Revised: November 23, 2013
Accepted: December 13, 2013
Published online: January 26, 2014
Processing time: 135 Days and 11.7 Hours
Several recent reports have described the occurrence of longitudinal stent deformation (LSD, defined as the distortion or shortening of a stent along the longitudinal axis), following its successful deployment. However, few reports have described LSD prior to any stent deployment. This previously unrecognized complication is the result of modifications to stent design. It has been noted that the new-generation stent platforms have a reduced number of connectors, which in turn causes a reduction in longitudinal stent strength. To corroborate previous findings by our lab and others (Vijayvergiya et al, 2013), we describe here two cases of LSD prior to stent deployment that occurred due to crushing of the proximal stent edge by the guide catheter while attempting to withdraw the crimped stent. In addition, we discuss the associated risk factors, such as the length of the stent, and specific management strategies, including technical guidelines and use of fluoroscopic guidance for maneuvering the stent during the procedure.
Core tip: We describe here two cases of longitudinal stent deformation before deployment. This report corroborates the findings previously made by us and others (Vijayvergiya et al, 2013) and emphasizes the risk of physical distortion of the stent prior to deployment. We also discuss specific risk factors of and management strategies for this unusual complication.
- Citation: Aminian A, Lalmand J, Dolatabadi D. Occurrence of longitudinal stent compression before stent deployment: Two case studies. World J Cardiol 2014; 6(1): 23-25
- URL: https://www.wjgnet.com/1949-8462/full/v6/i1/23.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i1.23
The case study published by Vijayvergiya et al[1] on longitudinal stent deformation (LSD) has been of great interest to us. The authors describe two cases of proximal LSD involving Promus Element stents that occurred before stent deployment. In both cases, the stent deformations were due to crushing of the proximal stent edge by the guide catheter that occurred while attempts were made to withdraw the crimped stent. Our group was the first to report a similar case involving a non-deployed 3.5 mm × 38 mm Taxus Element stent[2]. Since then, we have encountered two additional cases of LSD involving non-deployed stents.
The first case was a 69-year-old woman, who was required to undergo elective percutaneous coronary intervention (PCI) of the proximal and distal right coronary artery (RCA). Access was obtained via the right radial artery using a 6.5 Fr sheathless JR4 guide catheter (Asahi Intecc Co, Japan). Extensive guidewire-induced dissection of the RCA led to complications in the procedure. A 3.5 mm × 38 mm Multi-Link 8 stent (Abbott Vascular, United States) and a 3 mm × 38 mm Promus Element stent (Boston Scientific, United States) were placed in the proximal and mid-section of the RCA, respectively. Angiographic control showed the presence of residual dissection in the distal RCA. An attempt was made to place a 2.75 mm × 38 mm Promus Element stent in the distal RCA, but it failed to pass the mid-RCA. While withdrawing the crimped stent, deep engagement of the sheathless catheter occurred, and the proximal edge of the stent became blocked at the distal tip of the catheter. Further attempts to capture the crimped stent resulted in significant compression of the proximal stent edge. Hence, the operator decided to deploy the stent in the proximal RCA on a previously implanted stent. After performing serial high-pressure post-dilatation with a 3.75 mm non-compliant balloon and with the help of a guide catheter extension, a 3 mm × 28 mm Xience Prime stent (Abbott Vascular) was successfully deployed in the distal RCA. Notably, the patient recovered well and remained asymptomatic throughout the 20-mo clinical follow-up.
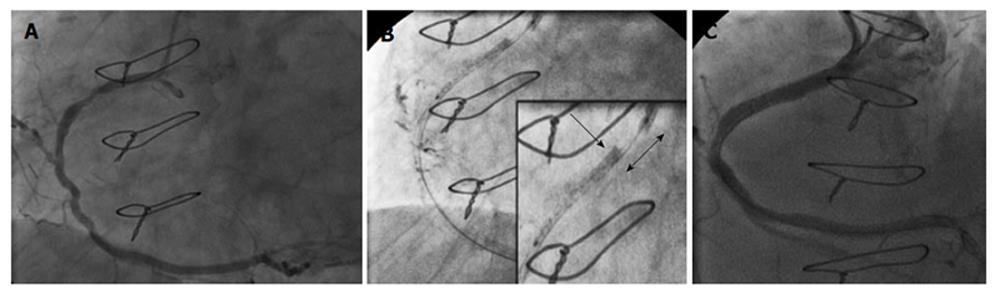
The second case was an 81-year-old woman, who was required to undergo elective PCI of the proximal and mid-RCA (Figure 1A). Access was obtained via the right femoral artery using a 6 Fr JR4 guide catheter. After performing serial predilatations with a 3 mm balloon, an attempt was made to place a 3.5 mm × 28 mm Multi-Link stent (Abbott Vascular) but it failed to pass the mid-lesion. While withdrawing the crimped stent, the guide catheter was pulled in and the proximal RCA was engaged. Concomitantly, the proximal edge of the stent was blocked at the distal tip of the guide catheter. After several attempts were made to pull the crimped stent into the guide catheter, it became angiographically evident that the guide catheter had crushed the proximal edge of the stent while the supporting balloon had partially entered the guiding catheter. As a result, the stent was shortened in length by approximately 8-10 mm before being deployed and had an accordioned aspect at the proximal stent edge (Figure 1B). It was decided to deploy the stent in the proximal RCA and to cover the mid-section with a second stent. Post-dilation was performed at high-pressure with a 3.5 mm non-compliant balloon and a 3 mm × 26 mm Integrity stent (Medtronic, United States) was placed on the residual mid-lesion. The final angiographic result was deemed acceptable (Figure 1C). The patient recovered well and remained asymptomatic throughout the 15-mo clinical follow-up.
Mamas et al[3] defined LSD as the distortion or shortening of a stent along the longitudinal axis following its successful deployment. We have reviewed the collective findings from the two cases reported by Vijayvergiya et al[1] and the three cases from our center, where LSD occurred prior to stent deployment. In all cases, the crushing of the proximal stent edge by the guide catheter during withdrawal of the balloon-stent system caused the LSD. A second important observation is that stent deformation led to an inability to recapture the crimped stent into the guide catheter and severely limited balloon-stent maneuverability. This difficulty arose in certain complex cases, mainly involving the Element Platform (4 out of 5 cases) and the Multi-Link Platform (1 case). Since all five cases utilized a long stent (38 mm long in 4 cases, and 28 mm long in 1 case), stent length could be an important risk factor in causing this unusual complication. Fluoroscopic images showed a clear separation between the proximal balloon marker and the proximal stent edge in all cases, making the diagnosis quick and conclusive. This case series confirms previous findings and highlights the risk of occurrence of LSD prior to stent deployment. The mechanism of the mishap reported here seems consistent with previous reports and involves a reduction in longitudinal stent strength frequently noted with newer stent-platforms.
Based on these reports, we recommend that in cases where withdrawal of a non-deployed new-generation stent into the guide catheter is difficult, the stent should be maneuvered carefully under fluoroscopic guidance. It is imperative to keep the guide catheter and the crimped stent in parallel axes. If resistance persists, advancement and careful rotation of the crimped stent could be attempted before its removal. When proximal stent deformation is visible or if a gap appears between the proximal balloon marker and the proximal stent edge, we strongly advocate avoiding forceful removal of the crimped stent, as it could worsen the stent deformation. In such cases, the operator has the option to either inflate the stent in another non-consequential location, or to pull back the stent and the guide catheter together. However, the latter strategy risks either loss of the coronary guidewire, which could be inconvenient in specific settings, such as dissections, or the previously reported difficulty in guidewire crossing through lesions. When choosing to deploy the deformed stent, efforts should be made to post-dilate the stent to avoid under-expansion and malapposition, which can lead to stent restenosis and thrombosis. We also recommend avoiding, as much as possible, deep intubation of the guide catheter during stent withdrawal, although sometimes this is unavoidable.
P- Reviewers: Chiu CC, Teng RJ S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Vijayvergiya R, Kumar A, Shrivastava S, Kamana NK. Longitudinal stent compression of everolimus-eluting stent: A report of 2 cases. World J Cardiol. 2013;5:313-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Aminian A, Lalmand J. Major longitudinal deformation of a new-generation drug-eluting stent during withdrawal into the guide catheter. J Invasive Cardiol. 2012;24:E318-E320. [PubMed] |
| 3. | Mamas MA, Williams PD. Longitudinal stent deformation: insights on mechanisms, treatments and outcomes from the Food and Drug Administration Manufacturer and User Facility Device Experience database. EuroIntervention. 2012;8:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |