Published online May 26, 2013. doi: 10.4330/wjc.v5.i5.154
Revised: October 13, 2012
Accepted: January 31, 2013
Published online: May 26, 2013
We present the case of a 66 year old male who presented with dyspnea and reduced exercise tolerance. Echocardiography demonstrated impaired left ventricular (LV) function and restrictive diastolic function with pronounced concentric left ventricular hypertrophy (LVH) without a history of hypertension and no aortic valve stenosis. Differential diagnostics of concentric LVH are discussed in detail. In the current case, cardiac amyloidosis (AL) amyloidosis was diagnosed and confirmed by serum amyloid P (SAP) scintigraphy and abdominal fat aspiration biopsy. This case shows the rapid decline in clinical condition with progression of cardiac involvement of AL. As discussed in detail, cardiac involvement in AL-amyloidosis generally denotes a poor prognosis, regardless of the method of treatment.
- Citation: Brugts JJ, Houtgraaf J, Hazenberg BP, Kofflard MJ. Echocardiographic features of an atypical presentation of rapidly progressive cardiac amyloidosis. World J Cardiol 2013; 5(5): 154-156
- URL: https://www.wjgnet.com/1949-8462/full/v5/i5/154.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i5.154
Amyloidosis is a disease that is characterised by the extracellular deposition of proteinaceous material (amyloid). A distinction has to be made between the (rare) AM-amyloidosis and the more common AL-amyloidosis on which this report will focus.
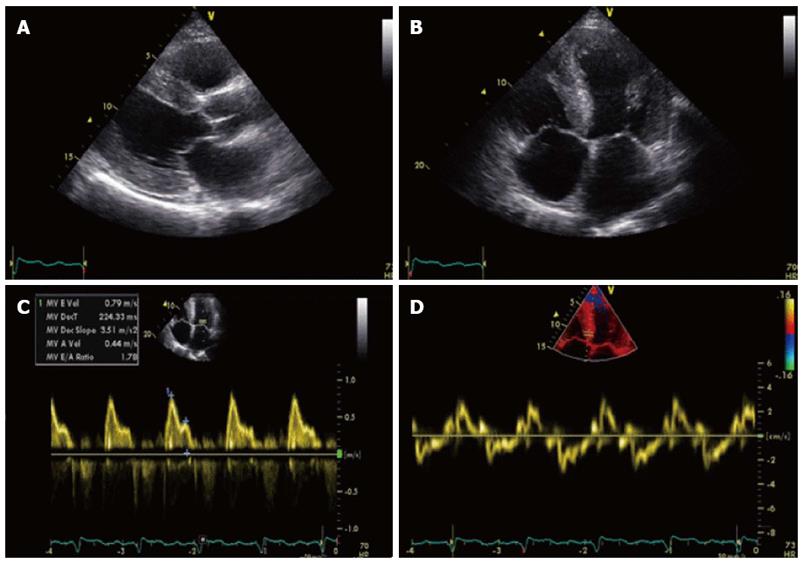
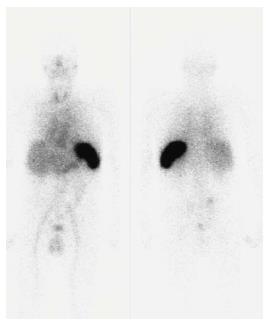
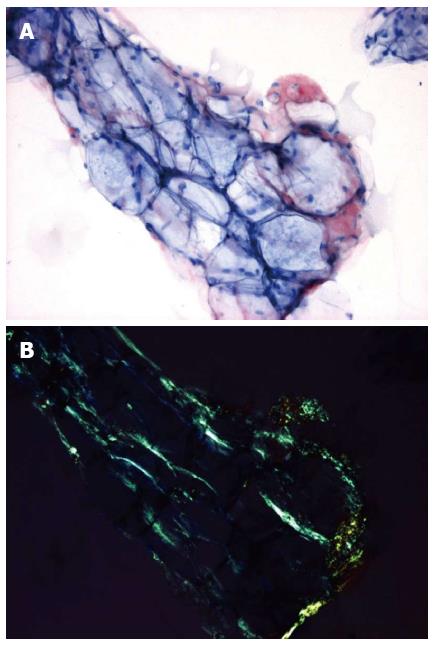
A 66-year old man was referred to our outpatient clinic for a second opinion because of slowly increasing shortness of breath on exertion, fatigue and reduced exercise tolerance over the previous year. His medical history included a non-ST segment elevation myocardial infarction with preserved left ventricular (LV) function and mild chronic obstructive pulmonary disease. Family history did not reveal any cardiovascular diseases or sudden cardiac death. On physical examination, blood pressure was 130/80 mmHg, a third heart sound was detected but there were no signs of heart failure. Electrocardiography showed microvoltages in the limb leads, a first degree atrio-ventricular block and Q-waves in the anterior and inferior wall leads. Laboratory tests revealed a ferriprive anaemia Hb 6.6; normal (N) = 8.5-11.0 mmol/L), elevated creatinine (150 μmol/L, N < 100 μmol/L), γ-glutamyltransferase (292 U/L, N < 35 E/L) and alkaline phosphatase (200 U/L, N < 120 E/L). Previous echocardiography 8 years before presentation demonstrated preserved LV function with ejection fraction (EF) of 64%, concentric LV hypertrophy with a width of the interventricular septum (IVS) and LV posterior wall of 18 and 12 mm, respectively. Diastolic function was normal (E/A ratio 0.80; Evel 0.49 m/s; Avel 0.61 m/s) with a normal right ventricular systolic pressure (RVSP). Subsequent echocardiograms demonstrated a progressive decline in EF, progressive diastolic dysfunction to grade II and pronounced concentric LV hypertrophy (LVH) without sparkling. During follow-up the patient remained asymptomatic until the year before his appearance at our centre. At presentation, echocardiography showed a moderately impaired LV function (EF 34%) with a sparkling IVS of 19 mm diameter. Diastolic dysfunction had worsened to grade III with E/A ratio of 1.8 [E-vel 0.80 A-vel 0.57; S’ 3.5 cm/s (N > 5 cm/s); E/E’ ratio 26.2 (N < 15)] with an increased RVSP of 41 mmHg with moderate tricuspid insufficiency (Figure 1). Values of S’ and E/E’ reflected the poor systolic function and raised filling pressures in our patient. The decline in ejection fraction and pronounced concentric LVH without a history of hypertension or aortic valve stenosis on echocardiography with new complaints of exertional dyspnea were reasons for further investigation to rule out or demonstrate other causes of concentric LVH such as amyloidosis, Fabry’s disease etc.[1,2]. Blood tests showed no para-proteinemia, but free light chains were found in urine (0.06 g/L) and serum samples. Based on the latter finding AL-amyloidosis was suspected[1,2]. This diagnosis was confirmed by serum amyloid P (SAP) scintigraphy (Figure 2) and abdominal fat aspiration biopsy (Figure 3). Bone biopsy revealed mild clonal plasma cell dyscrasia with excess of light chains and total plasma cells of 5 %. Cardiac magnetic resonance imaging (CMR) confirmed cardiac involvement with areas of fibrosis in the inferolateral wall[1,2]. Upon diagnosis, chemotherapy with Melfalan, Thalidomide and prednisolone was initiated according to the Palumbo-schedule[3,4]. Chemotherapy did not have any effect on the clinical condition and nine months after the diagnosis of cardiac amyloidosis, the patient died of heart failure.
In AL-amyloidosis the amyloid is produced by clonal light chains made by disrupted plasma cells (plasma cell dyscrasia). The extracellular deposition of AL-amyloid can occur in all tissues and organs, but predominates in heart, liver and kidney[3-5]. Cardiac involvement can vary from being absent to severe and is present in approximately 50% of cases. In half of these cases congestive heart failure (CHF) is the presenting symptom and when CHF is present, median survival is less than six months in untreated patients[3-5]. When the heart is involved, amyloid infiltration is generalised: ventricular and atrial myocardium, vasculature, conduction system and valves are equally affected. In 95% of patients with cardiac amyloidosis other organs or tissues are also affected, so signs or symptoms of extra-cardiac manifestations should not be ignored[3-5].
Electrocardiography usually shows low voltages in the limb leads and poor R wave progression in the precordial leads. Due to amyloid infiltration in the conduction system, several conduction disorders and arrhythmias can occur. Reduced myocardial relaxation is an early echocardiographic finding that usually progresses into restrictive patterns. There may also be left ventricular hypertrophy, granular sparkling, atrial dilation, valvular thickening and pericardial effusion[3-5].
The diagnosis of systemic amyloidosis can be confirmed by SAP scintigraphy and Congo red staining of abdominal fat aspiration biopsy[3-5]. Immunohistochemical staining determines the kind of protein from which the amyloid originates. When abdominal fat aspiration biopsy does not result in diagnosis, endomyocardial biopsy should be considered. The latter has a sensitivity of near 100%. Plasma cell dyscrasia in a bone marrow biopsy and free lambda or kappa (less common) light chains in serum and/or urine samples then confirm the diagnosis AL-amyloidosis[3-5].
This case shows the rapid decline in clinical condition with the progression of cardiac involvement in AL-amyloidosis[5]. Regardless of the method of treatment, cardiac involvement in AL-amyloidosis generally denotes a poor prognosis,. As in our patient, the median survival rate from the onset of symptoms of congestive heart failure is only 6 mo [6].
P- Reviewer Miyasaka Y S- Editor Cheng JX L- Editor Hughes D E- Editor Lu YJ
| 1. | Fernandez AB, Leitner J, Okolo J, Atalay MK, Goldstein L, Abbott JD. Value of cardiovascular magnetic resonance in suspected cardiac amyloidosis. J Cardiovasc Med (Hagerstown). 2012;13:590-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Innelli P, Galderisi M, Catalano L, Martorelli MC, Olibet M, Pardo M, Rotoli B, de Divitiis O. Detection of increased left ventricular filling pressure by pulsed tissue Doppler in cardiac amyloidosis. J Cardiovasc Med (Hagerstown). 2006;7:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007;50:2101-2110. [PubMed] |
| 4. | Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1:1331-1341. [PubMed] |
| 5. | Grogan M, Gertz MA, Kyle RA, Tajik AJ. Five or more years of survival in patients with primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2000;85:664-665, A11. [PubMed] |
| 6. | Rahman N, Toqeer M, Hawley I, Weston-Smith S, Whitehead MW, Rademaker JW, McWilliams E. Primary systemic amyloidosis presenting as idiopathic inflammatory colitis. BMJ Case Rep. 2011;2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |