Published online May 26, 2013. doi: 10.4330/wjc.v5.i5.151
Revised: April 3, 2013
Accepted: April 28, 2013
Published online: May 26, 2013
Processing time: 94 Days and 7.3 Hours
A 74-year-old man was admitted to the cardiac catheterization laboratory with acute myocardial infarction. After successful angioplasty and stent implantation into the right coronary artery, he developed cardiogenic shock the following day. Echocardiography showed ventricular septal rupture. Cardiac magnet resonance imaging (MRI) was performed on the critically ill patient and provided detailed information on size and localization of the ruptured septum by the use of fast MRI sequences. Moreover, the MRI revealed that the ventricular septal rupture was within the myocardial infarction area, which was substantially larger than the rupture. As the patient’s condition worsened, he was intubated and had intra-aortic balloon pump implanted, and extracorporeal membrane oxygenation was initiated. During the following days, the patient’s situation improved, and surgical correction of the ventricular septal defect could successfully be performed. To the best of our knowledge, this case report is the first description of postinfarction ventricular septal rupture by the use of cardiac MRI in an intensive care patient with cardiogenic shock and subsequent successful surgical repair.
Core tip: We report on the case of a 74-year-old man who developed cardiogenic shock and ventricular septal rupture following an episode of acute myocardial infarction. Cardiac magnet resonance imaging (MRI) provided detailed information on size, localization and tissue integrity of the ruptured septum with respect to the myocardial infarction zone, followed by successful surgical repair of the defect. To the best of our knowledge, this case report is the first description of post-infarction ventricular septal rupture by the use of cardiac MRI in an intensive care patient with cardiogenic shock and subsequent successful surgical repair.
- Citation: Gassenmaier T, Gorski A, Aleksic I, Deubner N, Weidemann F, Beer M. Impact of cardiac magnet resonance imaging on management of ventricular septal rupture after acute myocardial infarction. World J Cardiol 2013; 5(5): 151-153
- URL: https://www.wjgnet.com/1949-8462/full/v5/i5/151.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i5.151
Ventricular septal rupture after myocardial infarction is a rare complication but associated with a high mortality rate[1,2]. Cardiac magnetic resonance imaging (MRI) can provide detailed information on size and localization of the ruptured myocardium with respect to the myocardial infarction zone. We present a case of postinfarction ventricular septal rupture that was examined via cardiac MRI prior to surgical repair.
A 74-year-old man presented with a new episode of chest pain to his local general practitioner. Because the on-site electrocardiogram (ECG) showed ST-segment elevations, the patient was referred directly to our cardiac catheterization laboratory.
Physical examination showed hypotension but no dyspnea and no peripheral edema. The 12-lead ECG demonstrated sinus rhythm with a heart rate of 80/min, ST-segment elevation in the inferior leads (II, III, and aVF), and ST-segment depression in leads V3 to V5. Cardiac catheterization showed an occlusion of the right coronary artery, which was successfully recanalized by angioplasty and implantation of a bare metal stent. It was decided to postpone additional stenosis of the left main artery and the left circumflex artery for intervention in a later session. Afterwards, the patient was initially symptom-free with persistent hypotonic blood pressure. Echocardiography showed inferior wall akinesis with preserved left ventricular function and no aneurysm or ventricular septal defect.
The following day, the patient developed cardiogenic shock with supraventricular tachycardia. Echocardiography showed septal dyskinesia, a ventricular septal rupture basal inferoseptal of about 8 mm, and a left-to-right shunt of about 30%. Medical therapy included a daily dose of 100 mg aspirin, 75 mg clopidogrel, 40 mg simvastatin, and 2.5 mg fondaparinux. Norepinephrine was applied, adapted to the mean arterial blood pressure at a target pressure of 60-70 mmHg. Because the patient was stable at this mean arterial pressure of only 70 mmHg despite administration of norepinephrine and considering the high operative mortality, it was decided to first adopt a conservative approach and gain additional information in order to plan surgical repair.
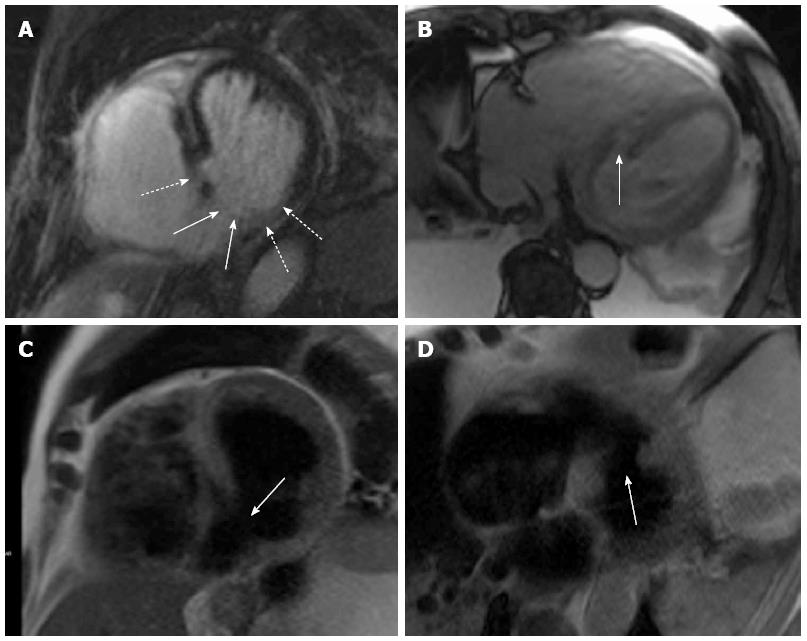
Therefore, the next day, a cardiac MRI in short axis and four-chamber view was performed on a MAGNETOM® Avanto 1.5 Tesla (Siemens AG Sector Healthcare, Erlangen, Germany). The main questions were size and localization of MI, and whether the rupture was located inside nonviable tissue or surrounded by viable tissue for surgical closure of the myocardial defect. The MRI was performed under emergency conditions and administration of analgesics and sedatives. Heart rate during MRI was 105 beats/min. The ventricular septal rupture first diagnosed by echocardiography was confirmed by cardiac MRI. Although the patient was under mild sedation, there was severe movement of the patient, making fast MRI sequences necessary. Therefore, a fast HASTE 2D for morphologic analysis, a fast SSFP LGE (7 heart beats), and a SSFP cine (not shown) were performed and allowed sufficient discrimination between scar, edema, and movement artifacts. Using these sequences, the rupture previously described by echocardiography was detected in the posterior septum with a defect size of about 2 cm and a surrounding wall edema with a diameter of about 4 cm. Late Gadolinium enhancement (LGE) imaging with PSIR-SSFP revealed an infarction area reaching from basal septal inferior to apical inferolateral, which, with a size of 3-4 cm, was substantially larger than the ventricular septal defect. Furthermore, it showed that the defect was within the infarction area (Figure 1).
As the patient’s condition worsened, he was intubated and had an intra-aortic balloon pump (IABP) implanted. Extracorporeal membrane oxygenation (ECMO) was initiated three days after the initial event, bridging the time until surgical repair in order to relieve secondary end organ failure, namely acute renal and liver failure.
During the following days, the patient’s situation improved, and surgery could be performed on day six after the onset of the myocardial infarction based on the results of cardiac MRI, knowing the extent of the septal defect, and the fact that viable tissue existed, making surgical repair of the defect possible.
Three target vessels were revascularized utilizing the left internal thoracic artery and saphenous vein grafts. The left ventricle was longitudinally opened posteriorly and parallel to the septum. The excision of the fragile infarction zone resulted in a large septum defect. The myocardial edges were stabilized with Teflon felts in sandwich technique, and the defect was covered with a Dacron patch reaching from the posterior mitral annulus to the left ventricular apex. The anterolateral papillary muscle had to be refixed. The intraoperative echo showed a competent mitral valve and no residual shunt.
The ECMO-support was continued until the first and the IABP-support until the fourth postoperative day. After prolonged weaning, the patient was eventually discharged to rehabilitation in subjective well-being almost two months after the initial event. He is alive and in NYHA class II six months after the operation.
In a patient with acute myocardial infarction, cardiac MRI was able to provide detailed information on size, localization, and tissue integrity of the ruptured septum with respect to the myocardial infarction zone.
Cardiac MRI has previously been utilized for characterization of ventricular septal defects, e.g., following chest trauma[3,4]. Nonetheless, none of these patients had been in a critical condition when cardiac MRI was performed. To the best of our knowledge, this case report is the first description of post-infarction ventricular septal rupture by the use of cardiac MRI in an intensive care patient with cardiogenic shock and subsequent successful surgical repair.
Interestingly, previous implantation of coronary bare-metal or drug-eluting stents is not a contraindication for cardiac MRI since various studies have confirmed the safety of both in MRI at 3 Tesla or less[5,6]. Operative mortality is about 54% if the surgical repair is performed within seven days from acute myocardial infarction[1]. Both timing and method of choice for correction of ventricular septal defect are still being debated[2], and percutaneous closure of ventricular septal defects is a new alternative to surgical repair[7]. However, to date, no studies have compared this new approach to surgical correction.
As postmyocardial infarction ventricular septal rupture is a severe and life-threatening complication, several limitations such as heart rate or circulatory stability exist for performing cardiac MRI in critically ill patients. Therefore, cardiac MRI may not be applicable in all patients, and its overall role in acute postinfarction ventricular septal defect can be considered marginal. However, when being performed, it can provide precise information on localization and size of the defect with respect to the myocardial infarction zone, which is of particular interest before surgical correction.
P- Reviewers Biondi-Zoccai GGL, Selvanayagam J S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Arnaoutakis GJ, Zhao Y, George TJ, Sciortino CM, McCarthy PM, Conte JV. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2012;94:436-443; discussion 443-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 2. | Moreyra AE, Huang MS, Wilson AC, Deng Y, Cosgrove NM, Kostis JB. Trends in incidence and mortality rates of ventricular septal rupture during acute myocardial infarction. Am J Cardiol. 2010;106:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Thuny F, Jacquier A, Riberi A, Avierinos JF, Renard S, Collart F, Luanika X, Bartoli JM, Métras D, Habib G. Images in cardiovascular medicine. Ventricular septal rupture after a nonpenetrating chest trauma: findings from real-time three-dimensional echocardiography and cardiac magnetic resonance. Circulation. 2005;112:e339-e340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Smíd M, Ferda J, Zlocha V. Post-traumatic ventricular septal defect. Eur Heart J. 2008;29:575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Sommer T, Maintz D, Schmiedel A, Hackenbroch M, Hofer U, Urbach H, Pavlidis C, Träber F, Schild H, Höher M. [High field MR imaging: magnetic field interactions of aneurysm clips, coronary artery stents and iliac artery stents with a 3.0 Tesla MR system]. Rofo. 2004;176:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Shellock FG, Forder JR. Drug eluting coronary stent: in vitro evaluation of magnet resonance safety at 3 Tesla. J Cardiovasc Magn Reson. 2005;7:415-419. [PubMed] |
| 7. | Maltais S, Ibrahim R, Basmadjian AJ, Carrier M, Bouchard D, Cartier R, Demers P, Ladouceur M, Pellerin M, Perrault LP. Postinfarction ventricular septal defects: towards a new treatment algorithm? Ann Thorac Surg. 2009;87:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |