, Almelo-Hengelo (Mr. M. Mintjens, Mrs. M. Holleman and Mrs. M. Rigterink-Hofman, Mr. H. Schutte, Mr. R. Heinen, Mr. E. IJspeerd, Mr. B. Gering, Mr. F. van de Bosch, Mr. J. Oolderink and Mr. M. Wildemors) and St. Lucas-Andreas Hospital, Amsterdam; St. Anna Hospital, Geldrop; and Gelre Hospital, Zutphen, the Netherlands. The assistance of the librarians of the medical library of Hospital Group Twente, Mrs. A. Geerdink and Mr. D. Maas during the preparation of the manuscript is highly appreciated.
Single coronary artery (SCA) is a rare congenital anomaly and occurs as an incidental finding in approximately 0.066% of the coronary angiography (CAG) population. SCA has been reported in association with and without atherosclerotic changes or in association with coronary artery fistulas, bicuspid aortic valves, and with hypertrophic cardiomyopathy.
CAG is the first diagnostic tool in the detection of a SCA. Once abnormal coronary arteries are suspected, multi-slice computed tomography (MSCT) and cardiac magnetic resonance (CMR) imaging scans are excellent tools for non-invasive determination of the course of the abnormal coronaries relative to the aorta and pulmonary artery. Determination of the course of incidentally found congenital coronary anomalies during routine CAG without the direct availability of CMR or MSCT scanning is challenging.
Percutaneous coronary intervention was successfully performed in 3 patients. Eight patients were managed medically. Arterial bypass graft was performed in 4 patients with the squeezed SCA. The literature addressing SCA is reviewed.
Congenital coronary artery anomalies, detected at necropsy, associated with sudden death and without antecedent signs have been recognized in calves. SCA is not limited to the human race, it has also been reported in other mammals such as horses, syrian hamsters and minipigs.
This paper showed that the availability and sophistications of MSCT facilitated the delineation of the course of a SCA. The authors presented a Dutch case series and review of the literature. This is an interesting report for clinical practice. Overall the report appears to be carefully examined and data adequately discussed.
P- Reviewers: Letsas K, Sakabe K S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Liu SQ
| 1. | Lipton MJ, Barry WH, Obrez I, Silverman JF, Wexler L. Isolated single coronary artery: diagnosis, angiographic classification, and clinical significance. Radiology. 1979;130:39-47. [PubMed] |
| 2. | Shirani J, Roberts WC. Solitary coronary ostium in the aorta in the absence of other major congenital cardiovascular anomalies. J Am Coll Cardiol. 1993;21:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Smith JC. Review of single coronary artery with report of 2 cases. Circulation. 1950;1:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Halperin IC, Penny JL, Kennedy RJ. Single coronary artery. Antemortem diagnosis in a patient with congestive heart failure. Am J Cardiol. 1967;19:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Desmet W, Vanhaecke J, Vrolix M, Van de Werf F, Piessens J, Willems J, de Geest H. Isolated single coronary artery: a review of 50,000 consecutive coronary angiographies. Eur Heart J. 1992;13:1637-1640. [PubMed] |
| 6. | Türkay C, Gölbasi I, Bayezid O. A single coronary artery from the right sinus of valsalva associated with atherosclerosis. Acta Cardiol. 2002;57:377-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Hara H, Ishii K, Nakamura M. A case of hypertrophic obstructive cardiomyopathy complicated by a single coronary artery treated by transcoronary septal ablation. J Invasive Cardiol. 2006;18:234-238. [PubMed] |
| 8. | El-Menyar AA, Das KM, Al-Suwaidi J. Anomalous origin of the three coronary arteries from the right aortic sinus Valsalva: role of MDCT coronary angiography. Int J Cardiovasc Imaging. 2006;22:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Bolognesi R, Tsialtas D, Barbaresi F, Manca C. Single coronary artery-right ventricular fistula with a partially thrombosed large aneurysm of its proximal tract in a 66-year-old man. Eur Heart J. 1994;15:1720-1724. [PubMed] |
| 10. | Hillestad L, Eie H. Single coronary artery. A report of three cases. Acta Med Scand. 1971;189:409-413. [PubMed] |
| 11. | Larsen AI, Ørn S, Barvik S, Nilsen DW. Anomalies of the coronary arteries originating from the right sinus of Valsalva. (1) Single coronary artery originating from the right sinus associated with fusion of the left and the non coronary cusp and atrophy of the left coronary ostium (2)Three separate coronary arteries originating from the right sinus of Valsalva. Int J Cardiol. 2007;115:e86-e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992;20:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 539] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 13. | Bunce NH, Lorenz CH, Keegan J, Lesser J, Reyes EM, Firmin DN, Pennell DJ. Coronary artery anomalies: assessment with free-breathing three-dimensional coronary MR angiography. Radiology. 2003;227:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Yucel EK, Anderson CM, Edelman RR, Grist TM, Baum RA, Manning WJ, Culebras A, Pearce W. AHA scientific statement. Magnetic resonance angiography : update on applications for extracranial arteries. Circulation. 1999;100:2284-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 578] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 16. | Virmani R, Burke AP, Farb A. Sudden cardiac death. Cardiovasc Pathol. 2001;10:211-218. [RCA] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Roberts WC. Major anomalies of coronary arterial origin seen in adulthood. Am Heart J. 1986;111:941-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 336] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Roberts WC, Shirani J. The four subtypes of anomalous origin of the left main coronary artery from the right aortic sinus (or from the right coronary artery). Am J Cardiol. 1992;70:119-121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Rigatelli G, Docali G, Rossi P, Bandello A, Rigatelli G. Validation of a clinical-significance-based classification of coronary artery anomalies. Angiology. 2005;56:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Rigatelli G, Rigatelli G. Congenital coronary artery anomalies in the adult: a new practical viewpoint. Clin Cardiol. 2005;28:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Cheitlin MD, MacGregor J. Congenital anomalies of coronary arteries: role in the pathogenesis of sudden cardiac death. Herz. 2009;34:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Liberthson RR. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 315] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Nguyen AL, Haas F, Evens J, Breur JM. Sudden cardiac death after repair of anomalous origin of left coronary artery from right sinus of Valsalva with an interarterial course: Case report and review of the literature. Neth Heart J. 2012;20:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1278] [Cited by in RCA: 1359] [Article Influence: 38.8] [Reference Citation Analysis (1)] |
| 25. | Cieslinski G, Rapprich B, Kober G. Coronary anomalies: incidence and importance. Clin Cardiol. 1993;16:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Kardos A, Babai L, Rudas L, Gaal T, Horvath T, Talosi L, Toth K, Sarvary L, Szasz K. Epidemiology of congenital coronary artery anomalies: a coronary arteriography study on a central European population. Cathet Cardiovasc Diagn. 1997;42:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Zhang LJ, Yang GF, Huang W, Zhou CS, Chen P, Lu GM. Incidence of anomalous origin of coronary artery in 1879 Chinese adults on dual-source CT angiography. Neth Heart J. 2010;18:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Morimoto H, Mukai S, Obata S, Hiraoka T. Incidental single coronary artery in an octogenarian with acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2012;15:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Fernandes F, Alam M, Smith S, Khaja F. The role of transesophageal echocardiography in identifying anomalous coronary arteries. Circulation. 1993;88:2532-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Soon KH, Selvanayagam J, Bell KW, Tang SH, Pereira J, Chan W, Lim YL. Giant single coronary system with coronary cameral fistula diagnosed on MSCT. Int J Cardiol. 2006;106:276-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Datta J, White CS, Gilkeson RC, Meyer CA, Kansal S, Jani ML, Arildsen RC, Read K. Anomalous coronary arteries in adults: depiction at multi-detector row CT angiography. Radiology. 2005;235:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Liesting C, Brugts JJ, Kofflard MJ, Dirkali A. Acute coronary syndrome in a patient with a single coronary artery arising from the right sinus of Valsalva. World J Cardiol. 2012;4:264-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Serota H, Barth CW, III , Seuc CA, Vandormael M, Aguirre F, Kern MJ. Rapid identification of the course of anomalous coronary arteries in adults: the “dot and eye” method. Am J Cardiol. 1990;65:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Kunimasa T, Sato Y, Ito S, Takagi T, Lee T, Saeki F, Moroi M. Absence of the right coronary artery detected by 64-detector-row multislice computed tomography. Int J Cardiol. 2007;115:249-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Jessurun GA, Willemsen MH, Vercoutere RA, Boonstra PW, Tio RA. Single coronary artery: a reappraisal. J Invasive Cardiol. 2004;16:40-41. [PubMed] |
| 36. | Hegde AN, Desai SB. Two cases of anomalous origins of left coronary artery with a course between the aortic root and the free standing subpulmonary infundibulum on CT coronary angiography. Interact Cardiovasc Thorac Surg. 2005;4:297-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, George R, Kaufmann P, Kopp AF, Knuuti J. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29:531-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 359] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 38. | Jahnke C, Nagel E, Ostendorf PC, Tangcharoen T, Fleck E, Paetsch I. Images in cardiovascular medicine. Diagnosis of a “single” coronary artery and determination of functional significance of concomitant coronary artery disease. Circulation. 2006;113:e386-e387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Zweiker R, Luha O, Klein WW. Rescue percutaneous transluminal coronary angioplasty in a patient with a single coronary artery arising from the right Sinus Valsalvae: previously unreported scenario and review of literature. J Intern Med. 2002;252:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Topaz O, DeMarchena EJ, Perin E, Sommer LS, Mallon SM, Chahine RA. Anomalous coronary arteries: angiographic findings in 80 patients. Int J Cardiol. 1992;34:129-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Garg N, Tewari S, Kapoor A, Gupta DK, Sinha N. Primary congenital anomalies of the coronary arteries: a coronary: arteriographic study. Int J Cardiol. 2000;74:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Tejada JG, Hernandez F, Sanchez I, Martin-Asenjo R. Stenting of anomalous left main coronary artery arising from the right sinus of Valsalva: a case report. Int J Cardiol. 2007;119:266-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Rigatelli G, Gemelli M, Zamboni A, Docali G, Rossi P, Rossi D, Grazio M, Franco G, Rigatelli G. Are coronary artery anomalies an accelerating factor for coronary atherosclerosis development? Angiology. 2004;55:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Kuon E, Ropers D. Single coronary artery--a rarity in the catheterization laboratory: case report and current review. Can J Cardiol. 2004;20:647-651. [PubMed] |
| 45. | Tejada JG, Albarran A, Velazquez MT, Sanz J, Pindado C, Tascon JC. Direct stenting in single coronary artery arising from the left sinus of Valsalva-a case report. Angiology. 2002;53:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Basalus M, Louwerenburg JW, van Houwelingen KG, Stoel MG, von Birgelen C. Primary percutaneous coronary intervention in the left main stem of a monocoronary artery. Neth Heart J. 2009;17:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Cheitlin MD, De Castro CM, McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva, A not-so-minor congenital anomaly. Circulation. 1974;50:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 519] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 48. | Shank AM, Bryant UK, Jackson CB, Williams NM, Janes JG. Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) in four calves. Vet Pathol. 2008;45:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Sans-Coma V, Arque JM, Duran AC, Cardo M. Origin of the left main coronary artery from the pulmonary trunk in the Syrian hamster. Am J Cardiol. 1988;62:159-161. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |