Published online Nov 26, 2014. doi: 10.4330/wjc.v6.i11.1192
Revised: August 15, 2014
Accepted: September 6, 2014
Published online: November 26, 2014
Processing time: 220 Days and 10 Hours
Myocardial pathologies are major causes of morbidity and mortality worldwide. Early detection of loss of cellular integrity and expansion in extracellular volume (ECV) in myocardium is critical to initiate effective treatment. The three compartments in healthy myocardium are: intravascular (approximately 10% of tissue volume), interstitium (approximately 15%) and intracellular (approximately 75%). Myocardial cells, fibroblasts and vascular endothelial/smooth muscle cells represent intracellular compartment and the main proteins in the interstitium are types I/III collagens. Microscopic studies have shown that expansion of ECV is an important feature of diffuse physiologic fibrosis (e.g., aging and obesity) and pathologic fibrosis [heart failure, aortic valve disease, hypertrophic cardiomyopathy, myocarditis, dilated cardiomyopathy, amyloidosis, congenital heart disease, aortic stenosis, restrictive cardiomyopathy (hypereosinophilic and idiopathic types), arrythmogenic right ventricular dysplasia and hypertension]. This review addresses recent advances in measuring of ECV in ischemic and non-ischemic myocardial pathologies. Magnetic resonance imaging (MRI) has the ability to characterize tissue proton relaxation times (T1, T2, and T2*). Proton relaxation times reflect the physical and chemical environments of water protons in myocardium. Delayed contrast enhanced-MRI (DE-MRI) and multi-detector computed tomography (DE-MDCT) demonstrated hyper-enhanced infarct, hypo-enhanced microvascular obstruction zone and moderately enhanced peri-infarct zone, but are limited for visualizing diffuse fibrosis and patchy microinfarct despite the increase in ECV. ECV can be measured on equilibrium contrast enhanced MRI/MDCT and MRI longitudinal relaxation time mapping. Equilibrium contrast enhanced MRI/MDCT and MRI T1 mapping is currently used, but at a lower scale, as an alternative to invasive sub-endomyocardial biopsies to eliminate the need for anesthesia, coronary catheterization and possibility of tissue sampling error. Similar to delayed contrast enhancement, equilibrium contrast enhanced MRI/MDCT and T1 mapping is completely noninvasive and may play a specialized role in diagnosis of subclinical and other myocardial pathologies. DE-MRI and when T1-mapping demonstrated sub-epicardium, sub-endocardial and patchy mid-myocardial enhancement in myocarditis, Behcet’s disease and sarcoidosis, respectively. Furthermore, recent studies showed that the combined technique of cine, T2-weighted and DE-MRI technique has high diagnostic accuracy for detecting myocarditis. When the tomographic techniques are coupled with myocardial perfusion and left ventricular function they can provide valuable information on the progression of myocardial pathologies and effectiveness of new therapies.
Core tip: This review addresses recent advances of measuring of extracellular volume (ECV) in ischemic and non-ischemic myocardial pathologies. The main approaches that are used for probing ECV are equilibrium contrast enhanced magnetic resonance imaging/multi-detector computed tomography and magnetic resonance imaging (MRI) longitudinal relaxation time mapping. These noninvasive techniques are currently used, but at a lower scale, as alternative to invasive endomyocardial biopsies to eliminate anesthesia, coronary catheterization and tissue sampling error. ECV measurements may aid in early detection of various myocardial pathologies. Delayed contrast enhanced-MRI (DE-MRI) and when T1-mapping demonstrated sub-epicardium, sub-endocardial and patchy mid-myocardial enhancement in myocarditis, Behcet’s disease and sarcoidosis, respectively. Furthermore, recent studies showed that the combined technique of cine, T2-weighted and DE-MRI technique has high diagnostic accuracy for detecting myocarditis. When the tomographic techniques are coupled with myocardial perfusion and left ventricular function it can provide valuable information on the progression of myocardial pathologies and effectiveness of new therapies.
- Citation: Saeed M, Hetts SW, Jablonowski R, Wilson MW. Magnetic resonance imaging and multi-detector computed tomography assessment of extracellular compartment in ischemic and non-ischemic myocardial pathologies. World J Cardiol 2014; 6(11): 1192-1208
- URL: https://www.wjgnet.com/1949-8462/full/v6/i11/1192.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i11.1192
Ischemic and non-ischemic cardiomyopathies have become a worldwide epidemic of the 21st century with increasing impact on healthcare systems. The 2012 European Society of Cardiology and 2013 American College of Cardiology Foundation/American Heart Association guidelines have set the stage for current therapy to reduce mortality and morbidity[1,2]. Revascularization of coronary arteries in acute myocardial infarct (AMI) have become the treatment of choice and revascularization procedures have evolved significantly. Because X-ray coronary angiography-the clinically accepted reference standard for demonstrating coronary artery disease is invasive and provides information only on the anatomical status of coronary obstructive lesions several noninvasive methods have been developed to aid in the assessment of the functional status of myocardium, namely contraction and perfusion as well as microvascular and cellular integrity, including positron emission tomography and contrast-enhanced echocardiography. More recently, delayed contrast-enhanced (DE) magnetic resonance imaging (MRI)[3-13]. Extracellular MR contrast media identifies hyperenhanced infarct, hypoenhanced microvascular obstruction zone and a moderately enhanced peri-infarct zone in acute myocardial infarction[5,6]. Delayed contrast enhanced-MRI (DE-MRI) has sensitivity of 99% for measuring AMI/scar infarct extent and 94% for measuring the transmural enhancement[3,7,8]. Transmural enhancement was used to predict recovery of regional function in enhanced segments[9]. A cutoff of 50% transmural enhancement was the threshold of recovery of regional function after intervention, where < 50% transmural enhancement predicted recovery in 53% of segments, while > 50% transmural enhancement was associated with negligible recovery (8% of segments)[10]. Furthermore, < 25% transmural enhancement predicted residual viability in 82% of segments. DE-MRI has been clinically used to diagnose and specify different types of ischemic and non-ischemic cardiomyopathies based on the pattern and location of enhancement.
In ischemic cardiomyopathy the sub-endocardium is always enhanced on DE-MRI[3,7,8], while in dilated cardiomyopathy, a patchy mid-myocardial pattern of enhancement is seen[12]. Patients with mid-myocardial enhancement are at higher risk of sudden cardiac death and arrhythmias[13]. Furthermore, patients with restrictive cardiomyopathy showed delayed myocardial enhancement over the entire sub-endocardial circumference[14]. DE-MRI and T1-mapping demonstrated sub-epicardial, sub-endocardial and patchy mid-myocardial enhancement in myocarditis specific cardiomyopathies such as Behcet’s disease and sarcoidosis, respectively. In Behcet’s disease, enhancement of sub-endocardial fibrosis in the right ventricle is considered a feature of the disease. Vignaux[15] observed delayed enhancement in sarcoidosis patients in specific locations [basal interventricular septum, lateral left ventricular (LV) wall] and distribution patterns (patchy or striate that do not involve the sub-endocardium) and in advanced cases of diffuse and focal pathologies. In non-ischemic dilated cardiomyopathy, Assomull et al[13] showed that the presence of delayed myocardial enhancement was associated with a 3-fold increase of hospitalization for heart failure or cardiac death and a 5-fold increase of sudden cardiac death or ventricular arrhythmias. In hypertrophic cardiomyopathy, the extent of differentially enhanced myocardium assessed on DE-MRI was linked with progressive disease and markers of clinical risk for sudden death[16].
Other MRI studies showed discordant results about the relationship between infarct enhancement and regional LV function. Beek et al[17] reported that 25% of LV segments with transmural enhancement showed potential improvement in function at 13 wk. In another recent study, Dall’Armellina et al[18] found that AMI does not necessarily equate with irreversible injury and severely underestimate salvaged myocardium on DE-MRI. Accordingly, new strategies have been developed to quantify diffuse myocardial fibrosis and small infarcted areas using equilibrium contrast enhanced magnetic resonance imaging/multi-detector computed tomography (MRI/MDCT) and T1-mapping techniques[19-27]. Lee et al[28] found that the extracellular volume (ECV) in healthy volunteers is stable between 8.5-23.5 min after gadolinium-based contrast media administration and in infarcted myocardium between 12-50 min[29]. These methods overcome the question of the relationship between myocardial enhancement, function and diffuse fibrosis on delayed enhancement. They also allowed for the detection of greater collagen content in the extracellular compartment of myocardium in aging, failing heart, congenital heart, infiltrative heart, hypertension and hypertrophic cardiomyopathy pathologies than normal myocardium[19,30-34].
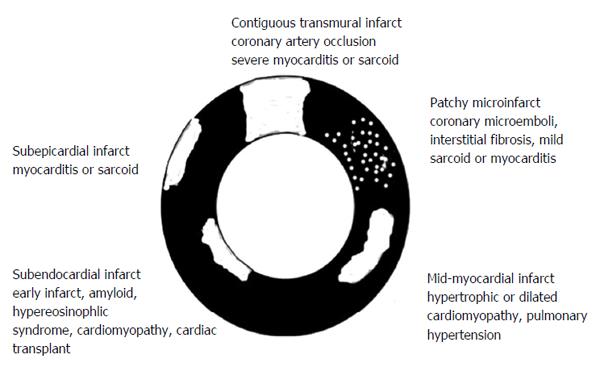
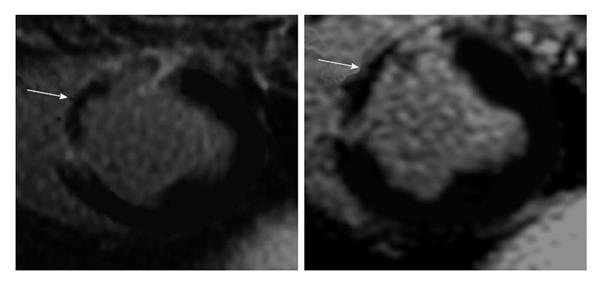
Visualization of small infarcted areas, peri-infarct zone, patchy microinfarct and diffuse fibrosis remains difficult using existing DE-MRI and DE-MDCT because of low sensitivity, minor/vague alterations in tissue structure, nonspecific enhancement or overlapping with other confounding diseases. On the other hand, experimental studies have shown expansion of ECV in conditions where myocardial damage is invisible on MRI[26,27]. A clinical MRI study found that the ECV of AMI is higher than the ECV in non-ischemic cardiomyopathies, suggesting that the damage is greater damage in the former. The study also showed that the location and pattern of enhancement differs between non-ischemic and ischemic cardiomyopathies[35] (Figure 1).
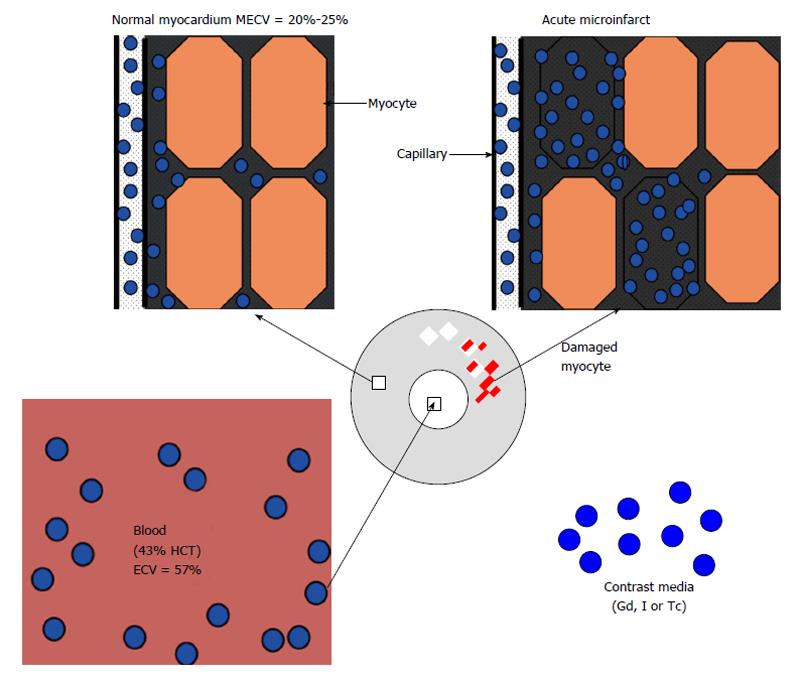
Microscopic studies revealed three fluid compartments in healthy myocardium, namely intravascular (approximately 10% of tissue volume), interstitial (approximately 15%) and intracellular (approximately 75%) compartment (Figure 2). It should be noted that the terms extracellular volume (ECV), volume of distribution, fibrosis index, and volume fraction of extravascular extracellular matrix share the same parameters for measuring the ECV by adjusting the contrast media partition coefficient with blood hematocrit[26,27,36].
Intracellular water accounts for 79% of total water or about 380 mL/100 g of dry tissue and varies between individuals and species[37]. The intracellular compartment includes myocardial cells, fibroblasts and vascular endothelial/smooth muscle cells. The main constituent proteins of the interstitial compartment are types I and III collagens. Water permeable membranes separate these compartments. Blood plasma and interstitial fluid exchange through pores and intercellular clefts in capillary endothelium.
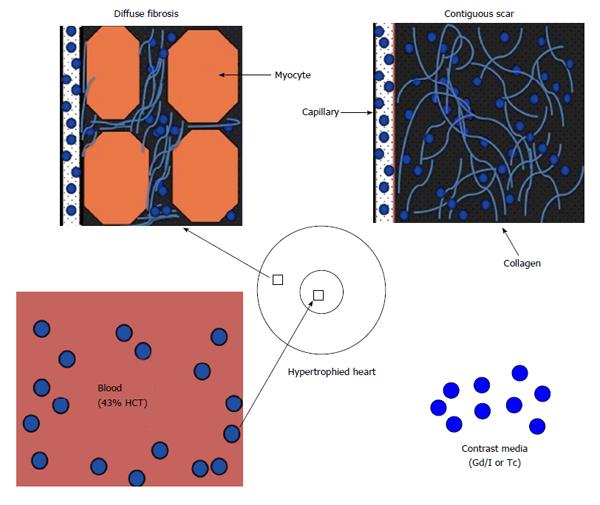
The fluid in the interstitial compartment consists of a water solvent containing sugars, salts, fatty acids, amino acids, coenzymes, hormones, neurotransmitters and cellular waste products. The exchange of fluid and accompanying solutes between compartments is governed by hydrostatic and oncotic forces. These forces are typically balanced to maintain a constant fluid volume in the compartments. The molecular pathways that contribute to extracellular compartment remodeling post-MI, however, are multifactorial and related to; (1) the increase in osmotic colloidal pressure resulting from the leakage of plasma proteins[38]; (2) the degradation of the extracellular matrix[39]; and (3) heterogeneous or homogeneous loss of membrane integrity of myocardial cells. Disturbance in microvascular permeability causes extravasation of plasma macromolecules that subsequently leads to water imbalance and interstitial edema. Loss of the membrane integrity of myocardial cells further expands the extracellular compartment; and that is the basis for assessing viability and fibrosis (Figure 2). Expansion of ECV in ischemic and non-ischemic heart diseases is strongly associated with adverse outcomes[40]. Expansion of ECV has been seen in myocarditis, hypertrophy, dilated cardiomyopathy, amyloidosis, congenital heart disease, aortic stenosis, restrictive cardiomyopathy, arrhythmogenic right ventricular dysplasia, hypertension and myocardial infarction (Figure 3).
Proton relaxation times (T1, T2, and T2*) reflect the composition of water protons in tissues. In 1992 several studies showed relationship between T1 change and extracellular MR contrast media content in myocardium[41-43]. Extracellular contrast media are rapidly distributed throughout the extracellular compartment in most tissues, but not in the brain, testis and retina. They are rapidly cleared from the circulation via the kidney. The quantity of contrast media distributed into a particular tissue is a function of physical extent of extracellular space and physiologic processes (blood flow, volume and diffusion) that distribute the agent into and remove it from the tissue. In myocardial infarct, investigators observed progressive alterations in structure and composition of the extracellular compartment[44,45]. Interstitial edema in infarcted myocardium causes increase in longitudinal (T1), transverse (T2) and T2* relaxation times[46] and administration of contrast media causes shortening[19,30-32]. The decrease in the T1 relaxation time is greater in infarcted than healthy myocardium, resulting in differential enhancement. T1 assessment has also been used to measure macromolecular content, water binding and water content in tissues. The T1 relaxation time is defined as the time when longitudinal proton magnetization recovers approximately 63% of its equilibrium value. T2* relaxation time refers to decay of transverse magnetization caused by a combination of spin-spin relaxation and magnetic field inhomogeneity. The differential attenuation of infarct and viable myocardium on MDCT relies on X-ray absorption by iodine.
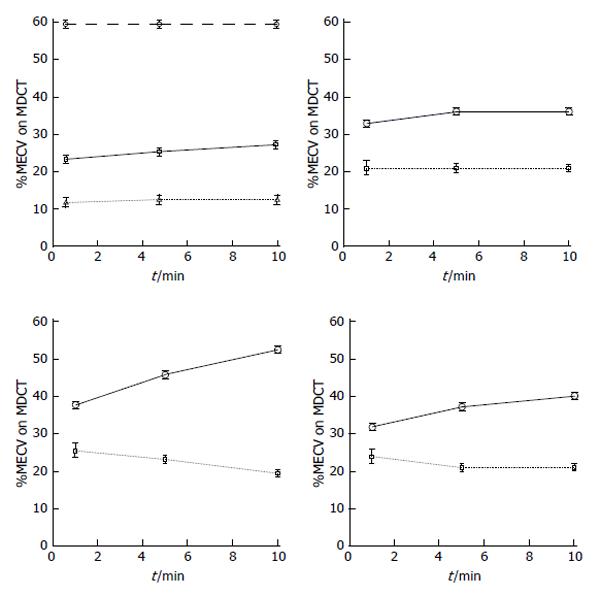
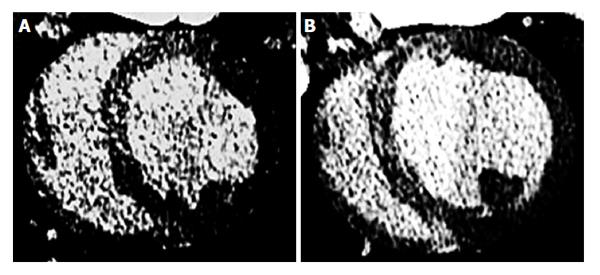
The gold standard method for estimation of ECV in patients has been sub-endocardial biopsy. This method, however, has relatively high inherent risk, is limited to small regions and is prone to sampling site error[47,48]. Visualization of large AMI and scar infarct on MRI and MDCT relies on the differences in signal intensity/attenuation between damaged and remote undamaged tissue to generate image contrast. It has been reported that undetected infarct account for at least 20% of all clinical cases of AMI and carry a prognosis as poor as detected ones[49]. Furthermore, signal intensity on DE-MRI is displayed on an arbitrary scale and tissue signals or contrast media concentration cannot be quantified. Patchy microinfarct and diffuse fibrosis in non-ischemic myocardial cardiomyopathies necessitate alternative techniques beyond current DE-MRI or DE-MDCT. Fast MRI and MDCT image acquisition, T1 sensitive sequences and contrast media allow the measurement of ECV. Look-Locker and echo planar MRI sequences as well as MDCT were used for non-invasive estimation of ECV. More recently, investigators have used MRI for T1 mapping and measuring ECV. The differences in regional T1 can be visualized as a grey-scale or color map[50-53]. Investigators also found that equilibrium contrast and T1 mapping methods provide information, beyond what is visually evident on DE-MRI/DE-MDCT[48,50]. These methods rely on three principles: (1) the measurement of global myocardial and blood T1 relaxation time/signal attenuation before contrast media administration; (2) a second measurement of T1 relaxation time/signal attenuation during contrast media equilibrium phase; and (3) a direct measurement of the blood contrast media volume of distribution. Extracellular inert gadolinium-based MR and iodinated computed tomography (CT) contrast media are crucial because they diffuse passively and rapidly between intravascular and extracellular compartments (Figure 4). Investigators have used longitudinal relaxation rate (1/longitudinal MR relaxation time; 1/T1) on MRI and myocardial signal attenuation on CT to quantify regional ECV[22,41-43,54]. The calculation of ECV is based on the ratio of the difference in signal attenuation or 1/T1 before and after administration of contrast medium in myocardium divided by the difference in signal attenuation or 1/T1 the blood pool. The increase in regional signal intensity on MRI and a decrease in attenuation on CT are attributed to the increase in ECV. Enhancement is expressed in Hounsfield or arbitrary units and employs tissue with lowest signal intensity as a reference for normality. The reason for using 1/T1 and not signal intensity on MRI is that signal intensity is not linearly correlated with contrast concentration. Unlike MR contrast media, signal attenuation after administration of CT contrast media is linearly correlated with contrast media concentration.
Our group was the first to find on MRI that the expansion in ECV is the mechanism for differential enhancement of infarct from healthy myocardium. We also demonstrated the peri-infarct zone[26,27,55]. Later, Klein et al[56] confirmed in patients with AMI that the partition coefficient is elevated in infarct compared to remote myocardium. Lee et al[28] found in healthy volunteers that the ECV is 27% ± 1% while Broberg et al[33] found it is slightly lower (22% ± 2%). Schelbert et al[29] claimed that similar ECV values can be obtained by bolus (21% ± 2%) and infusion (25% ± 2%) approaches.
Recent studies have also shown that MDCT allows for assessment of myocardial viability and visualization of coronary stenosis[57-59]. This imaging modality has been recently used for assessment of ECV in healthy volunteers[60] and infarcted swine hearts[61]. Investigators found on MDCT that the ECV in healthy volunteers is between 23%-26%[62,63]. We found in swine model of AMI that the ECV of iodinated contrast media is 24% in normal and 68% in infarcted myocardium (Table 1). Furthermore, the distribution volume of iodinated contrast medium was lower at the peri-infarct zone than infarct, suggesting that this zone contains admixture of viable and nonviable myocardial cells. In chronic infarct, the ECV in remote undamaged myocardium decreased to 18% as a result of compensatory hypertrophy (Table 1). Schelbert et al[29] and Schmidt et al[64] also observed extensive heterogeneity in scar infarct, derangement in myocardial structure and accumulation of interstitial collagen that alters electrical activity and stiffens the myocardium. A clinical study showed that microvascular obstruction (MVO) occurs in > 30% of patients after ST segment elevation in myocardial infarction[65]. The presence of MVO in the infarct is problematic in the assessment of ECV, because the rate of contrast wash-in and wash-out is severely reduced in MVO zone and the equilibrium state condition takes 18 min post contrast injection[56]. Furthermore, the signal intensity of MVO zone is similar to remote undamaged myocardium (Figure 5).
MRI has inherent challenges that can be summarized as follows: (1) the presence of dental prostheses, orthopedic hardware or LV assist devices in the scanner; (2) slow acquisition time associated with high cost; (3) unsuitable for claustrophobic or uncooperative patients; (4) high technical and personnel requirements; and (5) MR contrast media provides a non-linear relationship between signal intensity and concentration[66]. On the other hand, MDCT has the potential to accommodate a growing population of patients who are counter indicated for MRI. MDCT has different challenges such as: (1) presence of radiation exposure precludes serial assessment; (2) low contrast between infarct and normal myocardium; (3) requires post imaging reconstruction of images; and (4) lack of sequences analogous to MRI that provide circumferential/longitudinal LV strain data (such as tagging, phase contrast velocity encoded cine) or information on interstitial edema and hemorrhage (such as T2-weighted and T2*-weighted imaging).
Myocardial fibrosis (scar) can be related to either ischemic MI, non-ischemic cardiomyopathy, or the combination[67]. For example, diffuse and contiguous fibrosis has been reported in heart failure, aortic valve disease and hypertrophic cardiomyopathy[29,68-70], while solely diffuse fibrosis has been observed in myocarditis, hypertrophic/dilated cardiomyopathy, amyloidosis, congenital heart disease, aortic stenosis, restrictive cardiomyopathy (hypereosinophilic and idiopathic types), arrythmogenic right ventricular dysplasia and hypertension.
T1 mapping has been widely used to assess non-ischemic cardiomyopathies. Recent studies show that this technique also has the potential to assess ischemic MI. Klein et al[71] determined ECV in 11 patients with heart failure and found that the ECV is greater in the infarcted region (54% ± 1%) than remote myocardium (29% ± 2%). Ugander et al[20] measured ECV in 126 patients with myocardial infarct and non-ischemic myocardial fibrosis and detected sub-clinical abnormalities in remote myocardium using ECV measurements. They found that scar infarct has significantly higher ECV (51% ± 8%) than remote undamaged myocardium (27% ± 3%, P < 0.001, n = 36). In patients with non-ischemic cardiomyopathy, the ECV of atypically enhanced and remote myocardium were (37% ± 6% vs 26% ± 3%, P < 0.001, n = 30). They also observed in these patients that ECV of remote myocardium increased with the decrease of LV ejection fraction (r = -0.50, P = 0.02). A similar observation was reported in patients with heart failure[48]. It has been shown that beta-blockers and angiotensin-converting enzyme inhibitors reduce diffuse myocardial fibrosis in patients with heart failure and hypertensive heart disease, respectively[72,73], thus early measurement of ECV in suspected heart patients holds great promise for future clinical applications.
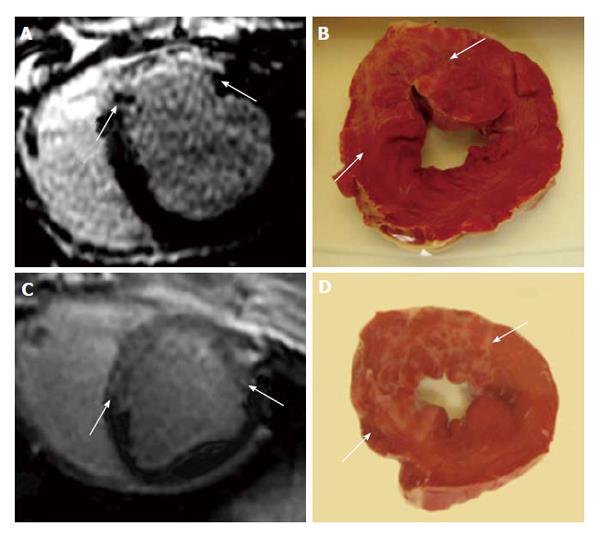
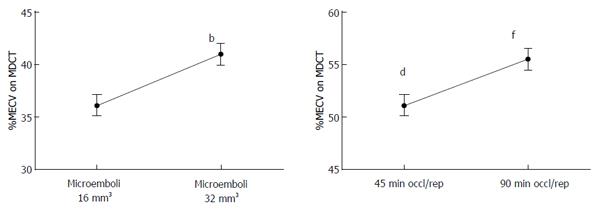
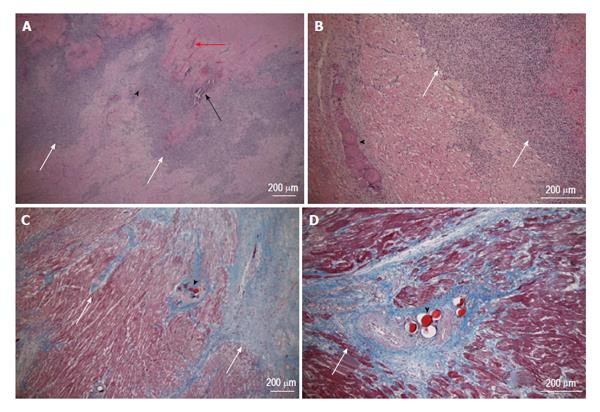
Coronary microembolization secondary to atherosclerotic plaque rupture occurs in spontaneously in patients with unstable angina/acute coronary syndromes[74-76] and accidentally during coronary interventions[77-83] with pathophysiological consequences, such as contractile dysfunction, perfusion-contraction mismatch, arrhythmias, myocardial ischemia and microinfarction[84-87]. Clinical studies showed that revascularization of an occluded coronary artery, using PCI, coronary artery stents, or bypass grafting, causes visible and invisible patchy microinfarct[88-91]. Both DE-MDCT and DE-MRI show promise in detecting patchy microinfarct caused by relatively large volumes of microemboli in a swine model (Figures 6 and 7)[92-99], while equilibrium contrast enhanced MDCT provides a quantitative estimation of ECV as a function of microemboli volumes and duration of coronary artery occlusion (Figure 8). Histologic examination reveals dislodged microemboli in blood vessels surrounded with microinfarct (Figure 9). Small particles cause MVO, patchy microinfarct[100], delayed infarct healing[101], perfusion deficits and disturbances in ECG signal conductivity[102,103]. ECV data derived from equilibrium contrast enhanced MDCT in a swine model are shown in Table 1.
Myocarditis is the most frequent disease in patients with acute coronary syndrome and normal coronary arteries[104]. Acute myocarditis is associated with systemic viral disease[105,106]. At the early stage, there is myocardial injury/infarction, edema and regional/global LV dysfunction. On DE-MRI, myocardial injury is focal and located in the sub-epicardium and mid-myocardium (Figure 1). This method was also used for quantifying myocarditis[107,108]. Furthermore, T2-weighted MRI sequence was also useful in detecting acute myocarditis for detecting interstitial edema, as an integral part of the inflammatory response, in acute myocarditis. This non-invasive method is useful for patients with acute chest pain, positive serum troponin and angiographicallly normal coronary arteries[109,110]. Mahrholdt et al[110] speculated that the differential enhancement in the early phase is related to myocardial necrosis, but in the late phase to scar tissue. The sensitivity of DE-MRI in detecting myocarditis has been variable because of the different patterns (diffuse vs focal and acute vs chronic) of enhancement[111-115]. More recently, Kellman et al[52,116] found that ECV is significantly higher (44% ± 6%) in myocarditic tissue compared with remote myocardium using T1 mapping.
LV hypertrophy is an independent risk factor for sudden death[117,118]. Diffuse fibrosis is a common feature of hypertrophic cardiomyopathy and characterized by expansion of ECV and accumulation of interstitial collagen/fibrosis, which are hallmarks of pathologic remodeling[119]. Because ventricular hypertrophy prevalence estimates in the general population are as high as 16% the public health implications are significant[120].
DE-MRI in hypertrophic cardiomyopathy showed that both diffuse fibrosis and necrosis share mid-myocardium and sub-epicardium of the ventricular septum[3,16,121,122] (Figure 1). Díez et al[123] provided evidence for the role of fibrotic remodeling in hypertensive heart disease. Myocardial fibrosis in ventricular hypertrophy can impair the electrical coupling of myocardial cells by separating these cells with collagen and create a substrate of tissue heterogeneity from which re-entrant arrhythmias may arise[124].
Recently, Shiozaki et al[125] used MDCT to measure myocardial fibrosis in 26 patients with asymptomatic or mildly symptomatic hypertrophic cardiomyopathy. Myocardial fibrosis was present in 25 of 26 patients (96%) with mean fibrosis mass of 21 ± 16 g, while patients with appropriate implantable cardioverter defibrillator shocks for ventricular tachycardia/fibrillation had significantly greater myocardial fibrosis than patients without (29 ± 19 g vs 14 ± 8 g; P = 0.01). For a myocardial fibrosis mass of at least 18 g, sensitivity and specificity for appropriate implantable cardioverter defibrillator firing were 73% and 71%, respectively[125].
Clinical studies showed that myocardial fibrosis is reversible and treatable with timely intervention, therefore early detection and assessment is crucial. Investigators proposed that ECV measured on MRI may be useful in serially assessing the effects of therapies focused on proliferation of fibrosis in myocardium, such as the ACE-inhibitor (lisinopril)[73] and the angiotensin II receptor antagonist losartan[73,123]. These therapies have been shown to reduce the LV wall stiffness and severity of myocardial fibrosis (measured on biopsy) and concomitantly improve diastolic function.
Dilated cardiomyopathy is an important cause of heart failure, sudden death and is the leading indication for cardiac transplantation in children and adults[126]. MRI provides accurate assessment of ventricular chamber size, wall thickness, and systolic function. The pattern of DE-MRI can differentiate ischemic vs non-ischemic heart disease[12]. For example, a sub-epicardial or mid-myocardial enhancement suggests non-ischemic cardiomyopathy. McCrohon et al[12] reported that specific patterns of enhancement have been purported in dilated cardiomyopathy to indicate a particular genetic association; however, these findings are nonspecific. Other investigators showed that 59% of patients with dilated cardiomyopathy and normal coronary arteries have no delayed enhancement. The other 28% of patients had mid-myocardial enhancement that is consistent with a non-ischemic cause and few patients had delayed endocardial enhancement that is consistent with ischemic cause. Others found in patients with dilated cardiomyopathy that the extent of fibrosis has been associated with increased risk of intraventricular systolic dyssynchrony[127].
Bandula et al[54] developed an equilibrium CT protocol, using iohexol at 300 mgI/mL delivered as a bolus of 1 mg/kg and a rate of 3 mL/s, followed immediately by an infusion of 1.88 mL/kg per hour with CT imaging before and at 25 min after injection of bolus of contrast agent. The ECV within the myocardial septum in 23 patients with severe aortic stenosis was measured using both equilibrium CT and equilibrium MRI in patients. Biopsy samples of the myocardial septum were collected during valve replacement surgery and used for histologic quantification of extracellular fibrosis. They found that the mean percentage of histologic fibrosis was 18% and a significant correlation between both equilibrium MDCT derived and equilibrium MRI derived ECV and percentage of histologic fibrosis (r = 0.71, P < 0.001 and r = 0.84[128], respectively). Equilibrium MDCT derived ECV was well correlated to equilibrium MRI derived ECV (r = 0.73). Broberg et al[33] also found the fibrosis index was significantly elevated in patients with congenital heart disease compared with normal controls (32% ± 5% vs 25% ± 2%; P = 0.001), ECV values were highest in patients with a systemic right ventricle (L-transposition of the great arteries or D-transposition with prior atrial redirection surgery) and cyanosis (35% ± 6%; P < 0.001 and 34% ± 6%; P < 0.001, respectively).
Ho et al[129] were unable to visualize diffuse myocardial fibrosis in the setting of dilated cardiomyopathy using DE-MRI, but by T1 mapping technique, they found that fibrotic tissue has lower T1 relaxation time compared with healthy myocardium. Nacif et al[22] successfully measured interstitial myocardial fibrosis in hypertrophied hearts using ECV measurement method. Others found that ECV is higher in subjects with hypertrophic cardiomyopathy (36% ± 3%) than control volunteers (27% ± 1%, P < 0.001). Furthermore, the ECV in hypertrophic hearts is heterogeneous and had substantially lower mean value than for scar infarct (69% ± 9%, P < 0.001)[116].
Neilan et al[130] studied patients with hypertension and recurrent atrial fibrillation referred for pulmonary vein isolation underwent a contrast-enhanced MRI for measurement of ECV and were followed up prospectively for a median of 18 mo. These patients had elevated LV volumes, LV mass, left atrial volumes, and increased ECV (patients with atrial fibrillation = 34% ± 3%; healthy control volunteers = 29% ± 3%; P < 0.001). They found positive associations between ECV and left atrial volume (r = 0.46, P < 0.01) and LV mass, but negative association between ECV and diastolic function (r = -0.55, P < 0.001). Furthermore, they demonstrated that each 10% increase in ECV is associated with a 29% increased risk of recurrent atrial fibrillation and concluded that ECV was the strongest predictor of the primary outcome of recurrent atrial fibrillation and the secondary composite outcome of recurrent atrial fibrillation, heart failure admission, and death.
Amyloidosis refers to soluble proteins become insoluble, which are deposited in the extracellular compartment of various tissues, resulted in disrupting function[131]. Amyloid heart disease is a systemic infiltrative disorder[132]. It has been hypothesized that amyloidosis in myocardium is facilitated by hypoxia that results from capillary dysfunction. Endomyocardial biopsy has been considered to be the gold standard for demonstrating amyloid deposition in the heart. The most useful stain in the diagnosis of amyloid is Congo red, which, combined with polarized light, makes the amyloid proteins appear apple green on microscopy.
Noninvasive diagnosis of myocardial amyloidosis on MRI is difficult when this disease is accompanied with LV wall thickening related to hypertension[133]. Amyloid heart reveals small infarcted areas on unenhanced T1 and T2 MRI[134]. DE-MRI in myocardial amyloidosis is inherently challenging because amyloid infiltration within the extracellular compartment reduces the differences in contrast between LV chamber blood and myocardium such that the two regions may null simultaneously[133,135]. On the other hand, other investigators found that the appearance of global and subendocardial enhancement On DE-MRI, is a unique characteristic of cardiac amyloid and correlates with prognosis[136]. ECV was also measured in this disease using T1 mapping and gadolinium-based contrast media[34,137]. It was found that the median ECV is significantly higher in infiltrative diseases (49% of tissue volume) compared with non-amyloid cardiomyopathy patients (33%) and volunteers (24%). The ECV strongly correlated with visually assessed segmental DE-MRI (r = 0.80) and LV mass index (r = 0.69), reflecting severity of myocardial infiltration. Sado et al[35] reported that the ECV expansion is higher in systemic amyloidosis than in any other measured myocardial diseases, such as Anderson-Fabry disease, dilated cardiomyopathy, hypertrophic cardiomyopathy and hypertrophic cardiomyopathy, outside of the infarct zone. In another MRI study, Bandula et al[54] studied 40 healthy volunteers and 67 patients with systemic amyloid light-chain amyloidosis of the upper abdomen using equilibrium MRI. They found that ECV was measured in the liver, spleen, and paravertebral muscle. ECV was highest in the spleen (34%), followed by liver (29%) and muscle (9%). In patients with amyloidosis ECVs measured within the spleen (39%), liver (31%), and muscle (16%) were significantly higher than in healthy controls.
Type 2 diabetes mellitus promotes the expansion of ECV and increase vulnerability to a variety of clinical problems. Expansion of ECV is associated with mechanical dysfunction[138-140] vasomotor dysfunction[141], arrhythmia[142] and mortality[40,142]. Several studies support the notion that expansion of ECV contributes to adverse outcomes in diabetic patients. This notion is based on medications blocking the renin-angiotensin-aldosterone system ameliorate expansion of ECV[143-145]. In a recent study, Wong et al[40] examined 1176 patients referred for MRI with and without diabetes. They found that diabetic patients (n = 231) had higher median ECV than non-diabetic patients (n = 945) (30.2% vs 28.1%, P < 0.001). More importantly, expansion of ECV measured by MRI appeared to be ameliorated with medications blocking the renin-angiotensin-aldosterone system. They concluded that diabetes is associated with increased ECV, which may be an important intermediate phenotype in diabetic individuals that is detectable by MRI-ECV and could be used as a biomarker to follow the effectiveness of diabetic treatment.
The ability to distinguish the sub-endocardial, mid-myocardium or sub-epicardial regions with true pathophysiology is a major limitation of ECV measurement. The presence of intra-myocardial fat also affects ECV measurements. The other major limitations of non-invasive MRI and MDCT in measuring ECV are the quality of the acquired images, presence of microvascular obstruction (may require continuous contrast infusion to reach equilibrium state of distribution)[23,146] and the binding of contrast media to serum albumin that increases the relaxivity of some extracellular contrast media and decreases its diffusion[147]. ECV measurement or T1 mapping are not intended to replace DE-MRI or DE-MDCT, which are excellent at depicting large infarction, but rather to be used in concert with cine and perfusion techniques. In tissues with a large intravascular compartment (such as the liver), the values of ECV may be overestimated by the equilibrium technique. The noise in the MDCT images stemming from beam-hardening artifacts originating from dense vertebral endplates is another limitation. Potential technical improvements in ECV measurements include faster processing of image data that reduce reliance on expert interpretation and increase the speed of image data processing.
There are still multiple limitations in using MRI and MDCT for the assessment of ECV, such as the radiation dose, availability of experienced staff, extensive labor and the usage of contrast media. The side effects and cost of contrast media as well as costs of the scanner time should be considered. Monitoring patients with severe heart failure or acute myocardial infarct inside the MR scanner is a difficult task.
New drugs, transplantation of different stem cells and local injection of genes have been recently introduced as potential therapies for infarct healing and myocardial regeneration. Chronological estimation of ECV may be useful for documenting the effectiveness of these therapies to promote myocardial viability. Recent clinical and experimental studies have shown that stem cells reduce infarct size and improve LV function in both AMI and scar[148-151]. In a recent study Wong et al[40] described an association between measurements of ECV and clinical outcomes in a large patient cohort undergoing MRI. They analyzed 793 patients with known or suspected coronary artery disease, cardiomyopathy, or arrhythmias. They excluded patients with cardiac amyloidosis, infiltrative disease, hypertrophic cardiomyopathy and areas of delayed contrast enhancement consistent with classic pattern of myocardial infarction. They found that ECV ranged from 22%-26% in healthy volunteers, whereas it ranged from 21%-46% in the patients. Over a median follow-up period of 6 mo, 39 patients died, and 43 experienced a major adverse event (composite of death/cardiac transplant/LV assist device implantation). In multivariable modeling, ECV was associated with adverse cardiac events. For every 3% increase in ECV, there was a 50% increased probability of an adverse cardiac event. Furthermore, the potential of MRI/MDCT in guiding intramyocardial therapies and providing reliable and reproducible assessment of myocardial viability, perfusion and function has been recently reviewed[152-154]. Further improvement in image resolution and processing of data would promise early detection and better pathophysiological understanding of diffuse fibrosis, myocardial infarct and help in timely intervention and therapy[155].
In conclusion, since ischemic and non-ischemic myocardial diseases are characterized by an increase in the ECV, these pathologies can be characterized and may be differentiated on equilibrium contrast enhanced MRI/MDCT and T1 mapping. ECV data may provide a useful tool for diagnosis and treatment monitoring in ischemic and non-ischemic myocardial diseases (such as patchy microinfarct after percutaneous coronary intervention), compensatory hypertrophy, inflammation, heart failure and hypertrophic cardiomyopathy. The challenges lie in developing fast and sensitive imaging sequences, simple software for analysis, which will facilitate the ECV assessment approach into clinical routine practice.
P- Reviewer: Kolettis TM, Lazzeri C, Lee TM S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task Force for the D, Treatment of A, Chronic Heart Failure of the European Society of C, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, Guidelines ESCCfP. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. European journal of heart. Eur J Heart Failure. 2012;14:803-869. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1841] [Article Influence: 167.4] [Reference Citation Analysis (1)] |
| 2. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2376] [Article Influence: 198.0] [Reference Citation Analysis (0)] |
| 3. | Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1774] [Cited by in RCA: 1744] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 4. | Judd RM, Lugo-Olivieri CH, Arai M, Kondo T, Croisille P, Lima JA, Mohan V, Becker LC, Zerhouni EA. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92:1902-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 307] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Saeed M, Lund G, Wendland MF, Bremerich J, Weinmann H, Higgins CB. Magnetic resonance characterization of the peri-infarction zone of reperfused myocardial infarction with necrosis-specific and extracellular nonspecific contrast media. Circulation. 2001;103:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Wu KC. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson. 2012;14:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Rogers WJ, Kramer CM, Geskin G, Hu YL, Theobald TM, Vido DA, Petruolo S, Reichek N. Early contrast-enhanced MRI predicts late functional recovery after reperfused myocardial infarction. Circulation. 1999;99:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 168] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Ichikawa Y, Sakuma H, Kitagawa K, Ishida N, Takeda K, Uemura S, Motoyasu M, Nakano T, Nozaki A. Evaluation of left ventricular volumes and ejection fraction using fast steady-state cine MR imaging: comparison with left ventricular angiography. J Cardiovasc Magn Reson. 2003;5:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation. 2001;104:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 405] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Pegg TJ, Selvanayagam JB, Jennifer J, Francis JM, Karamitsos TD, Dall’Armellina E, Smith KL, Taggart DP, Neubauer S. Prediction of global left ventricular functional recovery in patients with heart failure undergoing surgical revascularisation, based on late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 495] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 812] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 13. | Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 853] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 14. | Nihoyannopoulos P, Dawson D. Restrictive cardiomyopathies. Eur J Echocardiogr. 2009;10:iii23-iii33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. AJR Am J Roentgenol. 2005;184:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Kim RJ, Judd RM. Gadolinium-enhanced magnetic resonance imaging in hypertrophic cardiomyopathy: in vivo imaging of the pathologic substrate for premature cardiac death? J Am Coll Cardiol. 2003;41:1568-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Beek AM, Kühl HP, Bondarenko O, Twisk JW, Hofman MB, van Dockum WG, Visser CA, van Rossum AC. Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J Am Coll Cardiol. 2003;42:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Dall’Armellina E, Karia N, Lindsay AC, Karamitsos TD, Ferreira V, Robson MD, Kellman P, Francis JM, Forfar C, Prendergast BD. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ Cardiovasc Imaging. 2011;4:228-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 813] [Cited by in RCA: 745] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 20. | Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 21. | Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 417] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 22. | Nacif MS, Kawel N, Lee JJ, Chen X, Yao J, Zavodni A, Sibley CT, Lima JA, Liu S, Bluemke DA. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology. 2012;264:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 24. | Wendland MF, Saeed M, Yu KK, Roberts TP, Lauerma K, Derugin N, Varadarajan J, Watson AD, Higgins CB. Inversion recovery EPI of bolus transit in rat myocardium using intravascular and extravascular gadolinium-based MR contrast media: dose effects on peak signal enhancement. Magn Reson Med. 1994;32:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Saeed M, Higgins CB, Geschwind JF, Wendland MF. T1-relaxation kinetics of extracellular, intracellular and intravascular MR contrast agents in normal and acutely reperfused infarcted myocardium using echo-planar MR imaging. Eur Radiol. 2000;10:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, Dae MW, Wendland MF. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology. 1999;211:698-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 272] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Arheden H, Saeed M, Higgins CB, Gao DW, Ursell PC, Bremerich J, Wyttenbach R, Dae MW, Wendland MF. Reperfused rat myocardium subjected to various durations of ischemia: estimation of the distribution volume of contrast material with echo-planar MR imaging. Radiology. 2000;215:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, Sibley CT, Kellman P, Arai AE, Bluemke DA. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011;13:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, Kellman P, Jones BL, Ludwig DR, Schwartzman D. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049-1058. [PubMed] |
| 31. | Brooks A, Schinde V, Bateman AC, Gallagher PJ. Interstitial fibrosis in the dilated non-ischaemic myocardium. Heart. 2003;89:1255-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Udelson JE. Heart failure with preserved ejection fraction. Circulation. 2011;124:e540-e543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Mongeon FP, Jerosch-Herold M, Coelho-Filho OR, Blankstein R, Falk RH, Kwong RY. Quantification of extracellular matrix expansion by CMR in infiltrative heart disease. JACC Cardiovasc Imaging. 2012;5:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 35. | Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, Lachmann RH, Murphy E, Mehta A, Hughes DA. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 36. | Wendland MF, Saeed M, Lund G, Higgins CB. Contrast-enhanced MRI for quantification of myocardial viability. J Magn Reson Imaging. 1999;10:694-702. [PubMed] |
| 37. | Vinnakota KC, Bassingthwaighte JB. Myocardial density and composition: a basis for calculating intracellular metabolite concentrations. Am J Physiol Heart Circ Physiol. 2004;286:H1742-H1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Dauber IM, VanBenthuysen KM, McMurtry IF, Wheeler GS, Lesnefsky EJ, Horwitz LD, Weil JV. Functional coronary microvascular injury evident as increased permeability due to brief ischemia and reperfusion. Circ Res. 1990;66:986-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Zhao MJ, Zhang H, Robinson TF, Factor SM, Sonnenblick EH, Eng C. Profound structural alterations of the extracellular collagen matrix in postischemic dysfunctional (“stunned”) but viable myocardium. J Am Coll Cardiol. 1987;10:1322-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 170] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 407] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 41. | Diesbourg LD, Prato FS, Wisenberg G, Drost DJ, Marshall TP, Carroll SE, O’Neill B. Quantification of myocardial blood flow and extracellular volumes using a bolus injection of Gd-DTPA: kinetic modeling in canine ischemic disease. Magn Reson Med. 1992;23:239-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 137] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Wedeking P, Sotak CH, Telser J, Kumar K, Chang CA, Tweedle MF. Quantitative dependence of MR signal intensity on tissue concentration of Gd(HP-DO3A) in the nephrectomized rat. Magn Reson Imaging. 1992;10:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Tong CY, Prato FS, Wisenberg G, Lee TY, Carroll E, Sandler D, Wills J, Drost D. Measurement of the extraction efficiency and distribution volume for Gd-DTPA in normal and diseased canine myocardium. Magn Reson Med. 1993;30:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 128] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Jugdutt BI, Joljart MJ, Khan MI. Rate of collagen deposition during healing and ventricular remodeling after myocardial infarction in rat and dog models. Circulation. 1996;94:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | See F, Kompa A, Martin J, Lewis DA, Krum H. Fibrosis as a therapeutic target post-myocardial infarction. Curr Pharm Des. 2005;11:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Geschwind JF, Wendland MF, Saeed M, Lauerma K, Derugin N, Higgins CB. AUR Memorial Award. Identification of myocardial cell death in reperfused myocardial injury using dual mechanisms of contrast-enhanced magnetic resonance imaging. Acad Radiol. 1994;1:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 464] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 48. | Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 586] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 49. | Sheifer SE, Manolio TA, Gersh BJ. Unrecognized myocardial infarction. Ann Intern Med. 2001;135:801-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 1050] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 51. | Piechnik SK, Ferreira VM, Dall’Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 519] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 52. | Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 554] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 53. | Burt JR, Zimmerman SL, Kamel IR, Halushka M, Bluemke DA. Myocardial t1 mapping: techniques and potential applications. Radiographics. 2014;34:377-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | Bandula S, White SK, Flett AS, Lawrence D, Pugliese F, Ashworth MT, Punwani S, Taylor SA, Moon JC. Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: validation against histologic findings. Radiology. 2013;269:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 55. | Wendland MF, Saeed M, Arheden H, Gao DW, Canet E, Bremerich J, Dae MW, Higgins CB. Toward necrotic cell fraction measurement by contrast-enhanced MRI of reperfused ischemically injured myocardium. Acad Radiol. 1998;5 Suppl 1:S42-S44; discussion S45-S46. [PubMed] |
| 56. | Klein C, Schmal TR, Nekolla SG, Schnackenburg B, Fleck E, Nagel E. Mechanism of late gadolinium enhancement in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2007;9:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Schuleri KH, George RT, Lardo AC. Applications of cardiac multidetector CT beyond coronary angiography. Nat Rev Cardiol. 2009;6:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Mahnken AH. Computed tomography imaging in myocardial infarction. Expert Rev Cardiovasc Ther. 2011;9:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Thilo C, Hanley M, Bastarrika G, Ruzsics B, Schoepf UJ. Integrative computed tomographic imaging of cardiac structure, function, perfusion, and viability. Cardiol Rev. 2010;18:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Kitegawa K, Kurita Y, Ito T, Kurobe Y, Nakajima H, Nakamori S, Ishida M, Sakuma H. Regional and age-related variation of exgtracellular volume fraction in subjects without coronary artery disease assessed by cardiac ct. J Cardiovasc Comp Tomog. 2013;7:S75-S76. |
| 61. | Do L, Wilson MW, Suhail M, Hetts SW, Saeed M. Mdct quantification of extracellular volumes in acute and scarred myocardial injuries. J Heart Dis. 2013;10:50 (Abstract). |
| 62. | Bandula S, Banypersad SM, Sado D, Flett AS, Punwani S, Taylor SA, Hawkins PN, Moon JC. Measurement of Tissue interstitial volume in healthy patients and those with amyloidosis with equilibrium contrast-enhanced MR imaging. Radiology. 2013;268:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Kitegawa K, Kurita Y, Ito T, Kurobe Y, Nakajima H, Nakamori S, Ischida M, Sakuma H. Regional and age-related variation of extracellular volume fraction in subjects without coronary artery disease assessed by cardiac ct. J Cardiovasc Comp Tomog. 2013;7:S75-S76 (Abstract). |
| 64. | Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marbán E, Tomaselli GF. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006-2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 614] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 65. | Hombach V, Grebe O, Merkle N, Waldenmaier S, Höher M, Kochs M, Wöhrle J, Kestler HA. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 367] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 66. | George RT, Jerosch-Herold M, Silva C, Kitagawa K, Bluemke DA, Lima JA, Lardo AC. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest Radiol. 2007;42:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 67. | Anderson KR, Sutton MG, Lie JT. Histopathological types of cardiac fibrosis in myocardial disease. J Pathol. 1979;128:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 172] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Han Y, Peters DC, Dokhan B, Manning WJ. Shorter difference between myocardium and blood optimal inversion time suggests diffuse fibrosis in dilated cardiomyopathy. J Magn Reson Imaging. 2009;30:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Amano Y, Takayama M, Kumita S. Contrast-enhanced myocardial T1-weighted scout (Look-Locker) imaging for the detection of myocardial damages in hypertrophic cardiomyopathy. J Magn Reson Imaging. 2009;30:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, Alharethi R, Li D, Hershberger RE. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;295:H1234-H1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 71. | Klein C, Nekolla SG, Balbach T, Schnackenburg B, Nagel E, Fleck E, Schwaiger M. The influence of myocardial blood flow and volume of distribution on late Gd-DTPA kinetics in ischemic heart failure. J Magn Reson Imaging. 2004;20:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Hamdani N, Paulus WJ, van Heerebeek L, Borbély A, Boontje NM, Zuidwijk MJ, Bronzwaer JG, Simonides WS, Niessen HW, Stienen GJ. Distinct myocardial effects of beta-blocker therapy in heart failure with normal and reduced left ventricular ejection fraction. Eur Heart J. 2009;30:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 442] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 74. | Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation. 1985;71:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 743] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 75. | Baumgart D, Liu F, Haude M, Görge G, Ge J, Erbel R. Acute plaque rupture and myocardial stunning in patient with normal coronary arteriography. Lancet. 1995;346:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Porto I, Selvanayagam JB, Van Gaal WJ, Prati F, Cheng A, Channon K, Neubauer S, Banning AP. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation. 2006;114:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 77. | Califf RM, Abdelmeguid AE, Kuntz RE, Popma JJ, Davidson CJ, Cohen EA, Kleiman NS, Mahaffey KW, Topol EJ, Pepine CJ. Myonecrosis after revascularization procedures. J Am Coll Cardiol. 1998;31:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 362] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 78. | Kotani J, Nanto S, Mintz GS, Kitakaze M, Ohara T, Morozumi T, Nagata S, Hori M. Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation. 2002;106:1672-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 79. | Henriques JP, Zijlstra F. Frequency and sequelae of ST elevation acute myocardial infarction caused by spontaneous distal embolization from unstable coronary lesions. Am J Cardiol. 2003;91:708-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, van ‘t Hof AW, Hoorntje JC, Suryapranata H. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002;23:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 461] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 81. | Selvanayagam JB, Cheng AS, Jerosch-Herold M, Rahimi K, Porto I, van Gaal W, Channon KM, Neubauer S, Banning AP. Effect of distal embolization on myocardial perfusion reserve after percutaneous coronary intervention: a quantitative magnetic resonance perfusion study. Circulation. 2007;116:1458-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, Banning AP. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 306] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 83. | Selvangyagam JB, Rahimi K, Banning A, Cheng A, Pegg T, Karamitsos T, Taggart D, Neubauer S. Prognostic significance of post-procedural irreversible myocardial injury detected by cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2008;10:1 (Abstract). [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Dörge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, Erbel R, Heusch G. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 2000;279:H2587-H2592. [PubMed] |
| 85. | Dörge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker KA. Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol. 2002;34:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 86. | Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000;101:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 482] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 87. | Canton M, Skyschally A, Menabò R, Boengler K, Gres P, Schulz R, Haude M, Erbel R, Di Lisa F, Heusch G. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur Heart J. 2006;27:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Stoupakis G, Orlando J, Kalia H, Skurnick J, Saric M, Arora R. Preservation of myocardial microcirculation during mechanical reperfusion for myocardial ischemia with either abciximab or eptifibatide. J Invasive Cardiol. 2003;15:476-480. [PubMed] |
| 89. | Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, Erbel R. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 90. | Heusch G, Schulz R, Haude M, Erbel R. Coronary microembolization. J Mol Cell Cardiol. 2004;37:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Dizon JM, Brener SJ, Maehara A, Witzenbichler B, Biviano A, Godlewski J, Parise H, Dambrink JH, Mehran R, Gibson CM. Relationship between ST-segment resolution and anterior infarct size after primary percutaneous coronary intervention: analysis from the INFUSE-AMI trial. Eur Heart J Acute Cardiovasc Care. 2014;3:78-83. [PubMed] |
| 92. | Saeed M, Hetts SW, Do L, Sullivan S, Wilson MW. MDCT has the potential to predict percutaneous coronary intervention outcome in swine model: microscopic validation. Acta Radiol. 2012;53:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 93. | Saeed M, Hetts SW, Do L, Wilson MW. MRI study on volume effects of coronary emboli on myocardial function, perfusion and viability. Int J Cardiol. 2013;165:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Saeed M, Hetts SW, Do L, Wilson MW. Coronary microemboli effects in preexisting acute infarcts in a swine model: cardiac MR imaging indices, injury biomarkers, and histopathologic assessment. Radiology. 2013;268:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Saeed M, Hetts SW, English J, Do L, Wilson MW. Quantitative and qualitative characterization of the acute changes in myocardial structure and function after distal coronary microembolization using MDCT. Acad Radiol. 2011;18:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Saeed M, Hetts SW, Ursell PC, Do L, Kolli KP, Wilson MW. Evaluation of the acute effects of distal coronary microembolization using multidetector computed tomography and magnetic resonance imaging. Magn Reson Med. 2012;67:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Carlsson M, Jablonowski R, Martin AJ, Ursell PC, Saeed M. Coronary microembolization causes long-term detrimental effects on regional left ventricular function. Scand Cardiovasc J. 2011;45:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Breuckmann F, Nassenstein K, Bucher C, Konietzka I, Kaiser G, Konorza T, Naber C, Skyschally A, Gres P, Heusch G. Systematic analysis of functional and structural changes after coronary microembolization: a cardiac magnetic resonance imaging study. JACC Cardiovasc Imaging. 2009;2:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Nassenstein K, Breuckmann F, Bucher C, Kaiser G, Konorza T, Schäfer L, Konietzka I, de Greiff A, Heusch G, Erbel R. How much myocardial damage is necessary to enable detection of focal late gadolinium enhancement at cardiac MR imaging? Radiology. 2008;249:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | Amanieu C, Sanchez I, Arion S, Bonnefoy E, Revel D, Douek P, Boussel L. Acute myocardial infarction: early CT aspects of myocardial microcirculation obstruction after percutaneous coronary intervention. Eur Radiol. 2013;23:2405-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 101. | Bajwa HZ, Do L, Suhail M, Hetts SW, Wilson MW, Saeed M. MRI demonstrates a decrease in myocardial infarct healing and increase in compensatory ventricular hypertrophy following mechanical microvascular obstruction. J Magn Reson Imaging. 2014;40:906-914. [PubMed] |
| 102. | Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-2555. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1660] [Cited by in RCA: 1715] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 103. | Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 605] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 104. | Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 105. | Cooper LT. Myocarditis. N Engl J Med. 2009;360:1526-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 987] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 106. | Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT. Update on myocarditis. J Am Coll Cardiol. 2012;59:779-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 668] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 107. | Monney PA, Sekhri N, Burchell T, Knight C, Davies C, Deaner A, Sheaf M, Baithun S, Petersen S, Wragg A. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart. 2011;97:1312-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 108. | Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 109. | Zagrosek A, Wassmuth R, Abdel-Aty H, Rudolph A, Dietz R, Schulz-Menger J. Relation between myocardial edema and myocardial mass during the acute and convalescent phase of myocarditis--a CMR study. J Cardiovasc Magn Reson. 2008;10:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Mahrholdt H, Wagner A, Parker M, Regenfus M, Fieno DS, Bonow RO, Kim RJ, Judd RM. Relationship of contractile function to transmural extent of infarction in patients with chronic coronary artery disease. J Am Coll Cardiol. 2003;42:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 588] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 112. | Mahrholdt H, Wagner A, Holly TA, Elliott MD, Bonow RO, Kim RJ, Judd RM. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation. 2002;106:2322-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 113. | Mahrholdt H, Wagner A, Honold M, Wedemeyer I, Sechtem U. Images in cardiovascular medicine. Magnetic resonance assessment of cardiac function, infarct scar distribution, and ventricular remodeling in the setting of ischemic cardiomyopathy. Circulation. 2003;107:e103-e104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 114. | Mahrholdt H, Wagner A, Judd RM, Sechtem U. Assessment of myocardial viability by cardiovascular magnetic resonance imaging. Eur Heart J. 2002;23:602-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 115. | Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 620] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 116. | Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012;14:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 117. | Reinier K, Dervan C, Singh T, Uy-Evanado A, Lai S, Gunson K, Jui J, Chugh SS. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm. 2011;8:1177-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 469] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 119. | Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1234] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 120. | Chugh SS. Early identification of risk factors for sudden cardiac death. Nat Rev Cardiol. 2010;7:318-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 121. | Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 456] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 122. | Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2211] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 123. | Díez J, González A, López B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. 2005;2:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 124. | Assayag P, Carré F, Chevalier B, Delcayre C, Mansier P, Swynghedauw B. Compensated cardiac hypertrophy: arrhythmogenicity and the new myocardial phenotype. I. Fibrosis. Cardiovasc Res. 1997;34:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 125. | Shiozaki AA, Senra T, Arteaga E, Martinelli Filho M, Pita CG, Ávila LF, Parga Filho JR, Mady C, Kalil-Filho R, Bluemke DA. Myocardial fibrosis detected by cardiac CT predicts ventricular fibrillation/ventricular tachycardia events in patients with hypertrophic cardiomyopathy. J Cardiovasc Comput Tomogr. 2013;7:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 126. | Taylor DO, Edwards LB, Boucek MM, Trulock EP, Aurora P, Christie J, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report--2007. J Heart Lung Transplant. 2007;26:769-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 354] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 127. | Tigen K, Karaahmet T, Kirma C, Dundar C, Pala S, Isiklar I, Cevik C, Kilicgedik A, Basaran Y. Diffuse late gadolinium enhancement by cardiovascular magnetic resonance predicts significant intraventricular systolic dyssynchrony in patients with non-ischemic dilated cardiomyopathy. J Am Soc Echocardiogr. 2010;23:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 128. | Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 406] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 129. | Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 517] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 130. | Neilan TG, Mongeon FP, Shah RV, Coelho-Filho O, Abbasi SA, Dodson JA, McMullan CJ, Heydari B, Michaud GF, John RM. Myocardial extracellular volume expansion and the risk of recurrent atrial fibrillation after pulmonary vein isolation. JACC Cardiovasc Imaging. 2014;7:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 131. | Murakami T, Ishiguro N, Higuchi K. Transmission of systemic AA amyloidosis in animals. Vet Pathol. 2014;51:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 132. | Hassan W, Al-Sergani H, Mourad W, Tabbaa R. Amyloid heart disease. New frontiers and insights in pathophysiology, diagnosis, and management. Tex Heart Inst J. 2005;32:178-184. [PubMed] |
| 133. | Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 677] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 134. | Hosch W, Bock M, Libicher M, Ley S, Hegenbart U, Dengler TJ, Katus HA, Kauczor HU, Kauffmann GW, Kristen AV. MR-relaxometry of myocardial tissue: significant elevation of T1 and T2 relaxation times in cardiac amyloidosis. Invest Radiol. 2007;42:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 135. | Sparrow PJ, Merchant N, Provost YL, Doyle DJ, Nguyen ET, Paul NS. CT and MR imaging findings in patients with acquired heart disease at risk for sudden cardiac death. Radiographics. 2009;29:805-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 136. | Austin BA, Tang WH, Rodriguez ER, Tan C, Flamm SD, Taylor DO, Starling RC, Desai MY. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc Imaging. 2009;2:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 137. | Robbers LF, Baars EN, Brouwer WP, Beek AM, Hofman MB, Niessen HW, van Rossum AC, Marcu CB. T1 mapping shows increased extracellular matrix size in the myocardium due to amyloid depositions. Circ Cardiovasc Imaging. 2012;5:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 138. | Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 379] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 139. | Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436-2443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 736] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 140. | From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 357] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 141. | Schwartzkopff B, Brehm M, Mundhenke M, Strauer BE. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension. 2000;36:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 142. | Tamarappoo BK, John BT, Reinier K, Teodorescu C, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Vulnerable myocardial interstitium in patients with isolated left ventricular hypertrophy and sudden cardiac death: a postmortem histological evaluation. J Am Heart Assoc. 2012;1:e001511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 143. | Khavandi K, Khavandi A, Asghar O, Greenstein A, Withers S, Heagerty AM, Malik RA. Diabetic cardiomyopathy--a distinct disease? Best Pract Res Clin Endocrinol Metab. 2009;23:347-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 144. | Ng AC, Auger D, Delgado V, van Elderen SG, Bertini M, Siebelink HM, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T₁ mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ Cardiovasc Imaging. 2012;5:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 145. | Jellis C, Wright J, Kennedy D, Sacre J, Jenkins C, Haluska B, Martin J, Fenwick J, Marwick TH. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circ Cardiovasc Imaging. 2011;4:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 146. | Sado DM, Flett AS, Moon JC. Novel imaging techniques for diffuse myocardial fibrosis. Future Cardiol. 2011;7:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 147. | Wendland MF, Saeed M, Geschwind JF, Mann JS, Brasch RC, Higgins CB. Distribution of intracellular, extracellular, and intravascular contrast media for magnetic resonance imaging in hearts subjected to reperfused myocardial infarction. Acad Radiol. 1996;3 Suppl 2:S402-S404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 148. | Hassan N, Tchao J, Tobita K. Concise review: skeletal muscle stem cells and cardiac lineage: potential for heart repair. Stem Cells Transl Med. 2014;3:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Telukuntla KS, Suncion VY, Schulman IH, Hare JM. The advancing field of cell-based therapy: insights and lessons from clinical trials. J Am Heart Assoc. 2013;2:e000338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 150. | D’Amario D, Leone AM, Iaconelli A, Luciani N, Gaudino M, Kannappan R, Manchi M, Severino A, Shin SH, Graziani F. Growth properties of cardiac stem cells are a novel biomarker of patients’ outcome after coronary bypass surgery. Circulation. 2014;129:157-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 151. | Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 152. | Gerber BL, Belge B, Legros GJ, Lim P, Poncelet A, Pasquet A, Gisellu G, Coche E, Vanoverschelde JL. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 293] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 153. | le Polain de Waroux JB, Pouleur AC, Goffinet C, Pasquet A, Vanoverschelde JL, Gerber BL. Combined coronary and late-enhanced multidetector-computed tomography for delineation of the etiology of left ventricular dysfunction: comparison with coronary angiography and contrast-enhanced cardiac magnetic resonance imaging. Eur Heart J. 2008;29:2544-2551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 154. | Saeed M, Wilson M. Value of MR contrast media in image-guided body interventions. World J Radiol. 2012;4:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 155. | White SK, Sado DM, Flett AS, Moon JC. Characterising the myocardial interstitial space: the clinical relevance of non-invasive imaging. Heart. 2012;98:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |