Published online Mar 26, 2014. doi: 10.4330/wjc.v6.i3.100
Revised: December 17, 2013
Accepted: January 17, 2014
Published online: March 26, 2014
Processing time: 111 Days and 12.4 Hours
In patients with an acute ST-segment elevation myocardial infarction, timely myocardial reperfusion using primary percutaneous coronary intervention is the most effective therapy for limiting myocardial infarct size, preserving left-ventricular systolic function and reducing the onset of heart failure. Within minutes after the restoration of blood flow, however, reperfusion itself results in additional damage, also known as myocardial ischemia-reperfusion injury. An improved understanding of the pathophysiological mechanisms underlying reperfusion injury has resulted in the identification of several promising pharmacological (cyclosporin-A, exenatide, glucose-insulin-potassium, atrial natriuretic peptide, adenosine, abciximab, erythropoietin, metoprolol and melatonin) therapeutic strategies for reducing the severity of myocardial reperfusion injury. Many of these agents have shown promise in initial proof-of-principle clinical studies. In this article, we review the pathophysiology underlying myocardial reperfusion injury and highlight the potential pharmacological interventions which could be used in the future to prevent reperfusion injury and improve clinical outcomes in patients with coronary heart disease.
Core tip: As therapeutic interventions administered at the time myocardial reperfusion have been proven to reduce infarct size in both experimental and clinical models, the existence of a lethal reperfusion injury and its contribution to ischemic cardiac cell death can no longer be ignored. Patients presenting with an acute ST-segment elevation myocardial infarction will likely benefit from therapy aimed at the timely administration of drugs, most likely via primary percutaneous coronary intervention, for the reduction/prevention of lethal reperfusion injury. This approach will ensure that patients maximally benefit from the myocardial salvage that results from these therapies.
- Citation: Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ. Cardioprotection and pharmacological therapies in acute myocardial infarction: Challenges in the current era. World J Cardiol 2014; 6(3): 100-106
- URL: https://www.wjgnet.com/1949-8462/full/v6/i3/100.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i3.100
Acute myocardial infarction (AMI) is a major cause of mortality and morbidity worldwide. Each year, an estimated 785000 persons will have a new AMI in the United States alone and approximately every minute an American will succumb to one[1]. In addition, AMI has major psychological and legal implications for patients and society and is an important outcome measure in research studies. The prevalence of AMI provides useful data regarding the burden of coronary artery disease and offers insight into health care planning, policy and resource allocation[1].
The rapid time course of AMI and the temporal limitation on the maximal effectiveness of reperfusion constitute the pathobiological basis for the contemporary clinical strategies that emphasize early intervention within 1-2 h after the onset of symptoms[2]. Currently, timely myocardial reperfusion using either thrombolytic therapy or primary percutaneous coronary intervention forms the cornerstone of treatment for acute ST-segment elevation myocardial infarction (STEMI) patients[3]. However, mortality remains substantial in these patients, with in-hospital mortality ranging between 6% and 14%[4].
Reperfusion profoundly alters the outcome of an evolving AMI. If instituted in a timely manner, a potential transmural AMI can be prevented and the extent of necrosis greatly reduced and limited to the subendocardium. However, some injured cardiomyocytes at the edge of the wavefront become irreversibly injured during the reperfusion phenomenon, producing a component of lethal reperfusion injury[5]. After reperfusion, the salvaged myocardium exhibits impaired contractile function, a form of nonlethal reperfusion injury referred to as myocardial stunning. The earlier the reperfusion, the less total necrosis that occurs (including both the ischemia-induced and reperfusion-induced component), as well as the earlier the recovery of contractile function from the transient stunning. Conversely, reperfusion can be rendered less effective by the microvascular damage and obstruction that develop during the ischemic phase; this is known as the no-reflow phenomenon[6,7].
In this minireview, we provide an overview of myocardial reperfusion injury and highlight potential pharmacological interventions for preventing it in reperfused-STEMI patients.
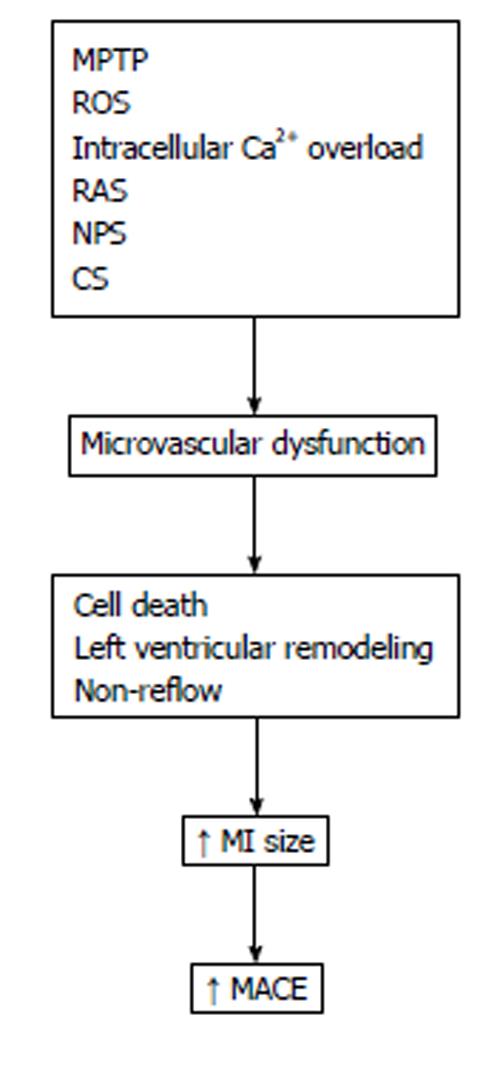
There are various pathophysiological mechanisms involved in myocardial injury reperfusion. It has been suggested that mitochondrial permeability transition pore opening, overproduction of oxygen-derived free radicals and intracellular calcium overload might be candidates responsible for reperfusion injury. However, other factors of importance in the pathogenesis of reperfusion injury must be included, such as platelet and neutrophil-mediated injury, the renin-angiotensin system and the complement activation[8,9] (Figure 1).
Multiple lines of evidence have converged to show that the mitochondria have a central role in the pathogenesis of cell injury[10,11]. In stressed cells, deleterious and salutary effects acting on mitochondria are mediated by death channels and salvage pathways, respectively[12]. The mitochondrial death channels include the mitochondrial permeability transition pore and the mitochondrial apoptosis channel.
The mitochondrial permeability transition pore is a voltage-dependent channel that is regulated by calcium and oxidative stress[13]. The opening in the first few minutes of reperfusion of the mitochondrial permeability transition pore, a non-selective channel of the inner mitochondrial membrane, in response to mitochondrial Ca2+ overload, oxidative stress, restoration of a physiological pH and ATP depletion, induces the cardiomyocyte death by uncoupling the biochemistry route of oxidative phosphorylation[14], which leads to a reduction in ATP production.
Cell membranes are composed mostly of phospholipids and proteins. Alterations in membrane proteins by free radicals are among the important factors in the evolution of myocardial ischemia reperfusion damage. Large quantities of oxygen-derived free radicals lead to overwhelming of the cellular endogenous antioxidant defences. This causes, among other effects, the peroxidation of lipid membranes and loss of membrane integrity which results in necrosis and cell death[15].
Re-introduction of abundant oxygen at the onset of reperfusion evokes a burst of additional toxic oxygen derivatives, including superoxide anion, hydroxyl radical and peroxynitrite, within the first few minutes of reflow. Moreover, oxidative stress also reduces the bioavailability of nitric oxide (vasodilator compound) during reperfusion[16].
Changes in intracellular calcium homeostasis play an important role in the development of reperfusion injury. Intracellular calcium release at the time of myocardial reperfusion is mediated by damage to the sarcolemmal membrane and oxidative stress-induced dysfunction of the sarcoplasmic reticulum. These changes result in cardiomyocyte hypercontracture, mitochondrial calcium overload and the opening of the mitochondrial permeability transition pore[17].
The complement system is activated during reperfusion injury. This contributes to the formation of the anaphylatoxins C3a, C4a and C5a, as well as the terminal complement complex, the membrane attack complex, which is deposited in cell membranes. The complement factors induce direct cell injury by increasing cell permeability and release of histamine and platelet activating factor. In addition, complement factors, especially C5a, are potent stimulators of neutrophil adherence and superoxide production[8].
Neutrophils are important for the development of reperfusion injury by releasing oxygen free radicals, proteases and pro-inflammatory mediators that further amplify the infiltration of neutrophils into the jeopardized myocardium[18]. Additionally, the hemorrheologic properties of neutrophils contribute to leukocyte entrapment in the capillaries, leading to microvascular plugging[19].
Local platelet aggregation and deposition and also microembolization are partially responsible for reperfusion injury, especially in relation to microvascular dysfunction. Reperfusion injury induces platelet activation and this exacerbates the damage to the myocardium. Platelet products may exacerbate microcirculatory spasm, leading to further microvascular congestion, thrombosis and sluggish coronary flow[8,20].
The key product of the renin-angiotensin system, angiotensin II, increases intracellular calcium levels of cardiomyocytes and smooth muscle cells, leading to positive inotropism, impairment of diastolic function and coronary vasoconstriction. At pathophysiological levels, angiotensin II is cardiotoxic and induces myocyte necrosis[8,21].
Progress in understanding the basic pathobiology of ischemic heart disease has led to many years of research aimed at developing pharmacological approaches for limiting myocardial ischemic damage. Although myocardial ischemia-reperfusion injury is clearly mediated by several elements (Figure 1), agents aimed against these components of ischemic injury have not been consistently effective in different clinical trials[2,22]. A number of reasons for the situation have been brought to light[2,7,23,24].
A number of pharmacological interventions have been tried in the clinical setting to prevent myocardial reperfusion injury in reperfused-STEMI patients, although the results have been largely disappointing. Moreover, several pharmacological agents for preventing myocardial reperfusion injury in reperfused-STEMI patients are currently being tested in proof-of-principal clinical studies (Table 1)[9,24].
| Mitochondrial permeability transition pore opening | Overproduction of oxygen-derived free radicals | Intracellular calcium overload | Neutrophil-mediated injury | Platelet- mediated injury |
| Cyclosporin A | Adenosine | Glucose- insulin- potassium | Adenosine | Abciximab |
| Melatonin | Metoprolol | Atrial natriuretic peptide | Abciximab | Melatonin |
| Erythropoietin | Erythropoietin | |||
| Glucose-insulin -potassium | Melatonin | |||
| Exenatide | ||||
| Melatonin |
Cyclosporine is known to inhibit the formation and opening of the mitochondrial permeability transition pore. In a proof-of-concept clinical trial involving 58 patients, cyclosporine administered as a 2.5 mg/kg intravenous bolus at the time of percutaneous coronary intervention was found to reduce the size of the myocardial infarct compared with placebo. Infarct size was reduced by 40%, as measured by creatine kinase release. Evaluation by magnetic resonance imaging also showed less myocardial damage[25,26]. The ongoing CIRCUS study (NCT01502774) is investigating whether this therapeutic approach can reduce patient death, hospitalization for heart failure and a 15% increase in left ventricular end-diastolic volume.
Exenatide a new antidiabetic drug, has been shown to reduce myocardial infarct size by 23% of area at risk at 90 d, as assessed by magnetic resonance imaging, when given as an intravenous infusion started 15 min prior to primary percutaneous coronary intervention and continued for 6 h[27,28].
Of all the agents that have been tested reduce myocardial infarct size or improve acute clinical outcome of STEMI, perhaps none is more controversial than glucose-insulin-potassium regimen. In the CREATE-ECLA trial, intravenous glucose-insulin-potassium infusion for 24 h was initiated after reperfusion of AMI. This trial had a negative outcome since it showed a difference in mortality at 30 d[29]. The IMMEDIATE trial has been recently published. In this trial, the intravenous glucose-insulin-potassium infusion for 12 h was started by paramedics in the ambulance prior to reperfusion. The composite of cardiac arrest or in-hospital mortality was lower in 4.4% of glucose-insulin-potassium patients compared to 8.7% in the placebo patients (P = 0.01)[30]. Thus, the use of glucose-insulin-potassium for AMI remains controversial and requires further studies.
Kitakaze et al[31] demonstrated that an infusion of carperitide (an atrial natriuretic peptide analogue) during 72 h after reperfusion reduced myocardial infarct size and preserved left ventricular ejection fraction in reperfused-STEMI patients.
Two large multicenter studies, AMI Study of Adenosine (AMISTAD) 1 and AMISTAD 2, showed that a high-dose 3-h intravenous infusion of adenosine started near the time of reperfusion significantly reduced anterior wall myocardial infarct size, as determined by nuclear imaging[32,33]. Other studies, however, were negative. A total of 112 patients with STEMI were randomized to 4 mg intracoronary adenosine or placebo. There was no benefit of adenosine on myocardial infarct size assessed by magnetic resonance imaging at 4 mo[34]. Fokkema et al[35] also studied the effect of high-dose intracoronary adenosine boluses on myocardial infarct size and parameters of myocardial reperfusion. Four hundred and forty-eight patients with acute STEMI were randomized to placebo or 2 bolus injections of intracoronary adenosine. Adenosine did not improve the myocardial infarct size. Thus, the efficacy of the use of adenosine for AMI remains unproven and requires further studies.
In a recent study by Stone et al[36], 452 patients presenting within 4 h of STEMI with proximal or mid-left anterior descending coronary artery occlusion and undergoing percutaneous coronary intervention plus bivalirudin as an anticoagulant were randomized to bolus intracoronary abciximab, no abciximab, and to manual aspiration thrombectomy versus no thrombectomy in a 2 × 2 factorial design. The authors concluded that in patients with large STEMI undergoing percutaneous coronary intervention with bivalirudin, the addition of intracoronary abciximab bolus significantly reduced myocardial infarct size. Not all recent clinical trials with abciximab have been positive.
Thiele et al[37] compared intracoronary abciximab bolus during primary percutaneous coronary intervention with an intravenous bolus in patients with STEMI. This large open-label, multicenter trial randomized > 2000 patients to intracoronary vs intravenous bolus abciximab followed by a 12 h intravenous infusion. The primary composite end point at 90 d (all-cause mortality, recurrent myocardial infarction or new congestive heart failure) was similar in the intracoronary group versus the intravenous group. Whereas the incidence of death and reinfarction did not differ between groups, fewer patients in the intracoronary group developed new congestive heart failure. The authors concluded that intracoronary abciximab bolus is safe and might be considered to reduce the rates of congestive heart failure. However, other secondary end points in this study, including enzymatic myocardial infarct size, were negative.
The large REVEAL study showed no reduction of infarct size[38] and several other recent trials were negative for infarct size reduction[39,40].
The capacity of β-blockers to reduce infarct size was evaluated extensively in the pre-reperfusion era, with inconsistent results[41]. In the context of reperfusion as the treatment of choice for STEMI, this has been poorly investigated. Experimental data suggest that the β-blocker metoprolol may reduce infarct size only when administered intravenously before reperfusion[42,43].
Recently, the results have been demonstrated of the Effect of Metoprolol in Cardioprotection During an AMI trial, the first randomized, clinical trial prospectively evaluating the effect of early intravenous β-blockade on infarct size in conjunction with primary angioplasty. A total of 270 patients with anterior STEMI (Killip class II or less) revascularized within 6 h after symptom onset were randomized to receive intravenous metoprolol or not before reperfusion. All patients received oral metoprolol according to clinical guidelines (first dose, 12-24 h after infarction). Infarct size, evaluated by magnetic resonance imaging and creatine kinase release, was significantly reduced in the intravenous metoprolol group with no excess side effects. The left ventricular ejection fraction was higher in the intravenous metoprolol group[44].
Melatonin, a circadian endocrine product of the pineal gland, is formed and released predominantly during night time. Melatonin has a diverse functional repertoire with actions in essentially all organs, including the heart and other portions of the cardiovascular system[45-47]. Melatonin reduces the pathophysiological mechanisms that are involved in these benefits, in part due to the detoxification myocardial reperfusion injury, with respect to radical oxygen species and radical of oxygen and nitrogen-based reactants melatonin and its metabolites[48,49]. Moreover, melatonin has indirect beneficial effects by increasing the activity of principal antioxidant enzymes[50]. Recent data also suggest that the mechanism of protection of melatonin appears to involve, at least in part, the inhibition of mitochondrial permeability transition pore opening via prevention of cardiolipin peroxidation[51]. The lack of these cardioprotective effects due to insufficient high endogenous melatonin levels might be associated with several cardiovascular pathologies, including ischemic heart disease[47,50,52].
Several studies show that humans with cardiovascular disease have noticeably lower circulating melatonin levels than age-matched subjects without significant cardiovascular deterioration[53]. Recent investigations in patients with STEMI undergoing primary percutaneous coronary intervention confirmed a relationship between melatonin concentrations and ischemia-modified albumin, a biomarker of myocardial ischemia. These data suggest that melatonin can act as a potent antioxidant agent, reducing myocardial damage induced by ischemia/reperfusion[54].
Because of the available scientific evidence, our group carried out a phase II clinical trial (ClinicalTrials.gov no. NCT00640094) using melatonin. We attempted to document whether intravenous and intracoronary melatonin administration reduces infarct size in STEMI patients treated by primary percutaneous coronary intervention by performing a multicenter, randomized, controlled clinical trial[55]. The importance of these studies is emphasized by the fact that melatonin is quickly distributed throughout the organism when exogenously administered (oral, intravenous or subcutaneous). It crosses all morphophysiological barriers and enters cardiac cells with ease. The highest intracellular concentrations of melatonin are found at a mitochondrial level[56]. This is especially important as the mitochondria is a major site of free radical generation and oxidative stress[57].
Unless the findings in animal investigations are totally misleading, it is expected that melatonin will have similar protective effects benefitting the human heart. Melatonin is easily synthesized in a pharmacologically pure form and is inexpensive. Because of its marked versatility in protecting against oxidative stress and reducing inflammation in patients with myocardial ischemia, melatonin may have significant potential to improve public health.
A major determinant of post-infarction mortality and morbidity is the extent of myocardial necrosis after STEMI; therefore, strategies to limit infarct size are important. Several pharmacological interventions have been proposed as potential cardioprotective therapies but their use in clinical practice has been limited.
The list of cardioprotective agents that can be used as adjuvant therapy during to reperfusion is promising. Large multicenter clinical trials with enough statistical power will be necessary to establish the reported beneficial effects and to answer the question of whether they can improve clinical outcomes. To prevent translational failure, particular attention must be paid to proper selection of patients (who will benefit the most), application (relevant concentration in the early phase of reperfusion) and hard end points.
P- Reviewers: Berenguer AB, Izawa KB S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Liu SQ
| 1. | Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2363] [Cited by in RCA: 3207] [Article Influence: 246.7] [Reference Citation Analysis (0)] |
| 2. | Buja LM, Weerasinghe P. Unresolved issues in myocardial reperfusion injury. Cardiovasc Pathol. 2010;19:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Worner F, Cequier A, Bardají A, Bodí V, Bover R, Martínez-Sellés M, Sabaté M, Sionis A, Vázquez de Prada JA, Arós F. Comments on the ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation. Rev Esp Cardiol. 2013;66:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Mandelzweig L, Battler A, Boyko V, Bueno H, Danchin N, Filippatos G, Gitt A, Hasdai D, Hasin Y, Marrugat J. The second Euro Heart Survey on acute coronary syndromes: Characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J. 2006;27:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 375] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 340] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Buja LM, Vela D. Cardiomyocyte death and renewal in the normal and diseased heart. Cardiovasc Pathol. 2008;17:349-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2847] [Article Influence: 158.2] [Reference Citation Analysis (0)] |
| 8. | Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 9. | Fröhlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J. 2013;34:1714-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 10. | Webster KA. Programmed death as a therapeutic target to reduce myocardial infarction. Trends Pharmacol Sci. 2007;28:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1552-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 12. | Webster KA. Mitochondrial Death Channels. Am Sci. 2009;97:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Buja LM. The pathobiology of acute coronary syndromes: clinical implications and central role of the mitochondria. Tex Heart Inst J. 2013;40:221-228. [PubMed] |
| 14. | Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Lazzarino G, Raatikainen P, Nuutinen M, Nissinen J, Tavazzi B, Di Pierro D, Giardina B, Peuhkurinen K. Myocardial release of malondialdehyde and purine compounds during coronary bypass surgery. Circulation. 1994;90:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 524] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 17. | Piper HM, García-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 376] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43:860-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 436] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Dreyer WJ, Michael LH, West MS, Smith CW, Rothlein R, Rossen RD, Anderson DC, Entman ML. Neutrophil accumulation in ischemic canine myocardium. Insights into time course, distribution, and mechanism of localization during early reperfusion. Circulation. 1991;84:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 206] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Xiao CY, Hara A, Yuhki K, Fujino T, Ma H, Okada Y, Takahata O, Yamada T, Murata T, Narumiya S. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001;104:2210-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Neves LA, Almeida AP, Khosla MC, Campagnole-Santos MJ, Santos RA. Effect of angiotensin-(1-7) on reperfusion arrhythmias in isolated rat hearts. Braz J Med Biol Res. 1997;30:801-809. [PubMed] |
| 22. | Kloner RA. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res. 2013;113:451-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 25. | Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1001] [Cited by in RCA: 982] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 26. | Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet G. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Lønborg J, Kelbæk H, Vejlstrup N, Bøtker HE, Kim WY, Holmvang L, Jørgensen E, Helqvist S, Saunamäki K, Terkelsen CJ. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv. 2012;5:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 29. | Mehta SR, Yusuf S, Díaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C, Kazmi K. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 430] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 30. | Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D’Agostino RB, Ruthazer R, Atkins JM, Sayah AJ, Levy MK. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. 2012;307:1925-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 32. | Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 377] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol. 2005;45:1775-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 428] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 34. | Desmet W, Bogaert J, Dubois C, Sinnaeve P, Adriaenssens T, Pappas C, Ganame J, Dymarkowski S, Janssens S, Belmans A. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur Heart J. 2011;32:867-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Fokkema ML, Vlaar PJ, Vogelzang M, Gu YL, Kampinga MA, de Smet BJ, Jessurun GA, Anthonio RL, van den Heuvel AF, Tan ES. Effect of high-dose intracoronary adenosine administration during primary percutaneous coronary intervention in acute myocardial infarction: a randomized controlled trial. Circ Cardiovasc Interv. 2009;2:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Stone GW, Maehara A, Witzenbichler B, Godlewski J, Parise H, Dambrink JH, Ochala A, Carlton TW, Cristea E, Wolff SD. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. 2012;307:1817-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 402] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 37. | Thiele H, Wöhrle J, Hambrecht R, Rittger H, Birkemeyer R, Lauer B, Neuhaus P, Brosteanu O, Sick P, Wiemer M. Intracoronary versus intravenous bolus abciximab during primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction: a randomised trial. Lancet. 2012;379:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 38. | Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, Barsness GW, Prather K, Heitner JF, Kilaru R. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 2011;305:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 39. | Suh JW, Chung WY, Kim YS, Kim KI, Jeon EJ, Cho YS, Youn TJ, Chae IH, Kim CH, Choi DJ. The effect of intravenous administration of erythropoietin on the infarct size in primary percutaneous coronary intervention. Int J Cardiol. 2011;149:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Prunier F, Bière L, Gilard M, Boschat J, Mouquet F, Bauchart JJ, Charbonnier B, Genée O, Guérin P, Warin-Fresse K. Single high-dose erythropoietin administration immediately after reperfusion in patients with ST-segment elevation myocardial infarction: results of the erythropoietin in myocardial infarction trial. Am Heart J. 2012;163:200-7.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Rude RE, Buja LM, Willerson JT. Propranolol in acute myocardial infarction: the MILIS experience. Am J Cardiol. 1986;57:38F-42F. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Ibanez B, Prat-González S, Speidl WS, Vilahur G, Pinero A, Cimmino G, García MJ, Fuster V, Sanz J, Badimon JJ. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation. 2007;115:2909-2916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Ibanez B, Cimmino G, Prat-González S, Vilahur G, Hutter R, García MJ, Fuster V, Sanz J, Badimon L, Badimon JJ. The cardioprotection granted by metoprolol is restricted to its administration prior to coronary reperfusion. Int J Cardiol. 2011;147:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Ibanez B, Macaya C, Sánchez-Brunete V, Pizarro G, Fernández-Friera L, Mateos A, Fernández-Ortiz A, García-Ruiz JM, García-Álvarez A, Iñiguez A. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 45. | Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ. Melatonin and cardiovascular disease: myth or reality? Rev Esp Cardiol (Engl Ed). 2012;65:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Dominguez-Rodriguez A, Abreu-Gonzalez P. Myocardial ischemia-reperfusion injury: Possible role of melatonin. World J Cardiol. 2010;2:233-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ. Melatonin and circadian biology in human cardiovascular disease. J Pineal Res. 2010;49:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 843] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 49. | Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res. 2013;54:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 614] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 50. | Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: protective effects of melatonin. J Pineal Res. 2008;44:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Dominguez-Rodriguez A, Arroyo-Ucar E, Abreu-Gonzalez P. Role of melatonin in preventing mitochondrial dysfunction in myocardial ischemia-reperfusion injury. Am J Cardiol. 2010;106:1521-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Dominguez-Rodriguez A. Melatonin in cardiovascular disease. Expert Opin Investig Drugs. 2012;21:1593-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Dominguez-Rodriguez A, Abreu-Gonzalez P, Avanzas P. The role of melatonin in acute myocardial infarction. Front Biosci (Landmark Ed). 2012;17:2433-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez MJ, Samimi-Fard S, Reiter RJ, Kaski JC. Association of ischemia-modified albumin and melatonin in patients with ST-elevation myocardial infarction. Atherosclerosis. 2008;199:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez MJ, Kaski JC, Reiter RJ, Jimenez-Sosa A. A unicenter, randomized, double-blind, parallel-group, placebo-controlled study of Melatonin as an Adjunct in patients with acute myocaRdial Infarction undergoing primary Angioplasty The Melatonin Adjunct in the acute myocaRdial Infarction treated with Angioplasty (MARIA) trial: study design and rationale. Contemp Clin Trials. 2007;28:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García-Corzo L, López LC, Reiter RJ, Acuña-Castroviejo D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 460] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 57. | Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ. Melatonin and cardioprotection in the acute myocardial infarction: a promising cardioprotective agent. Int J Cardiol. 2012;158:309-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |