Published online Oct 26, 2014. doi: 10.4330/wjc.v6.i10.1108
Revised: July 25, 2014
Accepted: August 27, 2014
Published online: October 26, 2014
Processing time: 257 Days and 11 Hours
Quantitative assessment of myocardial perfusion by myocardial blush grade (MBG) is an angiographic computer-assisted method to assess myocardial tissue-level reperfusion in patients with acute coronary syndromes and microvascular integrity in heart transplant recipients with suspected cardiac allograft vasculopathy. This review describes the ability of quantitative MBG as a simple, fast and cost effective modality for the prompt diagnosis of impaired microvascular integrity during routine cardiac catheterization. Herein, we summarize the existing evidence, its usefulness in the clinical routine, and compare this method to other techniques which can be used for the assessment of myocardial perfusion.
Core tip: In this article, we highlight the ability of quantitative myocardial blush grade for the assessment of microvascular integrity in patients with acute coronary syndromes (ACS) and heart transplant (HT) recipients with cardiac allograft vasculopathy (CAV). Using an, in the meanwhile well-established, computational algorithm, a prompt diagnosis can be made in the catheterization lab, which can identify patients with ACS and increased risk for myocardial remodelling and congestive heart failure in the long-term. In addition, this computational algorithm can identify HT recipients with increased risk for CAV and adverse cardiovascular outcomes.
- Citation: Hofmann NP, Dickhaus H, Katus HA, Korosoglou G. Quantitative assessment of myocardial blush grade in patients with coronary artery disease and in cardiac transplant recipients. World J Cardiol 2014; 6(10): 1108-1112
- URL: https://www.wjgnet.com/1949-8462/full/v6/i10/1108.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i10.1108
Impaired myocardial perfusion by either epicardial coronary artery disease (CAD) or small vessel disease is a common challenge in cardiology worldwide. Both CAD and cardiac allograft vasculopathy (CAV) in heart transplant (HT) recipients significantly influence mortality in such patient cohorts. Considering the vast amount of health care costs for post-rehabilitation support after myocardial infarction[1] and HT[2], preventive medical care should primarily focus on the early detection of cardiac pathology and risk stratification of such patients. Quantitative assessment of epicardial and microvascular integrity can aid tailoring pharmacologic therapy of patients identified at high risk for future events, which may ultimately improve clinical outcomes. We therefore previously developed a computer-assisted program for the analysis of microvascular integrity in patients undergoing cardiac catheterization either during acute myocardial infarction for reperfusion of the infarcted tissue[3,4] or during surveillance coronary angiography in cardiac transplant recipients[5,6]. Furthermore, such measures of perfusion can also be applied in patients undergoing fractional flow reserve analysis (FFR) for the assessment of the functional significance of coronary lesions of moderate severity[7].
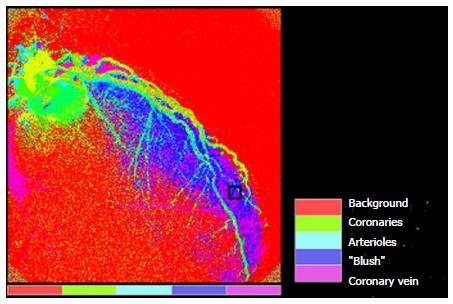
We previously introduced a computer-based algorithm for the quantification of MBG in patients with first time acute myocardial infarction[3]. This method aimed at the objective assessment of reperfused myocardial tissue and the estimation of infarct size and functional recovery of the myocardium at risk. This method is based on conventional cine angiographic films. In order to achieve maximal quality of the digital subtraction angiography images, the sequence is synchronized with the baseline electrocardiogram (ECG). The spatio-temporal spread of blood, or the so-called MBG, through epicardial vessels and then to the microvasculature and the myocardium, indicated by dye injection, represents a characteristic pattern for the myocardial perfusion. This dynamic temporal pattern is characterized by typical features as the maximal value of MBG intensity and the increase and decrease velocity which correspond to the different phases of flooding in and washout. Regions of interest are positioned in the distal part of each coronary artery in order to measure the plateau of mean grey level pixel intensity (Gmax which is measured on a standard gray scale of 0 to 255) as well as the time to maximal intensity rise (Tmax measured in seconds) (Figure 1). The ratio Gmax/Tmax is subsequently computed in each coronary vessel. Furthermore, based on the distribution of MBG over time in the epicardial vessels, arterioles and capillaries, parametric quantification can be applied as shown in Figure 2. To allow for quantification of MBG, frames should be recorded long enough in order to allow filling of the coronary veins, and images should be acquired during breath hold in order to avoid artefacts due to movement of the diaphragm. On the basis of 100 different temporal MBG profiles, an algorithm is established which classifies the acquired blush patterns into 4 different grades[8]. An example of a patient with post-interventional high Gmax/Tmax and full functional recovery after first acute non-ST-elevation myocardial infarction (NSTEMI) of the left anterior descending is illustrated in Figure 3.
In a swine model by Boyle et al[9], the quantitative myocardial blush grades could be assessed automatically and were closely related to established angiographic parameters of myocardial perfusion.
Our first clinical findings showed that quantitative MBG is applicable for the evaluation of microvascular tissue perfusion in patients with ST-elevation myocardial infarction (STEMI), being highly predictive for functional recovery of the myocardium at risk as assessed by echocardiography[3]. Hereby, multivariate analysis showed that MBG and Troponin T elevation were independent predictors of residual ejection fraction > 50%. Quantitative MBG by Gmax/Tmax showed the highest odds ratio and was therefore considered as the most robust variable for the prediction of the primary endpoint.
Furthermore, quantitative MBG was related to infarct transmurality and residual ejection fraction by cardiac magnetic resonance (CMR) in both STEMI and NSTEMI patients. This objective information can be acquired during routine cardiac catheterization, immediately after interventional treatment of the infarct related lesion, and can be used for the immediate risk stratification of patients with ACS[4]. Hereby, Gmax/Tmax was at least as accurate as infarct transmurality for the prediction of residual ejection fraction. Both clearly surpassed the accuracy of visual MBG.
Besides patients with CAD, we also investigated our computer-assisted program on the growing group of HT recipients. For this purpose, transplanted patients who underwent surveillance cardiac catheterization were subsequently analyzed by CMR, to assess myocardial relaxation and perfusion reserve during adenosine stress. Close correlations were observed between Gmax/Tmax with perfusion reserve and with mean diastolic strain rates. Visual and quantitative MBG had significantly higher accuracy than stenosis severity on coronary angiograms for the detection of diminished myocardial perfusion. Furthermore, quantitative MBG provided more robust prediction of survival compared to visually estimated blush and to coronary lumen narrowing assessment. Hereby, our findings indicate that quantitative MBG can be performed on coronary angiograms of HT recipients just as well, and may aid the detection of CAV in such individuals with impaired perfusion but with angiographically “normal” coronaries[5].
Impaired myocardial perfusion in transplanted hearts is closely associated with outcomes. In this regard, Gmax/Tmax is a simple to acquire and useful surrogate parameter of myocardial perfusion in HT recipients, which can predict cardiac outcomes. Gmax/Tmax differentiated between patients with high rate of cardiac events compared to those with higher quantitative MBG, who exhibited much better outcomes during a mean follow-up duration of 2.7 years. In addition, close correlations were observed between MBG and perfusion reserve measured by stress magnetic resonance imaging. Quantification of MBG may therefore be useful for the risk stratification of such patients[6].
Finally, preliminary clinical data indicate that MBG during adenosine infusion can be used to estimate angiographic perfusion reserve and is associated with FFR measures and with the myocardial perfusion reserve, assessed by CMR[7].
So far, different non-invasive and invasive imaging methods have been used for analysis and risk stratification of CAD and CAV patients, as, e.g., myocardial contrast echocardiography (MCE), CMR imaging and angiographic parameters including Thrombolysis in Myocardial Infarction (TIMI) flow grade, TIMI frame count, TIMI myocardial perfusion grade and MBG.
Myocardial perfusion and function can be assessed during MCE. This technique provides real-time visualisation of ischemic myocardium in regions of reduced blood flow. Although MCE is a practicable and non-invasive technique, it is limited by observer dependency and technical challenges pending on patients’ echogenic windows[10].
This is the current reference method for the assessment of cardiac anatomy, perfusion and function, viability and if required metabolism, all within a single examination, non-invasively and without ionizing radiation for the patients. Therefore, our studies mostly compare MBG to either CMR derived ejection fraction, remodelling, infarct size and transmurality or perfusion reserve index[4-6,11].
Reperfusion after myocardial infarction or ACS determines clinical outcome[12]. However, clinical and experimental data indicate that stenosis reduction during percutaneous coronary intervention (PCI) is not always associated with adequate myocardial tissue reperfusion, so that patients with TIMI 3 flow grade after PCI may still exhibit impaired microvascular integrity[3,13,14]. Thus, epicardial restoration of coronary blood flow is only prerequisite, but not a guarantee for myocardial recovery[15], the latter being a major predictor of mortality and morbidity in CAD patients.
Visually assessed MBG represents a reasonable alternative to TIMI flow grade and TIMI frame count, since it can distinguish between high and low risk constellations. The TIMI myocardial perfusion grade demonstrates a similar method that also considers the dynamic contrast agent washout. Unfortunately, the accuracy of both techniques is limited due to their categorical nature, which is associated with high observer variability, especially with non-expert readers.
The “Quantitative Blush Evaluator” (QuBE) from the TAPAS trial, an open-source computer program for quantification of myocardial perfusion, was used on angiograms in patients with acute STEMI[16]. The QuBE score correlated significantly with visual MBG as well as infarct size and microvascular dysfunction assessed by CMR[17]. Nevertheless, it has exclusively been used for STEMI patients so far, and further evaluation in other patient cohorts is warranted.
Quantitative assessment of MBG can be performed on coronary angiograms of either CAD or CAV patients. In this regard, Gmax/Tmax is a simple and useful surrogate parameter of microvascular integrity, which can (1) estimate clinical outcome in HT recipients with impaired perfusion reserve but without angiographically evident atherosclerosis and (2) infarct transmurality and functional recovery in both STEMI and NSTEMI. These results can be used for tailoring pharmacological treatment and aid early risk stratification in both CAD and CAV patients.
P- Reviewer: Avanzas P, Pastromas S S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Stargardt T, Schreyögg J, Kondofersky I. Measuring the relationship between costs and outcomes: the example of acute myocardial infarction in German hospitals. Health Econ. 2014;23:653-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Digiorgi PL, Reel MS, Thornton B, Burton E, Naka Y, Oz MC. Heart transplant and left ventricular assist device costs. J Heart Lung Transplant. 2005;24:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Korosoglou G, Haars A, Michael G, Erbacher M, Hardt S, Giannitsis E, Kurz K, Franz-Josef N, Dickhaus H, Katus HA. Quantitative evaluation of myocardial blush to assess tissue level reperfusion in patients with acute ST-elevation myocardial infarction: incremental prognostic value compared with visual assessment. Am Heart J. 2007;153:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Riedle N, Dickhaus H, Erbacher M, Steen H, Andrassy M, Lossnitzer D, Hardt S, Rottbauer W, Zugck C, Giannitsis E. Early assessment of infarct size and prediction of functional recovery by quantitative myocardial blush grade in patients with acute coronary syndromes treated according to current guidelines. Catheter Cardiovasc Interv. 2010;76:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Korosoglou G, Riedle N, Erbacher M, Dengler TJ, Zugck C, Rottbauer W, Hardt S, Bekeredjian R, Kristen A, Giannitsis E. Quantitative myocardial blush grade for the detection of cardiac allograft vasculopathy. Am Heart J. 2010;159:643-651.e2. [PubMed] |
| 6. | Hofmann NP, Voss A, Dickhaus H, Erbacher M, Doesch A, Ehlermann P, Gitsioudis G, Buss SJ, Giannitsis E, Katus HA. Long-term outcome after heart transplantation predicted by quantitative myocardial blush grade in coronary angiography. Am J Transplant. 2013;13:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Korosoglou G, Hofmann NP, Erbacher M, Dickhaus H, Bekeredjian R, Neumann FJ, Katus HA, Hardt SE. Quantitative myocardial blush grade reserve during pharmacologic hyperemia is related to fractional flow reserve measures in patients with stable coronary artery disease. Clin Res Cardiol. 2013;102 (abstract). |
| 8. | Dickhaus H, Erbacher M, Kücherer H. Quantification of myocardial perfusion for CAD diagnosis. Stud Health Technol Inform. 2007;129:1339-1343. [PubMed] |
| 9. | Boyle AJ, Schuleri KH, Lienard J, Vaillant R, Chan MY, Zimmet JM, Mazhari R, Centola M, Feigenbaum G, Dib J. Quantitative automated assessment of myocardial perfusion at cardiac catheterization. Am J Cardiol. 2008;102:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Korosoglou G, Hansen A, Hoffend J, Gavrilovic G, Wolf D, Zehelein J, Haberkorn U, Kuecherer H. Comparison of real-time myocardial contrast echocardiography for the assessment of myocardial viability with fluorodeoxyglucose-18 positron emission tomography and dobutamine stress echocardiography. Am J Cardiol. 2004;94:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Hofmann NP, Steuer C, Voss A, Erbel C, Celik S, Doesch A, Ehlermann P, Buss SJ, Giannitsis E, Katus HA. Comprehensive Bio-Imaging using Myocardial Perfusion Reserve Index during Adenosine Cardiac Magnetic Resonance and the High-sensitive Troponin T for the Prognosis of Heart Transplant Recipients. Am J Transplant. 2014;Oct 7; Epub ahead of print. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Cannon CP. Importance of TIMI 3 flow. Circulation. 2001;104:624-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Galiuto L, DeMaria AN, del Balzo U, May-Newman K, Flaim SF, Wolf PL, Kirchengast M, Iliceto S. Ischemia-reperfusion injury at the microvascular level: treatment by endothelin A-selective antagonist and evaluation by myocardial contrast echocardiography. Circulation. 2000;102:3111-3116. [PubMed] |
| 14. | Hansen A, Kumar A, Wolf D, Frankenbergerova K, Filusch A, Gross ML, Mueller S, Katus H, Kuecherer H. Evaluation of cardioprotective effects of recombinant soluble P-selectin glycoprotein ligand-immunoglobulin in myocardial ischemia-reperfusion injury by real-time myocardial contrast echocardiography. J Am Coll Cardiol. 2004;44:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Ito H, Okamura A, Iwakura K, Masuyama T, Hori M, Takiuchi S, Negoro S, Nakatsuchi Y, Taniyama Y, Higashino Y. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation. 1996;93:1993-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 260] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Vogelzang M, Vlaar PJ, Svilaas T, Amo D, Nijsten MW, Zijlstra F. Computer-assisted myocardial blush quantification after percutaneous coronary angioplasty for acute myocardial infarction: a substudy from the TAPAS trial. Eur Heart J. 2009;30:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Porto I, Hamilton-Craig C, De Maria GL, Vergallo R, Cautilli G, Galiuto L, Burzotta F, Leone AM, Niccoli G, Natale L. Quantitative Blush Evaluator accurately quantifies microvascular dysfunction in patients with ST-elevation myocardial infarction: comparison with cardiovascular magnetic resonance. Am Heart J. 2011;162:372-381.e2. [PubMed] |