Published online Nov 26, 2014. doi: 10.4330/wjc.v6.i11.1166
Revised: July 2, 2014
Accepted: September 6, 2014
Published online: November 26, 2014
Processing time: 191 Days and 14.8 Hours
Coronary artery disease (CAD) represents an important cause of mortality. Cardiovascular magnetic resonance (CMR) imaging evolved as an imaging modality that allows the assessment of myocardial function, perfusion, contractile reserve and extent of fibrosis in a single comprehensive exam. This review highlights the role of CMR in the differential diagnosis of acute chest pain by detecting the location of obstructive CAD or necrosis and identifying other conditions like stress cardiomyopathy or myocarditis that can present with acute chest pain. Besides, it underlines the prognostic implication of perfusion abnormalities in the setting of acute chest pain. Furthermore, the review addresses the role of CMR to detect significant CAD in patients with stable CAD. It elucidates the accuracy and clinical utility of CMR with respect to other imaging modalities like single-photon emission computed tomography and positron emission tomography. Besides, the prognostic value of CMR stress testing is discussed. Additionally, it summarizes the available CMR techniques to assess myocardial viability and describes algorithm to identify those patient who might profit from revascularization those who should be treated medically. Finally, future promising imaging techniques that will provide further insights into the fundamental disease processes in ischemic cardiomyopathy are discussed.
Core tip: Coronary artery disease (CAD) represents an important cause of mortality. This review highlights the role of cardiovascular magnetic resonance (CMR) in the differential diagnosis of acute chest pain. It underlines the prognostic implication of perfusion abnormalities in the setting of acute chest pain and addresses the role of CMR to detect significant CAD in patients with stable CAD. Besides, the prognostic value of CMR stress testing is discussed. Additionally, it summarizes the available CMR techniques to assess myocardial viability. This review describes a treatment algorithm and presents new imaging techniques that might give further insights into the fundamental disease processes in ischemic cardiomyopathy.
- Citation: Doesch C, Papavassiliu T. Diagnosis and management of ischemic cardiomyopathy: Role of cardiovascular magnetic resonance imaging. World J Cardiol 2014; 6(11): 1166-1174
- URL: https://www.wjgnet.com/1949-8462/full/v6/i11/1166.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i11.1166
Coronary artery disease (CAD) has a high prevalence in industrialized countries[1] and is therefore an important cause of mortality in the Western world[2]. Cardiac magnetic resonance (CMR) imaging offers the unique opportunity to non-invasively detect coronary artery stenoses and has become the gold standard for the assessment of viability. The detection of coronary artery stenoses can be performed using either vasodilator stressors like adenosine to detect myocardial ischemia or inotropic agents such as dobutamine to identify regional wall motion abnormalities. Due to its excellent temporal and spatial resolution, the possibility to assess myocardial perfusion without exposure to ionizing radiation and the independence of an acoustic window, CMR offers plenty advantages over other imaging modalities like stress echocardiography or single-photon emission computed tomography (SPECT).
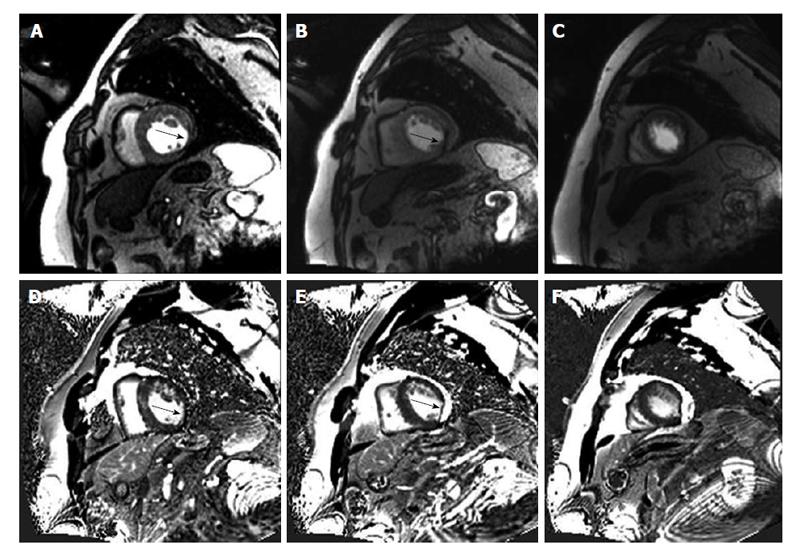
The exclusion of coronary artery stenoses in patients presenting with acute chest pain in the absence of diagnostic electrocardiographic changes or negative cardiac enzymes still remains a challenge. In these low risk patients CMR has proved to be a reliable risk-stratification tool. Kwong et al[3] was the first to demonstrate the utility of CMR for triage of patients with acute chest pain in the emergency department. He showed that the combination of CMR rest perfusion and late gadolinium enhancement (LGE) in patients presenting at an emergency department with angina and non-diagnostic electrocardiogram (ECG) had a sensitivity of 100% for non-ST-segment elevation infarction and a sensitivity of 84% sensitivity for acute coronary syndrome (ACS) as well as a specificity of 85% (Figure 1). Besides, CMR proved to be the strongest predictor of ACS and had an independent diagnostic value over clinical parameters including ECG, initial troponin-I, and the thrombolysis in myocardial infarction risk score. In a further study by Ingkanisorn et al[4], adenosine stress CMR was performed in 135 patients with chest pain and excluded myocardial infarction who presented at the emergency department. In this setting, adenosine perfusion abnormalities had 100% sensitivity and 93% to predict CAD. Furthermore, none of the patients with a normal adenosine stress examination was diagnosed with significant CAD or suffered from an adverse outcome during a follow-up period of one year. In a retrospective study by Hartlage et al[5] using either adenosine or dobutamine stress CMR in 255 patients presenting at the emergency department with acute low-risk chest pain and no prior history of CAD the negative predictive value for the primary endpoint of cardiac death, nonfatal acute myocardial infarction, obstructive CAD on invasive coronary angiography or recurrent chest pain, was 100% and 99%, respectively. Therefore, adenosine and dobutamine stress CMR proved to be reliable modalities to exclude obstructive CAD and a negative stress study provides an excellent intermediate-term prognosis. Besides, in patients with intermediate risk presenting at the emergency department, stress CMR reduced cardiac-related costs of the index visit and over the first year without increasing major cardiac events[6].
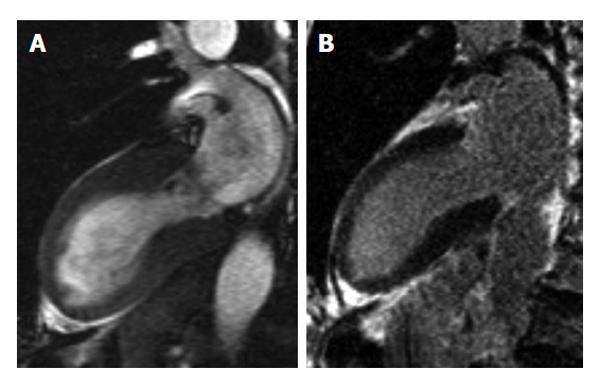
In addition, CMR can identify the underlying cause of conditions that present like ACS. In stress cardiomyopathy [Takotsubo cardiomyopathy (CMP), Figure 2], patients present with acute chest pain and/or dyspnea, modest elevation in cardiac troponin level and new ECG abnormalities despite the absence of significant (> 50%) obstructive coronary artery disease or angiographic evidence of acute plaque rupture. In these patients with marked apical or midventricular ballooning the absence of myocarditis or typical ischemic transmural LGE on CMR confirms the diagnosis[7-11]. Myocarditis (Figure 3) is another differential diagnosis in patients with acute chest pain that can be addressed with CMR allowing to visualize the key features of myocarditis: inflammation, hyperemia, edema, necrosis, myocardial dysfunction as well as accompanying pericardial perfusion in a single study[12-16].
The feasibility of stress CMR to detect coronary artery stenosis in patients with known or suspected CAD is well established[17-21]. In a meta-analysis[22] comparing 114 SPECT, 15 positron emission tomography (PET) and 37 CMR myocardial perfusion imaging studies for the detection of angiographically detected coronary artery stenoses ≥ 50%, all three imaging modalities proved to accurately detect obstructive CAD. Metaregression showed that CMR and PET have a significantly higher diagnostic accuracy than SPECT. In contrast to nuclear techniques, CMR perfusion is not affected by attenuation artifacts, has the highest spatial resolution and is therefore able to even subendocardial perfusion deficits[23]. The sensitivity and specificity to detect CAD ranged between 79%-88% and 81%-91% for dobutamine stress CMR or 67%-94% and 61%-85% for adenosine stress CMR, in meta-analysis[24-26] and a multicenter study[27]. The use of 3.0 T has shown to provide even higher diagnostic accuracy[28,29], however this technique is not widely available, yet and no data from multicenter studies exist so far.
However, CMR stress testing is not only able to detect CAD but also offers prognostic information. A study performing adenosine stress CMR using 1.5 and 3.0 T in 815 consecutive patients with stable CAD could show that the addition of inducible ischemia reclassified patient risk beyond standard clinical variables and improved discrimination of major adverse cardiac events[30]. These results were confirmed by another single center study[31] enrolling 1229 patients with stable angina. Recent meta-analysis[32,33] also proved that a negative adenosine or dobutamine stress CMR had a high negative predictive value for adverse cardiac events. Besides they showed that inducible perfusion defects as well as wall motion abnormalities had a comparable ability to identify low-risk patients.
Therefore, in the actual guidelines for the management of patients with stable CAD, stress imaging using either echocardiography, CMR or SPECT has become an integral part in the work-up of patients with a pretest probability (PTP) of CAD between 15%-65% and a left ventricular function (LVEF) ≥ 50% as well as in patients with a PTP of 66%-85% or a LVEF < 50%[34]. An imaging study should also be considered in symptomatic patients with prior revascularization [percutaneous coronary intervention (PCI) or coronary artery bypass graft][34]. In patients with coronary artery stenoses of angiographic intermediate severity causing a perfusion defect on CMR it could be shown that these patients are at higher risk for major adverse cardiac events (MACE) within the following 18 mo after the procedure, whereas deferring PCI in patients with intermediate coronary artery stenoses and no evidence of ischemia seemed to be save[18]. Thus, current guidelines suggest to consider an imaging stress test to assess the functional severity of intermediate lesions on coronary arteriography[34]. The decision to proceed to invasive angiography is not only based on symptoms and risk factors but also on the extent and severity of ischemia[34] (Figure 4).
In women, CAD develops 7 to 10 years later than in men. However, it is still the major cause of death in women[35]. Moreover, the risk of heart disease in women is often underestimated. Due to the underrecognition of heart disease and differences in clinical presentation in women, treatment strategies are less straightforward in women. In a study by Coelho-Filho et al[36] performing adenosine stress imaging in 237 men and 168 women referred for ischemia assessment, myocardial ischemia was the strongest predictor of MACE in both sexes. In a large study[37] using a combined adenosine and dobutamine stress CMR protocol in 471 men and 208 women, Jahnke et al[37] could show that CMR perfusion and wall motion abnormalities are equally suited for cardiac risk stratification in both sexes. In women, a negative stress CMR resulted in very low event rates during the following 4 years whereas, the event rates in men increased after the second year. These results might suggest that it is feasible to prolong the generally proposed 2-year warranty period of a negative CMR stress test to 4 years in women.
In clinical practice myocardial viability is characterized by functional recovery 6 wk to 6 mo after successful revascularization. CMR offers 3 methods to assess myocardial viability: end-diastolic wall thickness, low dose dobutamin stress CMR and LGE.
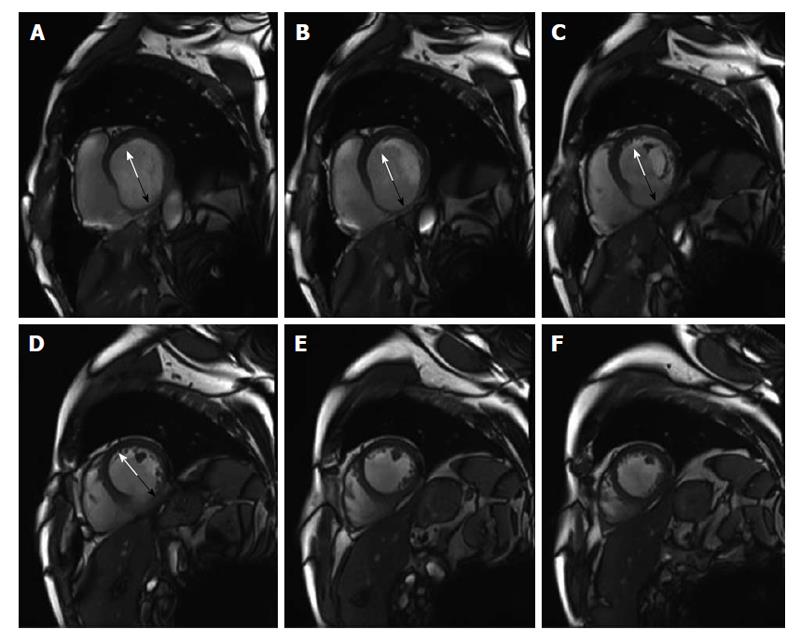
The easiest technique is to evaluate the maximal end-diastolic wall thickness (EDWT) because it only requires to determine the maximal EDWT on the cine images at rest. In the course of acute myocardial infarction structural changes are associated with myocardial thinning in the core zone of the infarction. In a study comparing EDWT on resting CMR and[18] fluorodeoxyglocose positron emission tomography (FDG PET) in 35 patients with myocardial infarction, Baer et al[38] could prove that myocardial segments with an EDWT ≥ 5.5 mm showed a normal FDG uptake, whereas myocardial segments with an EDWT < 5.5 mm revealed a significantly FDG uptake. Several studies using either a cut-off of 5.5 mm[39,40] or 6 mm[41,42] in patients with chronic ischemic myocardial dysfunction could show that myocardial segments with wall thinning below the cut-off have a low likelihood of functional recovery after revascularization.
Overall these studies[39-42] proved that EDWT has a good sensitivity and negative predictive value but only reasonable positive predictive value and poor specificity to predict functional recovery.
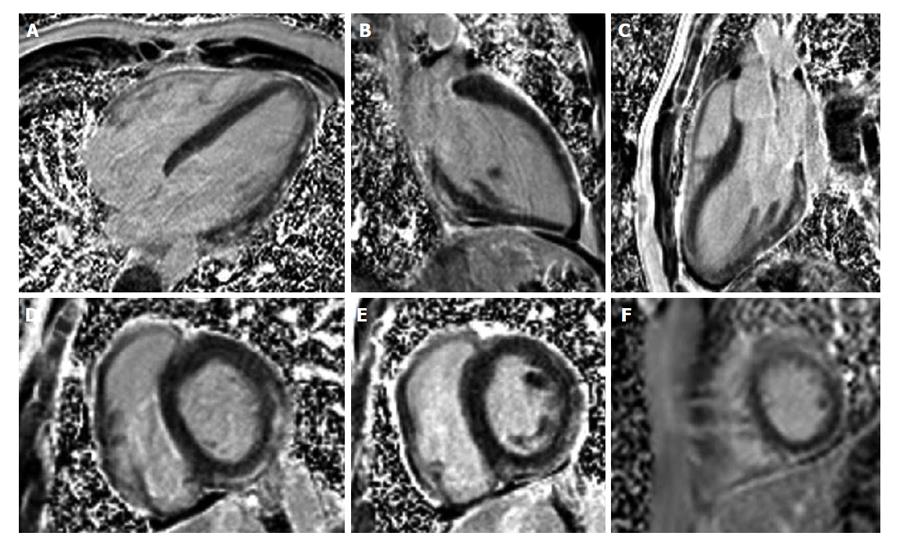
Figure 5 shows an example of a patient with a previous inferior myocardial infarction with severe thinning of the inferior myocardial wall segments.
Low dose dobutamine (≤ 10 μg/kg per minute) stress CMR is another technique used to evaluate myocardial viability. At low doses, dobutamine supports coronary vasodilatation and increases myocardial contractility[43]. Viable myocardium is distinguished by the identification of improved contractility under low dose dobutamine infusion. Several studies[39-41,44-46] proved that a CMR-derived systolic wall thickening > 2 mm during low dose dobutamine stress is able to identify myocardial segments with functional recovery after revascularization. According to these studies[39-41,44-46], the major strength of low dose dobutamine stress CMR is its high overall accuracy, specificity and positive predictive value.
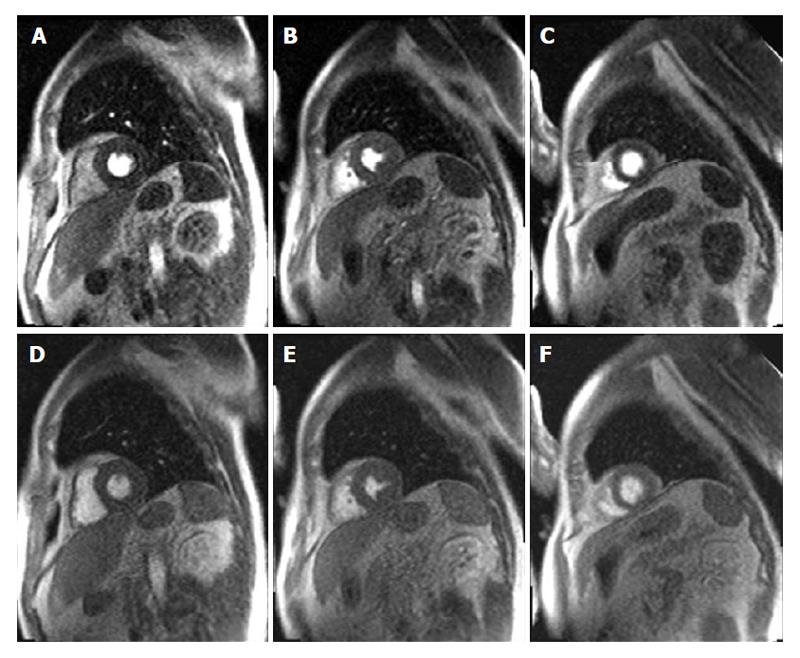
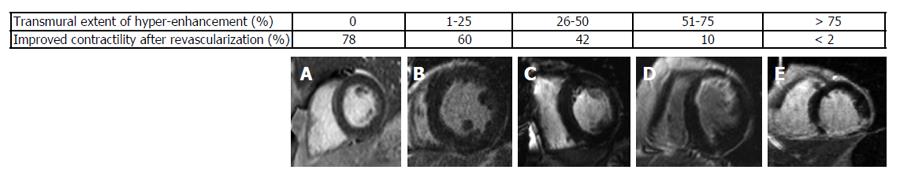
LGE that was first applied by Kim et al[47] has now become the gold standard for the evaluation of myocardial viability in ischemic heart disease. In nonviable tissue, the extracellular contrast agent spreads in a larger volume of distribution which results in delayed wash-out kinetics[48]. Moreover, late enhancement imaging sequences suppress the signal derived from remote myocardium resulting in high image contrast. Hence, this technique allows the detection of even very small myocardial infarctions (≥ 0.7 g of myocardial mass)[49]. In a meta-analysis by Romero et al[50] LGE with a cut-off of < 50% transmurality of scar tissue had a high sensitivity and a high negative predictive value to predict functional recovery. In patients with chronic ischemic heart disease the identification of viable myocardium is important to predict improvement of LVEF and survival after revascularization. In these patients the functional recovery was also linked to the transmural extent of scar[51]. Kim et al[51] could show that in segments without scar the functional recovery was 78% whereas in segments with a scar transmurality of more than 75%, the likelihood of contractility improvement after revascularization was less than 2% (Figure 6). As illustrated in Figure 6, the ability to predict functional recovery in segments with an intermediate scar transmurality between 1% and 75% ranged between 60% and 10%. In these patients with an intermediate scar transmurality an additional low-dose dobutamine stress examination helps to identify segments that show a contractile reserve. A combined approach of LGE enhancement imaging and low-dose dobutamin stress imaging proved to be the optimal approach to predict recovery after revascularization[44]. Therefore, in patients with wall motion abnormalities at rest the following algorithm as described by Nagel et al[52] should be applied. LGE imaging should be used as first line imaging modality to identify patients without a scar who should undergo revascularization. Patients with more than 50% LGE transmurality should be treated medically. In patients with less than 50% LGE transmurality an additional low dose dobutamine stress CMR should be performed to detect patients with an improved contractility who are likely to benefit from revascularization. Whereas patients with less than 50% LGE transmurality but without assessment of contractile reserve should be treated medically. This algorithm indicates that in patients without evidence of LGE or with a LGE > 50% transmurality, LGE imaging alone is sufficient. In case that an additional low dose dobutamin stress exam is required CMR allows to assess myocardial contractile reserve and LGE in a single comprehensive exam.
In patients with acute myocardial infarction and wall motion abnormalities CMR, Beek et al[53] could also proof that LGE CMR is able to detect hibernating myocardium that is able to functionally recover. Further studies[54,55] demonstrated that the transmural extent of delayed gadolinium enhancement correlates with the ability of functional improvement after acute myocardial infarction. Therefore, the distinction between reversible and irreversible dysfunctional myocardium in the acute setting after infarction also has a prognostic implication.
Moreover, myocardial scar has been demonstrated to be the cause of malignant reentrant ventricular arrhythmias causing sudden cardiac death in patients after myocardial infarction[56]. In patients with ischemic cardiamyopathy, Kwon et al[57] revealed that a greater extent of myocardial scar was associated with a significantly increased mortality or the need for cardiac transplantation, improving further risk stratification. In patients undergoing ICD implantation with CAD, the extent of myocardial scarring visualized by LGE CMR was significantly associated with appropriate device therapy and identified a subgroup of CAD patients with an increased risk of life-threatening ventricular arrhythmias[58].
Novel methods like precontrast T1 maps enable the detection of acute and chronic myocardial infarction[59] and might represent a further field to establish the use of CMR as a key to tissue characterization. In a combined clinical protocol native T1 mapping was suggested to reveal area at risk in ACS[60,61].
Extracellular volume (ECV) maps as a CMR marker for myocardial fibrosis can be generated if pre and post contrast T1 images are registered[62]. In contrast to LGE CMR, ECV is also able to visualize very early fibrotic changes[63].
In the future, T1 mapping and ECV may provide more profound insights into fundamental disease processes of the myocardium. Both techniques might affect clinical decision making, but to date are not yet part of the routine work-up. Besides, the reproducibility of the results still needs to be shown in multi-centre studies[64].
CMR is a non invasive imaging for the workup of patients with known or suspected CAD. It allows the detection of significant coronary stenoses in patients with acute and chronic chest pain. Moreover, it offers the unique opportunity to detect myocardial ischemia and viability or wall motion abnormalities and fibrosis in one examination. Novel techniques like T1 mapping and ECV will further expand the scope of application in the future.
P- Reviewer: Anan R, Fett JD, Toro R, Xiong XJ S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, Daly C, De Backer G, Hjemdahl P, Lopez-Sendon J. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341-1381. [PubMed] |
| 2. | Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154-e235. [PubMed] |
| 3. | Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, Christian TF, Balaban RS, Arai AE. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531-537. [PubMed] |
| 4. | Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, Paterson DI, Syed MA, Aletras AH, Arai AE. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427-1432. [PubMed] |
| 5. | Hartlage G, Janik M, Anadiotis A, Veledar E, Oshinski J, Kremastinos D, Stillman A, Lerakis S. Prognostic value of adenosine stress cardiovascular magnetic resonance and dobutamine stress echocardiography in patients with low-risk chest pain. Int J Cardiovasc Imaging. 2012;28:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Miller CD, Hwang W, Case D, Hoekstra JW, Lefebvre C, Blumstein H, Hamilton CA, Harper EN, Hundley WG. Stress CMR imaging observation unit in the emergency department reduces 1-year medical care costs in patients with acute chest pain: a randomized study for comparison with inpatient care. JACC Cardiovasc Imaging. 2011;4:862-870. [PubMed] |
| 7. | Haghi D, Fluechter S, Suselbeck T, Kaden JJ, Borggrefe M, Papavassiliu T. Cardiovascular magnetic resonance findings in typical versus atypical forms of the acute apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol. 2007;120:205-211. [PubMed] |
| 8. | Haghi D, Papavassiliu T, Flüchter S, Kaden JJ, Pörner T, Borggrefe M, Suselbeck T. Variant form of the acute apical ballooning syndrome (takotsubo cardiomyopathy): observations on a novel entity. Heart. 2006;92:392-394. [PubMed] |
| 9. | Haghi D, Suselbeck T, Borggrefe M. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J. 2007;71:1664; author reply 1665. [PubMed] |
| 10. | Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 390] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 11. | Doesch C, Burgstahler C, Seeger A, Miller S, May AE. Chest pain and reversible midventricular ballooning in a woman after witnessing sudden cardiac death: a possible variant of takotsubo cardiomyopathy. Can J Cardiol. 2009;25:e22. [PubMed] |
| 12. | Abdel-Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815-1822. [PubMed] |
| 13. | Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6:833-839. [PubMed] |
| 14. | Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802-1809. [PubMed] |
| 15. | Laissy JP, Hyafil F, Feldman LJ, Juliard JM, Schouman-Claeys E, Steg PG, Faraggi M. Differentiating acute myocardial infarction from myocarditis: diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology. 2005;237:75-82. [PubMed] |
| 16. | Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1951] [Cited by in RCA: 1747] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 17. | Ishida N, Sakuma H, Motoyasu M, Okinaka T, Isaka N, Nakano T, Takeda K. Noninfarcted myocardium: correlation between dynamic first-pass contrast-enhanced myocardial MR imaging and quantitative coronary angiography. Radiology. 2003;229:209-216. [PubMed] |
| 18. | Doesch C, Seeger A, Doering J, Herdeg C, Burgstahler C, Claussen CD, Gawaz M, Miller S, May AE. Risk stratification by adenosine stress cardiac magnetic resonance in patients with coronary artery stenoses of intermediate angiographic severity. JACC Cardiovasc Imaging. 2009;2:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Doesch C, Seeger A, Hoevelborn T, Klumpp B, Fenchel M, Kramer U, Schönfisch B, Claussen CD, Gawaz M, Miller S. Adenosine stress cardiac magnetic resonance imaging for the assessment of ischemic heart disease. Clin Res Cardiol. 2008;97:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Seeger A, Doesch C, Klumpp B, Kramer U, Fenchel M, Hoevelborn T, Gawaz M, Claussen CD, May AE, Miller S. [MR stress perfusion for the detection of flow-limiting stenoses in symptomatic patients with known coronary artery disease and history of stent implantation]. Rofo. 2007;179:1068-1073. [PubMed] |
| 21. | Nagel E, Klein C, Paetsch I, Hettwer S, Schnackenburg B, Wegscheider K, Fleck E. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432-437. [PubMed] |
| 22. | Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, Nelemans PJ, Schalla S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59:1719-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 23. | Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374-379 [PIMD: 12573373]. |
| 24. | Hamon M, Fau G, Née G, Ehtisham J, Morello R, Hamon M. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50:1343-1353. [PubMed] |
| 26. | de Jong MC, Genders TS, van Geuns RJ, Moelker A, Hunink MG. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: a systematic review and meta-analysis. Eur Radiol. 2012;22:1881-1895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, Schönberg SO, Luchner A, Strohm O, Ahlstrom H. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 28. | Bernhardt P, Walcher T, Rottbauer W, Wöhrle J. Quantification of myocardial perfusion reserve at 1.5 and 3.0 Tesla: a comparison to fractional flow reserve. Int J Cardiovasc Imaging. 2012;28:2049-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Cheng AS, Pegg TJ, Karamitsos TD, Searle N, Jerosch-Herold M, Choudhury RP, Banning AP, Neubauer S, Robson MD, Selvanayagam JB. Cardiovascular magnetic resonance perfusion imaging at 3-tesla for the detection of coronary artery disease: a comparison with 1.5-tesla. J Am Coll Cardiol. 2007;49:2440-2449. [PubMed] |
| 30. | Shah R, Heydari B, Coelho-Filho O, Murthy VL, Abbasi S, Feng JH, Pencina M, Neilan TG, Meadows JL, Francis S. Stress cardiac magnetic resonance imaging provides effective cardiac risk reclassification in patients with known or suspected stable coronary artery disease. Circulation. 2013;128:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 135] [Reference Citation Analysis (0)] |
| 31. | Buckert D, Dewes P, Walcher T, Rottbauer W, Bernhardt P. Intermediate-term prognostic value of reversible perfusion deficit diagnosed by adenosine CMR: a prospective follow-up study in a consecutive patient population. JACC Cardiovasc Imaging. 2013;6:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Gargiulo P, Dellegrottaglie S, Bruzzese D, Savarese G, Scala O, Ruggiero D, D’Amore C, Paolillo S, Agostoni P, Bossone E. The prognostic value of normal stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: a meta-analysis. Circ Cardiovasc Imaging. 2013;6:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:826-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 34. | Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2772] [Cited by in RCA: 2994] [Article Influence: 249.5] [Reference Citation Analysis (0)] |
| 35. | Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2010;18:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 555] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 36. | Coelho-Filho OR, Seabra LF, Mongeon FP, Abdullah SM, Francis SA, Blankstein R, Di Carli MF, Jerosch-Herold M, Kwong RY. Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient’s sex. JACC Cardiovasc Imaging. 2011;4:850-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Jahnke C, Furundzija V, Gebker R, Manka R, Frick M, Schnackenburg B, Marx N, Paetsch I. Gender-based prognostic value of pharmacological cardiac magnetic resonance stress testing: head-to-head comparison of adenosine perfusion and dobutamine wall motion imaging. Int J Cardiovasc Imaging. 2012;28:1087-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Baer FM, Voth E, Schneider CA, Theissen P, Schicha H, Sechtem U. Comparison of low-dose dobutamine-gradient-echo magnetic resonance imaging and positron emission tomography with [18F]fluorodeoxyglucose in patients with chronic coronary artery disease. A functional and morphological approach to the detection of residual myocardial viability. Circulation. 1995;91:1006-1015. [PubMed] |
| 39. | Schmidt M, Voth E, Schneider CA, Theissen P, Wagner R, Baer FM, Schicha H. F-18-FDG uptake is a reliable predictory of functional recovery of akinetic but viable infarct regions as defined by magnetic resonance imaging before and after revascularization. Magn Reson Imaging. 2004;22:229-236. [PubMed] |
| 40. | Baer FM, Theissen P, Schneider CA, Voth E, Sechtem U, Schicha H, Erdmann E. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31:1040-1048. [PubMed] |
| 41. | Gutberlet M, Fröhlich M, Mehl S, Amthauer H, Hausmann H, Meyer R, Siniawski H, Ruf J, Plotkin M, Denecke T. Myocardial viability assessment in patients with highly impaired left ventricular function: comparison of delayed enhancement, dobutamine stress MRI, end-diastolic wall thickness, and TI201-SPECT with functional recovery after revascularization. Eur Radiol. 2005;15:872-880. [PubMed] |
| 42. | Kløw NE, Smith HJ, Gullestad L, Seem E, Endresen K. Outcome of bypass surgery in patients with chronic ischemic left ventricular dysfunction. Predictive value of MR imaging. Acta Radiol. 1997;38:76-82. [PubMed] |
| 43. | Kobori M, Shida K, Negishi H, Masuda Y, Hosoyamada A. [Evaluation of dopamine and dobutamine for use in circulatory depression associated with induced total spinal block]. Masui. 1991;40:190-201. [PubMed] |
| 44. | Wellnhofer E, Olariu A, Klein C, Gräfe M, Wahl A, Fleck E, Nagel E. Magnetic resonance low-dose dobutamine test is superior to SCAR quantification for the prediction of functional recovery. Circulation. 2004;109:2172-2174. [PubMed] |
| 45. | Sandstede JJ, Bertsch G, Beer M, Kenn W, Werner E, Pabst T, Lipke C, Kretschmer S, Neubauer S, Hahn D. Detection of myocardial viability by low-dose dobutamine Cine MR imaging. Magn Reson Imaging. 1999;17:1437-1443. [PubMed] |
| 46. | Sayad DE, Willett DL, Hundley WG, Grayburn PA, Peshock RM. Dobutamine magnetic resonance imaging with myocardial tagging quantitatively predicts improvement in regional function after revascularization. Am J Cardiol. 1998;82:1149-1151, A10. [PubMed] |
| 47. | Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992-2002. [PubMed] |
| 48. | Mahrholdt H, Wagner A, Judd RM, Sechtem U. Assessment of myocardial viability by cardiovascular magnetic resonance imaging. Eur Heart J. 2002;23:602-619. [PubMed] |
| 49. | Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780-2783. [PubMed] |
| 50. | Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging. 2012;5:494-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453. [PubMed] |
| 52. | Nagel E, Schuster A. Myocardial viability: dead or alive is not the question! JACC Cardiovasc Imaging. 2012;5:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Beek AM, Kühl HP, Bondarenko O, Twisk JW, Hofman MB, van Dockum WG, Visser CA, van Rossum AC. Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J Am Coll Cardiol. 2003;42:895-901. [PubMed] |
| 54. | Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation. 2001;104:1101-1107. [PubMed] |
| 55. | Gerber BL, Garot J, Bluemke DA, Wu KC, Lima JA. Accuracy of contrast-enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation. 2002;106:1083-1089. [PubMed] |
| 56. | Scott PA, Morgan JM, Carroll N, Murday DC, Roberts PR, Peebles CR, Harden SP, Curzen NP. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol. 2011;4:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Kwon DH, Halley CM, Carrigan TP, Zysek V, Popovic ZB, Setser R, Schoenhagen P, Starling RC, Flamm SD, Desai MY. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc Imaging. 2009;2:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 58. | Alexandre J, Saloux E, Dugué AE, Lebon A, Lemaitre A, Roule V, Labombarda F, Provost N, Gomes S, Scanu P. Scar extent evaluated by late gadolinium enhancement CMR: a powerful predictor of long term appropriate ICD therapy in patients with coronary artery disease. J Cardiovasc Magn Reson. 2013;15:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JP, Sivananthan MU. Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007;58:34-40. [PubMed] |
| 60. | Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG. T1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2003;5:353-359. [PubMed] |
| 61. | Dall’Armellina E, Piechnik SK, Ferreira VM, Si QL, Robson MD, Francis JM, Cuculi F, Kharbanda RK, Banning AP, Choudhury RP. Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction. J Cardiovasc Magn Reson. 2012;14:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 62. | Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012;14:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 63. | Schalla S, Bekkers SC, Dennert R, van Suylen RJ, Waltenberger J, Leiner T, Wildberger J, Crijns HJ, Heymans S. Replacement and reactive myocardial fibrosis in idiopathic dilated cardiomyopathy: comparison of magnetic resonance imaging with right ventricular biopsy. Eur J Heart Fail. 2010;12:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 795] [Cited by in RCA: 856] [Article Influence: 71.3] [Reference Citation Analysis (0)] |