Published online Oct 26, 2014. doi: 10.4330/wjc.v6.i10.1100
Revised: March 6, 2014
Accepted: September 16, 2014
Published online: October 26, 2014
Processing time: 319 Days and 7.1 Hours
Cardiovascular disease is the leading cause of death in patients with peripheral arterial disease (PAD). Coronary artery disease (CAD) is highly prevalent, and often times coexist, in patients with PAD. The management of patients with PAD that requires a high-risk vascular surgical procedure for intermittent claudication, critical limb ischemia or expanding abdominal aortic aneurysm requires risk stratification with the revised cardiac risk index, optimization of medical therapies, and limited use of cardiac imaging prior to surgery. Preventive revascularization in patients with stable CAD, with the sole intention to mitigate the risk of cardiac complications in the peri-operative period, is not effective and may be associated with significant bleeding and thrombotic risks, in particular if stents are used. A strategy of universal use of cardiac troponins in the perioperative period for active surveillance of myocardial ischemia may be more reasonable and cost-effective than the current standard of care of widespread use of cardiac imaging prior to high-risk surgery. An elevated cardiac troponin after vascular surgery is predictive of long-term mortality risk. Medical therapies such as aspirin and statins are recommended for patients with post-operative myocardial ischemia. Ongoing trials are assessing the role of novel anticoagulants. Additional research is needed to define the role of cardiac imaging and invasive angiography in this population.
Core tip: Patients with advanced peripheral arterial disease who need vascular surgery have a high prevalence of coronary atherosclerosis and are at increased risk of perioperative myocardial infarction. Coronary revascularization prior to the vascular operation is not an effective intervention to mitigate this risk. A strategy of widespread use of cardiac troponins in the perioperative period is recommended to detect perioperative ischemic events associated with a long-term mortality risk. The selective use of medical interventions, cardiac imaging and coronary angiography in this population deserves further study.
- Citation: Garcia S, McFalls EO. Perioperative clinical variables and long-term survival following vascular surgery. World J Cardiol 2014; 6(10): 1100-1107
- URL: https://www.wjgnet.com/1949-8462/full/v6/i10/1100.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i10.1100
The approach to patients with peripheral arterial disease (PAD) is best appreciated in the broader context of the epidemiology of the disease, risk factors, and surgical and endovascular interventions to improve symptoms, preserve limb viability or prevent aneurismal rupture.
Peripheral arterial disease (PAD) and coronary artery disease (CAD) often coexist in the same patient and share a common risk factor profile, pathophysiology, and array of therapeutic interventions[1-3]. Cardiovascular disease is the leading cause of death in patients with PAD, responsible for about two of every three deaths[4].
Vascular surgery is considered a high-risk operation with one in four patients experiencing a peri-operative myocardial infarction (PMI), which is associated with increased long-term mortality[5,6]. Identifying the clinical variables associated with increased risk of PMI prior to surgery as well as defining the best strategy for surveillance of PMI after high-risk surgery are of critical importance in clinical practice to mitigate risk and improve outcomes.
The definition of PAD is based on a resting ankle-brachial index (ABI) of ≤ 0.90[1]. Noticeably, the presence of symptoms is not required to diagnose PAD. For every patient with symptoms of PAD there are 4 with no symptoms as defined by ABI or duplex ultrasonography[7]. Screening for PAD is therefore recommended to detect the disease in individuals with a high pre-test probability. In the PARTNERS (PAD Awareness, Risk, and Treatment: New Resources for Survival) study the prevalence of PAD defined by ABI was 29%[8]. Therefore, current ACC/AHA guidelines recommend screening for PAD in patients aged ≥ 70 or 50-69 years with a risk factor for vascular disease[1]. Intermittent claudication (IC) is the most common presenting symptom of symptomatic PAD. IC is characterized by leg pain (muscular pain) with activity that is relieved by physical rest. Claudication tends to occur one anatomical level below the arterial level of obstruction or occlusion. For example a patient with superficial femoral artery (SFA) occlusion will likely have calf symptoms. The prevalence of IC in the general population is low but increases significantly with age so that in patients aged 60 or older is about 6%[9].
The risk factors for developing PAD and CAD show significant overlap and include male gender, age, hypertension, hyperlipidemia, renal insufficiency, black race, and more importantly diabetes mellitus (DM) and smoking, both of which have odds ratios (ORs) over 3 for symptomatic PAD[2,10-14]. Likewise, diabetics and smokers have a 3 to 4-fold increase in the risk of developing critical limb ischemia and amputations[2,12].
The prevalence of CAD in patients with PAD depends on the setting and the sensitivity of the method used to identify occult CAD. In the REACH (Reduction of Atherotrombosis for Continued Health) outpatient registry (Figure 1), 50% of patients with PAD and polyvascular disease had coexistent CAD[3]. In a landmark angiographic study of 1000 patients undergoing coronary angiography prior to vascular surgery conducted at the cleveland clinic by Hertzer et al[15] only 8% had normal coronary arteries prior to surgery, 2/3 had severe CAD, 10% had inoperable CAD and 18% had moderate CAD.
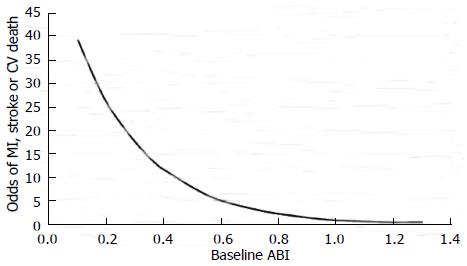
The annual rate of major adverse cardiovascular events (MACE) (myocardial infarction, stroke, and vascular death) in patients with PAD is 5%-7%[1,2]. Critical limb ischemia (CLI) patients have 20% mortality only in the first year after initial presentation. CAD is responsible for 40%-60% of deaths among patients with PAD while cerebral arterial disease accounts for another 10%-20% of deaths[1,2,4]. The severity of PAD, as quantified by ABI, correlates with the risk of MACE so that for every 0.10 decrease in ABI there is a corresponding 10% increase in MACE[16]. There is a strong association between MACE and ABI ≤ 0.60 in patients with diabetes[16] (Figure 2).
Variables to assess prior to a vascular operation include the type of operation (open vs endovascular), the risk of concomitant CAD and the functional status of the patient[17]. Open abdominal aneurismal repair with cross-clamp of the aorta and non-elective operations carry the highest risk of cardiovascular complications[18] in part due to the hemodynamic stress of the surgery, CAD burden, and the acuity of the condition that often hampers the ability to start preoperative interventions to mitigate cardiac risk.
Evaluating the functional status of subjects undergoing vascular surgery is an important step in assessing if a patient can tolerate the hemodynamic stress of a prolonged surgery. If a patient is unable to achieve a metabolic demand of 4-METS, which is a level compatible with routine activities of daily living, the risk of surgical complications increases and additional testing may be warranted. Stress imaging testing, usually with pharmacological agents such as adenosine or dobutamine, has been recommended prior to high-risk vascular surgery in patients with functional capacity < 4 METS[17]. The presence of large or multiple ischemic segments or transient ischemic dilatation of the left ventricle may indicate either multivessel or left main CAD. These findings are considered high risk and are associated with an increased risk of perioperative cardiac complications and reduced long-term survival[19]. Coronary angiography is recommended to patients that have high-risk findings on non-invasive imaging, as certain angiographic subsets (i.e., left main CAD) derive a long-term benefit from revascularization[20]. An initial approach that combines clinical and stress-imaging variables is cost-effective[21].
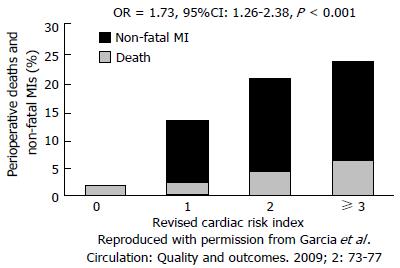
The Revised Cardiac Risk Index (RCRI) is a risk score comprised of six clinical variables (Table 1) that has been validated in a general surgery population as a tool to predict the risk of cardiac adverse events at 30 d[22]. A RCRI ≥ 3 is associated with > 5% risk of a serious cardiac complication in the postoperative period. However, in vascular surgery the RCRI tends to underestimate the risk of cardiac complications. In the Coronary Artery Revascularization Prophylaxis (CARP) trial a RCRI > 1 was predictive of a 10% risk of MI or death at 30 d in the preoperative revascularization (PR) group and 15% in the medical arm (Figure 3)[23].
| High-risk procedures (i.e., vascular surgery) |
| History of cerebrovascular disease |
| History of coronary artery disease |
| History of congestive heart failure |
| Creatinine > 2.0 mg% |
| Diabetes (insulin-dependent) |
The CARP Trial was a randomized, multisite VA study designed to assess the role of PR in patients with CAD undergoing elective vascular surgery[24]. A total of 510 patients were enrolled and randomized to either PR or no PR prior to elective vascular surgery. Indications for surgery included an expanding AAA in 33% of patients and arterial occlusive disease of the lower limbs in 67%. The index revascularization procedure consisted of percutaneous coronary intervention (PCI) in 59% and coronary artery bypass graft (CABG) surgery in 41% of patients. At 2.7 years, mortality in the PR group was 22% and in the no PR group was 23% (P = 0.92; RR = 0.98, 95%CI: 0.70-1.37) (Figure 4). Similarly, no difference in outcomes was seen within 30-d, mortality was 3.1% in the PR group and 3.4% in the no PR group (P = 0.87) and a MI occurred in 11.6% of the PR group and 14.3% of the no PR group (P = 0.37). The main conclusion of the CARP study is that preoperative coronary artery revascularization prior to vascular surgery does not result in better short- or long-term clinical outcomes in patients with stable CAD.
The pilot Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echo (DECREASE) - V study randomized 101 patients with stress-induced ischemia and multivessel or left main CAD to PR or no PR prior to high-risk vascular surgery[25]. At 1-year, the composite of non-fatal myocardial infarction and mortality between groups (49% vs 44%, P = 0.48) was no different. Taken together these data do not support a strategy of PR prior to elective vascular surgery in patients with stable CAD.
The Third Universal Definition of myocardial infarction (MI) proposed by the ESC/ACCF/AHA/WHF task force requires a rise and fall of cardiac biomarkers, preferably troponins, with at least one value above the 99th percentile of the upper reference limit (URL) coupled with a clinical correlate of ischemia such as ischemic symptoms, electrocardiographic ischemic changes, or imaging criteria of new loss of previously viable myocardium[26]. However, owing to the effects of anesthesia, and other factors such as widespread use of narcotics, the vast majority of perioperative ischemic events are clinically silent. In the Perioperative ischemic evaluation (POISE) trial 65% of patients with a perioperative ischemic event did not experience ischemic symptoms[27]. The risk of death at 30 d was 9.7% in patients with a symptomatic MI and 12.5% in patients with an asymptomatic MI. Thus, the universal definition of MI may not be as sensitive in the perioperative period to detect ischemic events that are associated with poor intermediate- and long-term outcomes. An isolated peak cardiac biomarker elevation (preferably troponins) above the 99th URL, with or without a correlate of ischemia, may be the most sensitive tool to detect perioperative ischemic events that are clinically important. In the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) registry[28], a peak postoperative troponin T (TnT) measured within the first 3 d after surgery was the strongest predictor of 30-d mortality and explained 41.8% of the deaths in population attributable risk analysis (Figure 5). A peak TnT of 0.02 ng/mL was associated with a 4% risk of death at 30 d[28].
Preoperative clinical variables that predicted 30-d mortality risk and were retained in the model that included peak TnT values included: age > 65, recent history of high-risk CAD, peripheral arterial disease, history of stroke, chronic obstructive pulmonary disease (COPD), cancer, urgent/emergency surgery, and major general or neurosurgical procedures. Of note, major vascular surgery and diabetes were not predictive of 30-d mortality in the model that included TnT[28].
In a cohort of 377 patients included in the CARP trial in whom cardiac troponin I was measured and analyzed by a core lab after the vascular surgery the proportion of patients with a perioperative myocardial infarction was 26%. Independent predictors of an MI included: age > 70 (OR = 1.84; 95%CI: 1.14-2.98; P = 0.01), abdominal aortic surgery (OR = 1.82; 95%CI: 1.09-3.03; P = 0.02), diabetes (OR = 1.86; 95%CI: 1.11-3.11; P = 0.02), angina (OR = 1.67; 95%CI: 1.03-2.64; P = 0.04), and baseline ST-T wave abnormalities (OR = 1.62; 95%CI: 1.00-2.6; P = 0.05)[29].
Clinical, angiographic, and pathological studies have shed light into the mechanisms underlying postoperative ischemic events[30-33]. Most of these events are caused by a mismatch between O2 supply and demand, usually with severe CAD in the background that is unmasked by the stress of the surgery. Landesberg et al[30] showed that tac ST-segment depression related to rapid heart rates is common in the perioperative period and predictive of long-term mortality. The duration of ST-segment depression and peak catecholamine levels after surgery are associated with infarct size. Chronic total occlusions (CTOs) are common in patients with a perioperative ischemic event or cardiac death (81%) relative to only 29% in patients without ischemic complications after surgery[31]. Two pathological studies reported conflicting data on the incidence of plaque rupture after fatal postoperative MI[32-33]. Dawood et al[32] described evidence of plaque rupture in only 7% of patients (42 autopsies). Conversely, a higher incidence of plaque rupture (46%) was described by Cohen et al[33]. Differences in timing of the autopsy relative to the time of the MI may account for some of the discrepancies in the data.
Data from randomized clinical trials are lacking to guide therapy in the postoperative period. Small studies have shown that interventions aimed at improving oxygen delivery and minimizing myocardial oxygen consumption are beneficial in this setting[34]. The main goal of therapy is to preserve coronary perfusion pressure during diastole. This is best achieved with judicious utilization of beta-blockers, analgesia, and fluid administration with the intention to avoid tachycardia and hypotension. In the POISE trial for every 10-beats/min increase in heart rate there was a 31% relative increase in the odds of perioperative MI[27]. Current guidelines recommend aggressive blood pressure control in patients with PAD, in particular in patients with diabetes and/or chronic kidney disease (goal < 130/80 mmHg)[35]. In the HOPE (Heart Outcomes Prevention Evaluation) trial ramipril 10 mg was associated with a 22% reduction in cardiovascular events and is currently recommended for high-risk patients, including those with PAD[36].
Statins contribute to plaque stabilization by decreasing circulating levels of inflammatory cytokines and reactive oxygen species while increasing expression of nitric oxide synthase[37]. Additionally, evidence from randomized clinical trials and observational studies support its use in clinical practice. In the DECREASE-III study a 53% reduction in CV death and myocardial infarction was seen with high-dose fluvastatin in patients undergoing vascular surgery[38]. In another trial of 100 patients randomly assigned to 20 mg of atorvastatin or placebo prior to vascular surgery, the use of statins was associated with a significant reduction in cardiac events, from 26% to 8% at 6 mo[39]. An observational study of 164 veterans undergoing vascular surgery at our medical center demonstrated that utilization of statin drugs was associated with a reduction in long-term mortality[5]. Guidelines recommend the use of statins in patients with peripheral arterial disease to reduce cardiovascular events[2].
Owing to concerns for bleeding after non-cardiac surgery, the use of medical therapies and interventional strategies commonly used to treat spontaneous MIs such as antiplatelet agents, anticoagulants and invasive coronary angiography are rarely used in this setting and have not been extensively studied in clinical trials. The management of myocardial infarction After NoncArdiac surgery (MANAGE) trial (NCT01661101) will be the first study to randomize patients (n = 3200) with a PMI after noncardiac surgery to dabigatran or placebo. The primary end point is the occurrence of a major vascular complication (vascular mortality, nonfatal MI, nonfatal stroke, and pulmonary embolism). The trial plans to complete enrollment in November 2015.
Another strategy for prevention of myocardial ischemia during surgery is ischemic preconditioning, which describes the protection afforded by application of non-lethal episodes of myocardial ischemia prior to the index ischemic event[40,41]. The Cardiac Remote Ischemic Preconditioning Prior to Elective Vascular Surgery (CRIPES, NCT: 01558596) was designed to determine the feasibility and safety of using remote ischemic preconditioning (RIPC) prior to vascular surgery, and to obtain preliminary estimates of its effects on detectable postsurgical increases in cardiac troponin I[42]. A similar strategy of RIPC has been evaluated prior to coronary angioplasty[43] and coronary artery bypass surgery[44] with positive initial results.
Patients with PAD in need of elective vascular surgery have a high prevalence of coronary atherosclerosis and are at increased risk of perioperative myocardial infarction. Coronary revascularization prior to the vascular operation is not an effective intervention to mitigate this risk. A strategy of widespread use of cardiac troponins in the perioperative period is recommended to detect perioperative ischemic events associated with a long-term mortality risk. The selective use of medical interventions, cardiac imaging and coronary angiography in this population deserves further study.
P- Reviewer: Al-Mohammad A, Athanasios G, Nemes A S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 746] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 2. | Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5-S67. [PubMed] |
| 3. | Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1143] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 4. | Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1889] [Cited by in RCA: 1814] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 5. | Marston N, Brenes J, Garcia S, Kuskowski M, Adabag S, Santilli S, McFalls EO. Peak postoperative troponin levels outperform preoperative cardiac risk indices as predictors of long-term mortality after vascular surgery Troponins and postoperative outcomes. J Crit Care. 2012;27:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | McFalls EO, Ward HB, Moritz TE, Littooy F, Santilli S, Rapp J, Larsen G, Reda DJ. Clinical factors associated with long-term mortality following vascular surgery: outcomes from the Coronary Artery Revascularization Prophylaxis (CARP) Trial. J Vasc Surg. 2007;46:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation. 1995;91:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 335] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1957] [Cited by in RCA: 1859] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 9. | Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 744] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 10. | Criqui MH, Vargas V, Denenberg JO, Ho E, Allison M, Langer RD, Gamst A, Bundens WP, Fronek A. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1311] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 12. | Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1029] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 13. | Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 861] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 14. | Muntner P, Wildman RP, Reynolds K, Desalvo KB, Chen J, Fonseca V. Relationship between HbA1c level and peripheral arterial disease. Diabetes Care. 2005;28:1981-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Hertzer NR, Beven EG, Young JR, O’Hara PJ, Ruschhaupt WF, Graor RA, Dewolfe VG, Maljovec LC. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 912] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 16. | Mehler PS, Coll JR, Estacio R, Esler A, Schrier RW, Hiatt WR. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation. 2003;107:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol. 2007;50:1707-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 300] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | McFalls EO, Ward HB, Moritz TE, Apple FS, Goldman S, Pierpont G, Larsen GC, Hattler B, Shunk K, Littooy F. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J. 2008;29:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Eagle KA, Brundage BH, Chaitman BR, Ewy GA, Fleisher LA, Hertzer NR, Leppo JA, Ryan T, Schlant RC, Spencer WH. Guidelines for perioperative cardiovascular evaluation for noncardiac surgery: an abridged version of the report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Mayo Clin Proc. 1997;72:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Garcia S, Moritz TE, Ward HB, Pierpont G, Goldman S, Larsen GC, Littooy F, Krupski W, Thottapurathu L, Reda DJ. Usefulness of revascularization of patients with multivessel coronary artery disease before elective vascular surgery for abdominal aortic and peripheral occlusive disease. Am J Cardiol. 2008;102:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Boersma E, Poldermans D, Bax JJ, Steyerberg EW, Thomson IR, Banga JD, van De Ven LL, van Urk H, Roelandt JR. Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA. 2001;285:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 303] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2439] [Cited by in RCA: 2145] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 23. | Garcia S, Moritz TE, Goldman S, Littooy F, Pierpont G, Larsen GC, Reda DJ, Ward HB, McFalls EO. Perioperative complications after vascular surgery are predicted by the revised cardiac risk index but are not reduced in high-risk subsets with preoperative revascularization. Circ Cardiovasc Qual Outcomes. 2009;2:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, Pierpont G, Santilli S, Rapp J, Hattler B. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 782] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 25. | Poldermans D, Schouten O, Vidakovic R, Bax JJ, Thomson IR, Hoeks SE, Feringa HH, Dunkelgrün M, de Jaegere P, Maat A. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49:1763-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B. Universal definition of myocardial infarction. Circulation. 2007;116:2634-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1862] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 27. | Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, Leslie K, Rao-Melacini P, Chrolavicius S, Yang H. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 461] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 28. | Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 744] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 29. | Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, Xavier D, Chrolavicius S, Greenspan L, Pogue J. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839-1847. [PubMed] |
| 30. | Landesberg G, Mosseri M, Zahger D, Wolf Y, Perouansky M, Anner H, Drenger B, Hasin Y, Berlatzky Y, Weissman C. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37:1839-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Ellis SG, Hertzer NR, Young JR, Brener S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am J Cardiol. 1996;77:1126-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 85] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol. 1996;57:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 219] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol. 1999;8:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Martinez E, Kim L, Rosenfeld B, Faraday N, Bass E, Perler B, Williams GN, Dorman T, Pronovost P. Early detection and real-time intervention of postoperative myocardial ischemia: the STOPMI (Study for the Treatment of Perioperative Myocardial Ischemia) Study. Abstract presented at: Association of University Anesthesiologists; May 16-18 2008; Durham NC. |
| 35. | European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2620] [Cited by in RCA: 2419] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 36. | Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6011] [Cited by in RCA: 5642] [Article Influence: 225.7] [Reference Citation Analysis (0)] |
| 37. | Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39-III43. [PubMed] |
| 38. | Schouten O, Boersma E, Hoeks SE, Benner R, van Urk H, van Sambeek MR, Verhagen HJ, Khan NA, Dunkelgrun M, Bax JJ. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361:980-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Durazzo AE, Machado FS, Ikeoka DT, De Bernoche C, Monachini MC, Puech-Leão P, Caramelli B. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39:967-975; discussion 975-976. [PubMed] |
| 40. | Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1003] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 41. | Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 422] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 42. | Garcia S, Rector TS, Zakharova MY, Magras A, Sandoval Y. Cardiac Remote Ischemic Preconditioning Prior to Elective Major Vascular Surgery (CRIPES): Study Design and Rationale. J Clin Trials. 2013;S5:002. |
| 43. | Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O’Sullivan M. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 44. | Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 523] [Article Influence: 29.1] [Reference Citation Analysis (0)] |