Published online Oct 26, 2014. doi: 10.4330/wjc.v6.i10.1122
Revised: June 1, 2014
Accepted: July 25, 2014
Published online: October 26, 2014
Processing time: 204 Days and 1.3 Hours
Abnormal connections between the ascending aorta and the cardiac chambers are rare, especially in the context of right-sided infective endocarditis (IE). Transthoracic echocardiography (TTE) with color-flow Doppler, transesophageal echocardiography (TEE), or both may be required for diagnosis. We present the case of a woman admitted with right-sided heart failure (HF) symptoms. She had a previous history of tricuspid valve IE 30 years ago. TTE and TEE revealed an aorto-right atrium fistula located just under the non-coronary cusp into the right atrium at the level of the previously affected tricuspid valve. The Patient refused surgery and was discharged home on HF medications. She has been stable for the last 3 years. The peculiarity of this case is the late symptomatic presentation of the aorto-atrial fistula and the unusual association to tricuspid valve IE.
Core tip: Aorto-cardiac fistulas are rare, usually associated to prosthetic aortic valve infective endocarditis. The median duration of symptoms to echocardiographic detection of fistulization is about one month. We present a case of aorto-atrial fistula at late presentation, 30 years after tricuspid valve infective endocarditis. This article describes the epidemiology, clinical manifestations, pathophysiology, diagnostic modalities, treatment and outcomes of aorto-cardiac fistulas.
- Citation: Villablanca PA, Sukhal S, Maitas O, Onuegbu A, Muñoz-Peña JM, Joseph A, Requena C, Mohananey D. Aorto-right atrial fistula: Late complication of tricuspid valve infective endocarditis. World J Cardiol 2014; 6(10): 1122-1126
- URL: https://www.wjgnet.com/1949-8462/full/v6/i10/1122.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i10.1122
Aorto-cardiac fistulas (ACF) are a rare complication of infective endocarditis (IE); it is usually a complication of prosthetic aortic valve IE. We report a case of a patient who was found to have an Aorto-right atrial fistula 30 years after his tricuspid valve IE was treated. No similar late complication of tricuspid valve IE has been reported.
A 51-year-old woman presented to the emergency department (ED) with worsening decreased exercise tolerance over the past 2 mo. Her past medical history was significant for a previous culture-negative tricuspid valve IE in 1980 that was treated medically with antibiotics, permanent atrial fibrillation, asthma and hypothyroidism. Home medications included aspirin, furosemide, metoprolol and albuterol.
The patient stated that in the last 2 mo she developed worsening shortness of breath, lower extremity edema, chest tightness, palpitations, weakness-fatigue and abdominal discomfort. She denied any inciting events. Within the last two weeks, her New York Heart Association (NYHA) functional class deteriorated from NYHA class I to NYHA class III, manifested by shortness of breath on minimal exertion, relieved only by rest. The patient denied orthopnea, paroxysmal nocturnal dyspnea, hemoptysis, chest pain, fevers, chills and weight loss.
On physical examination, her heart rate was 134 beats/min, blood pressure 115/62 mmHg and a temperature of 36 °C. Cardiac examination revealed a markedly elevated jugular venous pressure of 12 cm, grade 4/6 low frequency pansystolic murmur in the lower left sternal border, irregularly irregular rhythm, hepatojugular reflux and lower extremity edema up to the knees. The rest of the physical examination was normal.
Laboratory work up included BMP, CBC, ESR, CRP, which were unremarkable, except for microcytic anemia of 8.2 g/dL with normal ferritin and a BNP of 1046 pg/dL. The EKG demonstrated atrial fibrillation, right axis deviation and an incomplete right bundle branch block. The chest X-ray was significant for moderate cardiomegaly with right atrial and ventricular enlargement. She was started on diuretics and beta-blockers for heart failure (HF) exacerbation secondary to atrial fibrillation with rapid ventricular response and then transferred to the general medicine floor.
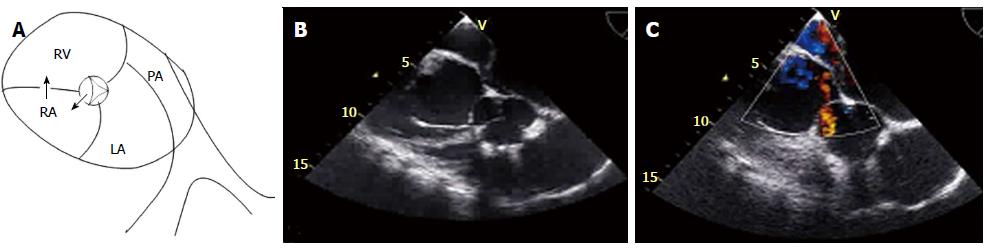
On the next day of admission, a transthoracic echocardiography (TTE) was performed, showing normal left ventricular size and systolic function, severe dilation of the right ventricle with mark hypocontractility, severe tricuspid regurgitation with the anterior leaflet calcified with flail segments from previous IE. There was a diastolic flow into the right atrium from the aorta (non coronary cusp). There was no evidence of aortic insufficiency, outflow tract gradient, or ventricular septal defect. Given our suspicion for aorto-atrial fistula, a transesophageal echocardiogram (TEE) was done, showing a small fistula from just under the non-coronary cusp into the right atrium at the level of the previously affected tricuspid valve (Figure 1). The patient underwent cardiac catheterization, which showed normal coronary arteries and confirmed the echocardiographic findings of an Aorto-right atrial fistula and tricuspid regurgitation.
Cardiac surgery service was consulted for tricuspid valve replacement and repair of the fistula. Patient refused surgery due to religious issues and was discharged home on diuretics, beta-blockers, angiotensin receptor 2 blockers and spironolactone. At 3-year follow up, patient has been stable, with no further exacerbation. Surgery has been offered repeatedly, we explained the risks and benefits of performing the surgery and the good results that can be accomplished with an acceptable morbidity and mortality, yet she only wants to continue with medical management.
IE has been associated with a myriad of complications such as HF and stroke[1]. The frequency and type of complications due to IE have changed with advances in diagnosis and therapy. Uncontrolled rare extra-valvular cardiac complications of IE, such as fistulous intra-cardiac connections, which were previously common complications of IE, are infrequent in the antibiotic era. Reported for the first time in 1924 as an incidental finding on autopsy[2], the incidence of ACF has been described in less than 2.2% of cases of native valve IE[3] and 3.3% of prosthetic valve IE[4] in retrospective studies. Staphylococcus aureus has been documented as the most common etiology reported on both autopsies and retrospective studies[3-5] with Streptococcus spp., Enterococcus spp., and other bacterial and fungal infections as other documented etiologies[6].
ACF has been documented in a variety of clinical scenarios, most frequently occurring in cases of aortic valve IE, and is more common in prosthetic than native aortic valve. ACF is present in less than 1% of right-sided IE cases, and is usually associated with concomitant native aortic IE[7,8]. There are isolated cases in the English literature that report ACF secondary to native tricuspid valve[9]. It has been described also with blunt trauma[10], stab wound of the chest[11], ruptured aneurysms of the sinus of valsalva (SV)[12], aortic dissection[13], congenital disorder[14], cardiac valve surgery[15], percutaneous cardiac valve implantation[16], heart transplantation[17], and autoimmune vasculitis[18].
The proposed theory to explain the fistulization mechanism between the aorta and the cardiac chamber is through the bacterial invasion and spread of the affected valve into the adjacent tissues and structures, resulting in the formation of a periannular abscess and erosion of the SV. The aortic abscesses involving the SV may rupture internally with erosion of the sinus and subsequent development of aorto-cavitary or aorto-pericardial fistulas[3,5,7,19,20]. Perivalvular abscesses have been reported as the cause of 6%-9% of fistula cases[21,22]. Due to its relative avascularity and infected regurgitation of jet striking subvalvular structures[23], the intervalvular fibrosa is more susceptible to infection[24]. The ACF creates a left to right shunt from any of the three aortic valve sinuses to any of the four cardiac chambers with no preponderance from any specific aortic sinus to a specific cavity, resulting in further hemodynamic deterioration[5,7]. These pathologic communications are highly morbid and lead to hemodynamic instability secondary to the shunt effect[19].
Diagnosing ACF can be challenging, and the clinical presentation will depend on the size of the shunt. Patients with a small ACF may be completely asymptomatic with an associated murmur only[25,26], but the clinical presentation may range from refractory HF[20] to a chest pain syndrome due to acute coronary syndrome and aortic dissection[17,27]. Cardiac auscultation may cause a continuous murmur[28], a thrill[29] or both[25], and can be the key to further pursue this diagnosis with appropriate imaging modalities. The median duration of symptoms to echocardiographic detection of fistulization is about 25 d as reported in a retrospective multi-center study[7]. There are isolated cases reported years after prosthetic valve implantation[15]. A high index of suspicion is required, especially in the background of recent surgery or previous IE.
Although aortography is the gold standard for diagnosis, non-invasive methods such as contrast enhanced CT, MRI, and echocardiography are currently preferred. TTE is the initial test of choice in the routine assessment of patients presenting with HF symptoms or murmurs, and is therefore usually the first image modality that allows us to confirm or suspect the presence of an ACF. However, TEE is superior to TTE for better delineation of function and morphology when intra-cardiac complications, such as ACF, are suspected[30,31]. The high rate of echocardiographic diagnosis is likely due to the high-pressure differences between the aorta and the cardiac chambers, which enables observation of the highly turbulent flow that is easily detectable by color Doppler[7]. Three–dimensional echocardiography has been reported to have the potential to delineate anatomic structures, allowing a greater understanding of the pathological process and also obtaining unconventional views of cardiac structures[32]. It can delineate structures that are otherwise not visible in TEE and TTE, allowing cropping, full-volume data, and slicing in various planes[33,34]. Computed tomography, magnetic resonance imaging, and aortography can allow better description, position, dimension and anatomic conditions of the ACF, and may be required as an important adjunctive tool to confirm the diagnosis and delineate the anatomy before closure[35-38].
Surgery, which is the primary treatment of ACF, may carry severe complications, particularly with critically unstable patients with an increased postoperative mortality after surgical correction[4]. Factors associated with adverse outcomes include staphylococcal infection, urgent or emergency surgery, moderate to severe HF, renal failure, increased age and residual fistula[3,7,19,20,22,39]. With the high postoperative mortality with surgical closure of ACF and with the advancement of endovascular technologies, more emphasis is now placed on percutaneous closure with devices such as an Amplatzer plug[40,41], though it should be avoided in in patients with active infection.
We report a case of a patient who was found to have an Aorto-right atrial fistula 30 years after his tricuspid valve was treated for IE. To our knowledge and after a systematic review of the English literature, no similar late complication of treated IE has been reported.
A 51-year-old female with a history of tricuspid valve infective endocarditis presented with shortness of breath.
Right side heart failure symptoms.
Aorto-cardiac fistula vs valvular heart disease vs new infective endocarditis.
Hb of 8.2 g/dL with normal ferritin and a BNP of 1046 pg/dL; inflammatory markers (erythrocyte sedimentation rate, serum C-reactive protein, blood cell count) were within normal limits.
Transthoracic and transesophageal echocardiography demonstrated Aorto-right atrial fistula.
The patient was medically managed for her heart failure after she refused surgical treatment.
Echocardiography images and explanatory figure are provided in the case report.
A high index of suspicion for aorto-cardiac fistula is required, especially in the background of recent surgery or previous infective endocarditis.
This is an interesting paper.
P- Reviewer: Firstenberg MA, Oteo JA S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Mansur AJ, Grinberg M, da Luz PL, Bellotti G. The complications of infective endocarditis. A reappraisal in the 1980s. Arch Intern Med. 1992;152:2428-2432. [PubMed] |
| 2. | Boyd L. A study of four thousand cases of aneurysm of the thoracic aorta. AM J MED SCI. 1924;168:654-667. |
| 3. | Anguera I, Miro JM, Evangelista A, Cabell CH, San Roman JA, Vilacosta I, Almirante B, Ripoll T, Fariñas MC, Anguita M. Periannular complications in infective endocarditis involving native aortic valves. Am J Cardiol. 2006;98:1254-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Anguera I, Miro JM, San Roman JA, de Alarcon A, Anguita M, Almirante B, Evangelista A, Cabell CH, Vilacosta I, Ripoll T. Periannular complications in infective endocarditis involving prosthetic aortic valves. Am J Cardiol. 2006;98:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Arnett EN, Roberts WC. Valve ring abscess in active infective endocarditis. Frequency, location, and clues to clinical diagnosis from the study of 95 necropsy patients. Circulation. 1976;54:140-145. [PubMed] |
| 6. | Williams-Phillips S. Aorto-cavitary fistula: a complication of infective endocarditis. West Indian Med J. 2012;61:756-759. [PubMed] |
| 7. | Anguera I, Miro JM, Vilacosta I, Almirante B, Anguita M, Muñoz P, Roman JA, de Alarcon A, Ripoll T, Navas E. Aorto-cavitary fistulous tract formation in infective endocarditis: clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur Heart J. 2005;26:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Yuan SM. Right-sided infective endocarditis: recent epidemiologic changes. Int J Clin Exp Med. 2014;7:199-218. [PubMed] |
| 9. | Akowuah EF, Casula R, Thanos A, Cooper GJ. Aorto-right atrial fistula associated with native tricuspid valve endocarditis. J Cardiovasc Surg (Torino). 2002;43:841-842. [PubMed] |
| 10. | Muehlschlegel JD, Alomar-Melero E, Staples ED, Janelle GM. Acute high-output failure from an aortoventricular fistula due to a ruptured sinus of Valsalva aneurysm after blunt chest trauma. Anesth Analg. 2006;103:1408-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Barbosa FM, Quiroga JM, Otero AE, Girela GA. Aortic valve regurgitation with aorto-right ventricular fistula following penetrating cardiac injury. Interact Cardiovasc Thorac Surg. 2011;13:653-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Onorato E, Casilli F, Mbala-Mukendi M, Perlasca E, Santoro F, Bortone F, Arena V. Sudden heart failure due to a ruptured posterior Valsalva sinus aneurysm into the right atrium: feasibility of catheter closure using the Amplatzer duct occluder. Ital Heart J. 2005;6:603-607. [PubMed] |
| 13. | Matsuhisa H, Obo H, Nakagiri K, Mukohara N, Shida T. Aorto-right atrial fistula caused by type A aortic dissection. Ann Thorac Surg. 2004;78:2173-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Pinaud F, Pezard P, Merheb M, Sibileau E, Baufreton C. Congenital aorto-right ventricular fistula in an adult. Eur Heart J. 2009;30:2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Roy D, Saba S, Grinberg I, Zughaib M, Sakwa M, Clancy P, McKendrick G. Aorto-right ventricular fistula: a late complication of aortic valve replacement. Tex Heart Inst J. 1999;26:140-142. [PubMed] |
| 16. | Muñoz-García AJ, Rodríguez-Bailón I, Briales JH, Navarro MJ, García JM, de Teresa-Galván E. Aorto-right ventricular fistula after percutaneous aortic valve implantation of a CoreValve prosthesis. Tex Heart Inst J. 2011;38:728-729. [PubMed] |
| 17. | Caruso A, Iarussi D, Materazzi C, Dialetto G, Covino F, Bossone E, Cotrufo M. Aortic dissection with fistula to right atrium after heart transplantation: diagnosis by transthoracic and transesophageal echocardiography. Echocardiography. 2000;17:337-340. [PubMed] |
| 18. | Melua A, Campbell N, McCluskey D, MacGowan SW. Aorto-atrial fistula without aneurysm formation in Behçet’s disease. Heart. 1998;80:200-201. [PubMed] |
| 19. | Anguera I, Quaglio G, Miró JM, Paré C, Azqueta M, Marco F, Mestres CA, Moreno A, Pomar JL, Mezzelani P. Aortocardiac fistulas complicating infective endocarditis. Am J Cardiol. 2001;87:652-654, A10. [PubMed] |
| 20. | Archer TP, Mabee SW, Baker PB, Orsinelli DA, Leier CV. Aorto-left atrial fistula. A reversible cause of acute refractory heart failure. Chest. 1997;111:828-831. [PubMed] |
| 21. | San Román JA, Vilacosta I, Sarriá C, de la Fuente L, Sanz O, Vega JL, Ronderos R, González Pinto A, Jesús Rollán M, Graupner C. Clinical course, microbiologic profile, and diagnosis of periannular complications in prosthetic valve endocarditis. Am J Cardiol. 1999;83:1075-1079. [PubMed] |
| 22. | Choussat R, Thomas D, Isnard R, Michel PL, Iung B, Hanania G, Mathieu P, David M, du Roy de Chaumaray T, De Gevigney G. Perivalvular abscesses associated with endocarditis; clinical features and prognostic factors of overall survival in a series of 233 cases. Perivalvular Abscesses French Multicentre Study. Eur Heart J. 1999;20:232-241. [PubMed] |
| 23. | Chandra S, Ameta D, Kharwar RB, Goyal M, Kumar D, Dwivedi SK, Saran RK. Three-dimensional echocardiographic delineation of an acquired aorto-left atrial fistula complicating native aortic valve endocarditis - “advantage of three dimensions”. Echocardiography. 2013;30:E326-E330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Kim HW, Chung CH. Mitral-aortic intervalvular fibrosa pseudoaneurysm resulting in the displacement of the left main coronary artery after aortic valve replacement. J Thorac Cardiovasc Surg. 2010;139:e18-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Chow WH, Lee PK, Cheung KL, Mok CK. Two-dimensional and pulsed Doppler echocardiographic diagnosis of an acquired aortic right ventricular fistula. Clin Cardiol. 1989;12:544-545. [PubMed] |
| 26. | Lorenz J, Reddy CV, Khan R, Hoover E, Hsu HK, El-Sherif N. Aortico-right ventricular shunt following aortic valve replacement. Chest. 1983;83:922-925. [PubMed] |
| 27. | Amabile N, Gil JM, Sarran A. Aorto-right ventricular fistula presenting 10 years after aortic surgery as an acute coronary syndrome. Arch Cardiovasc Dis. 2009;102:153-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Cakir C, Duygu H, Kilicaslan B, Ertas F, Ozen N, Nazli C, Ergene O. Postoperative diagnosis of aorto-right ventricular outflow tract fistula caused by stab wound: a case report. J Am Soc Echocardiogr. 2007;20:1415.e5-1415.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Jackson DH, Murphy GW, Stewart S, DeWeese JA, Schreiner BF. Delayed appearance of left-to-right shunt following aortic valvular replacement. Report of two cases. Chest. 1979;75:184-186. [PubMed] |
| 30. | Ananthasubramaniam K. Clinical and echocardiographic features of aorto-atrial fistulas. Cardiovasc Ultrasound. 2005;3:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Patsouras D, Argyri O, Siminilakis S, Michalis L, Sideris D. Aortic dissection with aorto-left atrial fistula formation soon after aortic valve replacement: A lethal complication diagnosed by transthoracic and transesophageal echocardiography. J Am Soc Echocardiogr. 2002;15:1409-1411. [PubMed] |
| 32. | Maffè S, Zenone F, Dellavesa P, Paffoni P, Paino AM, Signorotti F, Cucchi L, Pardo NF, Parravicini U. Usefulness of three-dimensional transthoracic echocardiography in particular clinical settings: a case of aorto-cavitary fistula in periprosthetic aortic valve abscess. Echocardiography. 2012;29:E141-E144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Chan KL, Liu X, Ascah KJ, Beauchesne LM, Burwash IG. Comparison of real-time 3-dimensional echocardiography with conventional 2-dimensional echocardiography in the assessment of structural heart disease. J Am Soc Echocardiogr. 2004;17:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Patel V, Fountain A, Guglin M, Nanda NC. Three-dimensional transthoracic echocardiography in identification of Aorto-right atrial fistula and aorto-right ventricular fistulas. Echocardiography. 2010;27:E105-E108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Park H, Park TH, Lee DY, Ahn J, Baek HK, Kim MH, Kim YD, Park KJ, Wu JS. A case of aortic dissection with fistula from aorta to right ventricle. Korean Circ J. 2012;42:629-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Rajiah P, Kanne JP. Cardiac MRI: Part 1, cardiovascular shunts. AJR Am J Roentgenol. 2011;197:W603-W620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Dwivedi SK, Vijay SK, Chandra S, Ahmad N, Saran RK, Singh SK. Giant aorto-right ventricular fistula with single coronary artery. Circulation. 2012;125:e462-e465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Siebers C, Schramm R, Friedmann A, Weig T. Severe cardiogenic shock due to acute onset of an aorto-to-right atrial shunt in a patient with aortic valve endocarditis. Int J Surg Case Rep. 2014;5:108-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Ellis SG, Goldstein J, Popp RL. Detection of endocarditis-associated perivalvular abscesses by two-dimensional echocardiography. J Am Coll Cardiol. 1985;5:647-653. [PubMed] |
| 40. | Lu TL, Beregi JP, Rey C, Midulla M, Lions C. Percutaneous closure of an aorto-right ventricular fistula with an Amplatzer plug. J Vasc Interv Radiol. 2011;22:100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Lebreiro AM, Silva JC. Transcatheter closure of an iatrogenic aorto-right ventricular fistula. Catheter Cardiovasc Interv. 2012;79:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |