INTRODUCTION
The estimated prevalence of permanent left bundle branch block (LBBB) in the general population is 1.5%[1], 0.43% for men and 0.28% for women of middle age[2]. Exercise-induced transient LBBB (EI-LBBB) is infrequent and occurs in 0.4%-0.5% of patients undergoing exercise tolerance tests (ETT)[3-5]. Grady et al[5] have shown that transient EI-LBBB is an independent predictor for major cardiovascular morbidity and mortality. A longitudinal study demonstrated that coronary artery disease and heart failure were more prevalent in patients with EI-LBBB[4]. The mechanism of transient EI-LBBB remains unclear, it may reflect concomitant valvular heart disease, cardiomyopathy, congenital heart disease, conduction abnormalities or coronary artery disease (CAD). Generally, permanent LBBB may be associated with a deterioration of left ventricular function, mechanical dyssynchrony and heart failure[6]. Furthermore, it has also been reported that transient EI-LBBB may result in reversible left ventricular dyssynchrony[7].
In some patients, EI-LBBB may be the first manifestation of diffuse heart disease and its presence is associated with a poorer prognosis compared to normal intraventricular conduction and right bundle branch block (RBBB) without concomitant cardiac disorders[8]. We present two adult patients with exercise-induced LBBB in the presence or absence of CAD and an attempt is made to review the international literature.
CASE REPORT
Case 1
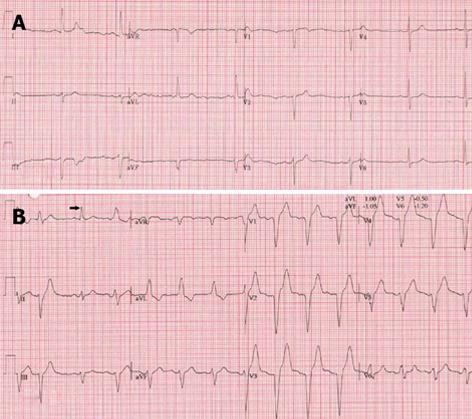
An 80-year-old male with well treated hypertension and paroxysmal atrial fibrillation was evaluated for chest pain exaggerated by physical stress with a good reaction to sublingual nitroglycerin. On examination his body mass index (BMI) was 29 kg/m2, rest blood pressure was 129/81 mmHg and rest heart rate of 60 bpm. Neither cardiac murmur or signs of congestive heart failure were present. The rest of the examination was normal. Resting electrocardiogram (ECG) depicted sinus rhythm (SR) with left axis deviation and slow progression of R-waves in V1-4 (Figure 1A). Transthoracic echocardiography (TTE) revealed a normal left ventricle ejection fraction (LVEF) of 0.64, LV hypertrophy, inferior wall hypokinesia, biatrial dilatation, mild mitral and tricuspid regurgitation with normal estimated pulmonary artery pressure of 20 mmHg. On routine ETT 120% of target exercise tolerance was reached and 87% of the maximal heart rate. He developed EI-LBBB at frequency of 80 bpm (Figure 1B), which lasted, through the third minute of the recovery period, till the end of the test at a heart rate of 83 bpm. At coronary angiography, significant stenoses were found in the left anterior descending and circumflex coronary arteries. Percutaneous coronary intervention was performed and both lesions were dilated with placement of DES. Accordingly a drug therapy was started composed of aspirin for 1 mo, clopidogrel for 1 year and oral anticoagulant, lipid lowering drug, hydrochloorthiazide and irbesartan as a maintenance drug regimen.
Figure 1 Resting electrocardiogram demonstrating sinus rhythm at 48 bpm with slow progression of R wave in the right precordial leads V1-V4 without delayed conduction (A) and during exercise tolerance testing at a heart rate of 80 bpm a left bundle branch block occurred which persisted into the recovery period (B).
Case 2
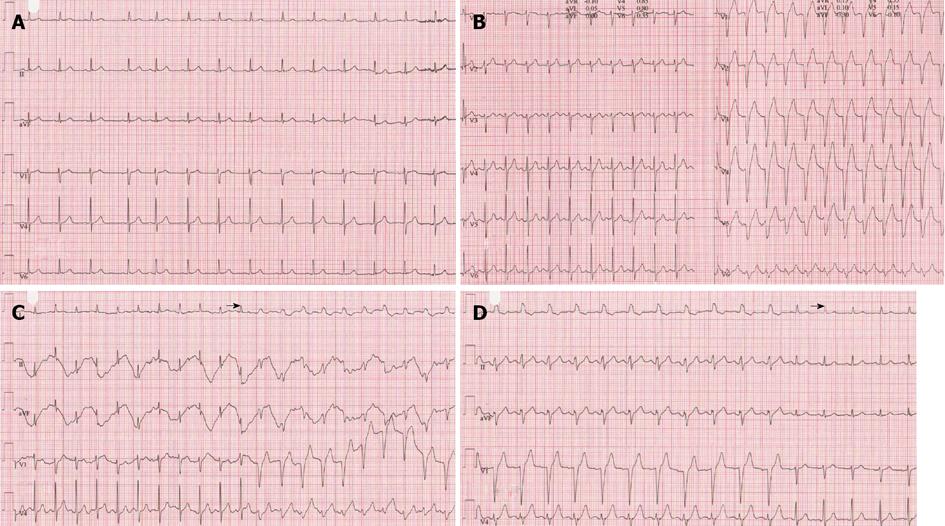
A 58-year-old female with known subclinical hypothyroidism without risk factors for CAD was analyzed for exercise-related oppressive chest pain. She had no previous history of cardiovascular disease. On physical examination, BMI was 21 kg/m2, rest blood pressure of 117/70 mmHg and rest heart rate regular of 85 bpm, central venous pressure was not elevated and further physical examination was otherwise unremarkable. Resting ECG (Figure 2A) revealed SR with normal findings and biochemical values, all were within normal limits. Findings of TTE were all normal. On echocardiography no intraventricular dyssynchrony was observed as TTE was performed at rest during normal conduction. The LVEF was 0.65. Nuclear stress myocardial perfusion studies revealed no reversible or irreversible defects, but EI-LBBB developed during the exercise phase at a heart rate of 141 bpm (Figure 2B) and a QRS width of 138 ms which was accompanied with chest discomfort. EI-LBBB disappeared at a frequency of 96 bpm. Beta blocker was initiated and on repeated bicycle ergometer test performed one month later, EI-LBBB accompanied with chest pain occurred at a slower heart rate of 129 bpm (Figure 2C) which recovered at a rate of 100 bpm (Figure 2D). Due to persistence of the chest complaints and occurrence of EI-LBBB during myocardial perfusion test despite normal findings (resting ECG and failure to demonstrate any reversible/irreversible defects in the nuclear stress myocardial perfusion), a decision was made to perform CAG which revealed normal coronary arterial tree. The β-blocker therapy was discontinued. An explanation for the failure of beta-blocker to abolish EI-LBBB may be related to inappropriate dose of the drug used for an “unknown” substrate.
Figure 2 Resting electrocardiogram depicting normal sinus rhythm at a rate of 85 bpm with normal conduction (A), during exercise tolerance testing normal conduction till a frequency of 129 bpm (left panel) and at a heart rate of 141 bpm (right panel) a left bundle branch block occurred which recovered at a frequency of 96 bpm (not shown) (B), on repeat exercise tolerance tests, under β-blocker therapy, exercise-induced left bundle branch block occurred at a heart rate of 129 bpm (C) and disappeared at a frequency of 100 bpm (D).
Black arrows indicate the transition from normal to abnormal conduction and vice versa.
Definitions
Maximal heart rate: Was calculated as (220 - age), predicted maximal heart rate and directly measured maximal heart rate from the ECG.
Target heart rate: About 85% of the maximal heart rate, based on age, gender and length. Exercise tolerance test (ETT): were performed on a bicycle ergometer in accordance with the guidelines for exercise testing[9]. All exercise tests were assessed by a cardiologist, specialized nurse and/or a nurse practitioner. Exercise test end points were defined by the following: (1) Positive: ECG evidence of myocardial ischemia, ≥ 1.0 mm horizontal shift of the ST segment at 80 ms after the J point in comparison with the baseline ECG and/or a 30 mmHg decrease in systolic blood pressure and/or ventricular arrhythmia and/or typical limiting anginal complaints; (2) Negative: in the absence of any of the above cited criteria; (3) Intermediate: < 1.0 mm ST segment depression as compared to baseline ECG and/or non-specific anignal complaints in the absence of ECG evidence of ischemia; and (4) Non-interpretable: if less than 85% of the target heart rate was reached and absence of the criteria of a positive test.
Left bundle branch block: The diagnosis of complete LBBB was made from the 12-lead ECG if all the following criteria were accordingly met: (1) a QRS duration ≥ 120 ms, (2) predominantly upright complexes with broad-slurred R waves in leads I and V6, (3) a QS or rS pattern in V1 and (4) absence of q wave in leads I or V6.
DISCUSSION
Intermittent bundle branch block was first described by Lewis in 1913[10]. Exercise-induced LBBB associated with angina pectoris in the presence of normal coronary arteries is infrequent but a well known clinical entity and has been reported earlier by Vieweg et al[11] in 1976. It has been found in association with underlying structural heart disease[12], slow arterial coronary flow[13], coronary spasm[14] and also in subjects without heart disease[15]. Virtanen et al[16] defined the chest complaints as atypical characterized by sudden onset, starting simultaneously with the appearance of EI-LBBB, not radiating and associated with palpitation and walk through phenomenon. EI-LBBB occurs when the frequency reaches or exceeds the refractory period of one of the bundles. Both transient RBBB and LBBB may be induced during exercise tolerance test. On follow-up, significant CAD was detected in all patients with EI-RBBB and in only 70% of patients with EI-LBBB[3]. EI-LBBB is more prevalent (74%) than RBBB (26%), with a heart rate at onset varying from 74 to 170 bpm and associated with high prevalence of significant CAD (70%)[3]. In the first patient, EI-LBBB ensued at a heart rate of 94 bpm, later coronary angiography showed multi-vessel disease requiring percutaneous intervention which was successfully performed. In our second patient, EI-LBBB accompanied with chest pain started at a frequency of 141 bpm with a QRS width of 138 ms and following treatment with B-blocker, EI-LBBB occurred at a heart rate of 129 bpm without alteration of QRS width. An explanation for the failure of beta-blocker to abolish EI-LBBB may be related to inappropriate dose of the drug used for an “unknown” substrate.
In a study by Schultz et al[17] in 1986, in 4 patients with EI-LBBB compared with normal controls, significant differences between the left and the right ventricle were observed in a mean phase imaging. When typical angina was present in association with EI-LBBB, abnormal myocardial perfusion scans were significantly frequent (68%) vs (25%) of atypical chest pain[18]. In our second case, no abnormalities were depicted on stress myocardial perfusion test. Mechanism of rate-dependent-LBBB has been delineated by Neuss et al[19] in 1974, they postulated that rate-dependent-LBBB during rapid atrial stimulation is due to increased recovery time of the involved bundle branch, failure of anticipated shortening of action potential with increasing heart rate and postdrive depression of conductivity. Another possible mechanism for EI-LBBB, is the presence of microcirculatory ischemia undetectable by coronary angiography as proposed by Loubeyre et al[20]. Furthermore, paradoxical septal motion may be responsible for the chest discomfort in EI-LBBB [16].
In 2011, Tanaka et al[7] demonstrated in a case report that EI-LBBB occurring at a heart rate of 100 bpm during treadmill exercise testing is associated with significant left ventricular intraventricular mechanical dyssynchrony, confirmed by speckle tracking radial time strain which has resolved after pharmacological intervention with β-blocker and angiotensin II antagonist.
On the other hand, transient LBBB at a heart frequency of 100 bpm on a “resting” ECG and echocardiographic confirmation of interventricular dyssynchrony (80 ms difference in aortic and pulmonary preejection time) have been described in a patient with flecainide self intoxication, both have resolved after discontinuation of the drug[21]. None of our patients was on flecainide therapy.
The prognostic significance of EI-LBBB has been variably reported[22]. Grady et al[5] have shown that EI-LBBB independently predicts a higher risk of death (29%) and major cardiac events (19%) compared with matched control group, (25%) and (10%), respectively. The total event rate was 76% for EI-LBBB (28/37) group versus 24% for the control group (9/37). In the study of Candell Riera et al[15], they found that the prognosis of patients with EI-LBBB associated with chest pain and normal coronary arteries is favorable but on follow-up permanent LBBB developed (5 out of 8 patients) and atrioventricular block rarely occurred requiring pacemaker implantation (1 out of 8 patients)[15]. Hertzeanu et al[22] suggested that heart rate at which EI-LBBB is a prognostic factor, i.e., the onset of EI-LBBB at a heart rate of ≤ 120-125 bpm correlated with the occurrence of occlusive CAD, as was the case in our first patient, whereas subjects who develop EI-LBBB at a heart rate of ≥ 120-125 bpm have a normal coronary arterial tree and a better prognosis which was demonstrated in our second patient. However, when coronary artery disease is the cause of asymptomatic (silent ischemia) EI-LBBB occurring even at a heart rate of 188 bpm, the overall prognosis tends to be poor[12]. It has been suggested that the onset of EI-LBBB at a heart rate of ≥ 125 bpm is highly correlated with the presence of normal coronary arteries[23], but in some cases it has been observed to occur at a lower heart rate of 105 bpm[24]. Our second patient developed EI-LBBB at heart rate of 141 bpm without β-blocker and at 129 bpm under β-blocker therapy without evident significant CAD.
The importance of history taking regarding the nature of presenting chest pain, typical or atypical has been elaborated by Vasey et al[23]; CAD was present in EI-LBBB with classic angina but otherwise absent when atypical chest pain was prevalent. In the eighties, in the study of Hardarson et al[2], no increase in mortality rate due to CAD or hypertension was observed among subjects with permanent LBBB. Recently, it has been shown in pharmacologically and invasively treated patients with permanent LBBB and high-risk myocardial perfusion SPECT, that cardiac deaths, mostly sudden, occurred in 18% of patients[25]. These findings renders the current treatment policy of such group of patients to be revisited.
Not only EI-LBBB may be associated with CAD in 70% of patients but also CAD had been documented in 100% of patients with EI-RBBB[3]. Recently, Bussink et al[26], delineated that newly acquired RBBB in adult population is associated with increased risk of myocardial infarction and pacemaker implantation but not with chronic heart failure, atrial fibrillation or chronic obstructive pulmonary disease. On the contrary to the findings of Breithardt[8], RBBB is found to be associated with increased cardiovascular risk and all-cause mortality in men and women. Thus even the development of RBBB in asymptomatic individuals should attract our attention for cardiovascular risk[26].