Revised: March 25, 2013
Accepted: March 28, 2013
Published online: April 26, 2013
Processing time: 108 Days and 19.6 Hours
The sphingolipid metabolites ceramide, sphingosine, and sphingosine-1-phosphate (S1P) and its enzyme sphingosine kinase (SphK) play an important role in the regulation of cell proliferation, survival, inflammation, and cell death. Ceramide and sphingosine usually inhibit proliferation and promote apoptosis, while its metabolite S1P phosphorylated by SphK stimulates growth and suppresses apoptosis. Because these metabolites are interconvertible, it has been proposed that it is not the absolute amounts of these metabolites but rather their relative levels that determine cell fate. The relevance of this “sphingolipid rheostat” and its role in regulating cell fate has been borne out by work in many labs using many different cell types and experimental manipulations. A central finding of these studies is that SphK is a critical regulator of the sphingolipid rheostat, as it not only produces the pro-growth, anti-apoptotic messenger S1P, but also decreases levels of pro-apoptotic ceramide and sphingosine. Activation of bioactive sphingolipid S1P signaling has emerged as a critical protective pathway in response to acute ischemic injury in both cardiac and cerebrovascular disease, and these observations have considerable relevance for future potential therapeutic targets.
Core tip: The sphingolipid pathway has received considerable attention recently, because its active metabolites appear to have salutary effects on cytoprotection in experimental cardiac and cerebral ischemia. Both inhibitors and antagonists of the sphingolipid sphingosine-1-phosphate (S1P) pathway appear to limit ischemic injury through a variety of mechanisms. Because of the clinical availability of Fingolimod (FTY720), a S1P analog, for use in multiple sclerosis, preclinical and clinical studies should focus on the development of this and similar pharmaceuticals for a new indication.
- Citation: Kawabori M, Kacimi R, Karliner JS, Yenari MA. Sphingolipids in cardiovascular and cerebrovascular systems: Pathological implications and potential therapeutic targets. World J Cardiol 2013; 5(4): 75-86
- URL: https://www.wjgnet.com/1949-8462/full/v5/i4/75.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i4.75
Sphingolipids were first described in late 19th century and have long been viewed as merely ubiquitous components of the cell membrane. Recently, sphingolipids have been increasingly revaluated because they are now recognized to not only regulate vital cell functions, but also form cell membrane microdomain “lipid rafts” for integrating cell signaling[1,2]. Sphingolipids are formed via the metabolism of sphingomyelin, a ubiquitous constituent of the plasma membrane, or by de novo synthesis. Enzymatic pathways result in the formation of several different lipid mediators such as ceramide, sphingosine, and sphingosine-1-phosphate (S1P). Several studies now showed that these sphingolipid mediators and their enzymes, especially sphingosine kinase (SphK), are likely to have an integral role in different cell processes including proliferation, inflammation, apoptosis and migration. The mode of action of each sphingolipid is different. A significant body of research now indicates that sphingolipids are intimately involved in disease progression and that these lipids, together with associated enzymes and receptors, can provide effective drug targets for the treatment of pathological states. This review will highlight the current knowledge of research where sphingolipids are involved with focus on cardiovascular and cerebrovascular disease, and the mechanisms of action of each sphingolipid mediator. In addition, the therapeutic potential of drugs that alter sphingolipid actions with focus on SphK/S1P signaling pathway that appears to be a target of interest for therapeutic manipulation.
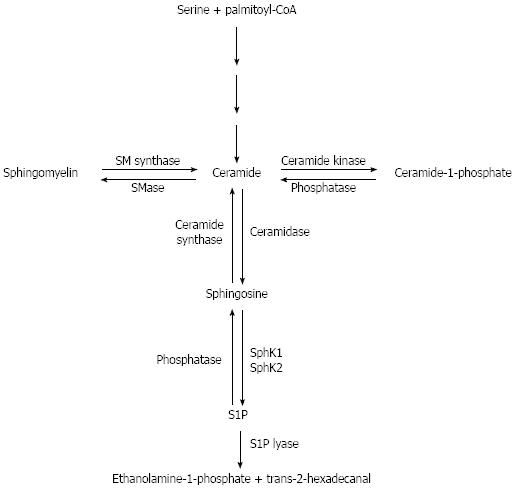
Sphingolipids are complex lipids comprised of a sphingoid base, and are one of the major lipid components of cell membrane as well as glycerophospholipid and cholesterol. A schematic diagram of sphingolipid metabolism is depicted in Figure 1. De novo biosynthesis of sphingolipids begins with the conversion of serine and palmitoyl-CoA into 3-ketosphinganine. 3-ketosphinganine is then converted to dihydrosphingosine. Dihydroceramide synthase acrylates dihydrosphingosine to form dihydroceramide, which is then reduced to ceramide by dihydroceramide desaturase. Sphingomyelin can also be converted to ceramide by sphingomyelin synthase, and a reverse reaction is catalyzed by sphingomyelinase. Ceramide can also be degraded by ceramidase to form sphingosine, which can, in turn, be phosphorylated to S1P by two enzymes SphK1 or 2. The bioactive lipid ceramide-1-phosphate (C1P) is formed by phosphorylation of ceramide by ceramide kinase; it can also be reverted to ceramide by ceramide phosphatase. The reverse reaction from S1P is catalyzed by sphingosine-1-phophate phosphatases and ceramide synthase that yield sphingosine and ceramide respectively[3]. S1P can be further metabolized by S1P lyase, yielding hexadecenal and ethanolamine phosphate[4].
Ceramides are a family of lipids that consist of sphingosine covalently linked to a fatty acid and are densely located at the cell membrane. Ceramides are the key component lipids that constitute sphingomyelin, the major source of the human sphingolipids, and one of the major components which form the phospholipid bilayer[5]. Discovery over the last few decades reveal that all stress stimuli, such as inflammatory mediators, heat, ultraviolet radiation, hypoxia, chemotherapeutics, and oxidative stress increase ceramide production as part of an evolutionarily conserved cellular response[6-13] and toll like receptor 4 seems to be involved in ceramide synthesis[14]. Consecutively ceramides not only promote cell cycle arrest and promote apoptosis, a form of programmed cell death, but also play an important role in the regulation of autophagy, cell differentiation, and inflammatory responses[9,15-18]. Ceramide is also involved in dephosphorylation and inactivation of one major mediator of cell survival; protein kinase Akt, (Akt/PKB)[19-21]. On the other hand, recent data shows that phosphorylated ceramide, C1P seems to have the opposite effects from ceramide; by inducing prosurvival functions, such as cell growth and survival, control of inflammation and mediation of macrophage migration[22-24].
Sphingosine is also a bioactive sphingolipid formed from ceramide as a result of ceramidase activity. It was first described as the physiological inhibitor of the survival signal protein kinase C (PKC), and was also found to up-regulate caspase 3 in the cascade of apoptosis[25-28]. There are many reports showing that PKC is inhibited by exogenous sphingosine, and it has been demonstrated that endogenously generated sphingosine is a potent PKC inhibitor[29]. In turn, sphingosine can control the activity of other key enzymes involved in the regulation of metabolic or cell signaling pathways such as the Mg2+ dependent form of phosphatidate phosphohydrolase[30,31], phospholipase D (PLD)[32], or diacylglycerol kinase[33,34]. Although there is abundant evidence that sphingosine is toxic to cells[25,28], diverse function by concentration dependence of sphingosine has been reported. Vessey et al[35] recently reported that at lower dose (submicromolar), a more physiologic concentrations, sphingosine has been shown to be cardioprotective in isolated Langendorff-perfused rat hearts subjected to ischemia/reperfusion injury. Unlike S1P, sphingosine-induced cardioprotection seems to be mediated by cyclic nucleotide-dependent protein kinase A and G (PKA and PKG) pathways[35]. While, at the higher concentrations usually employed, sphingosine is toxic to cells[27].
S1P is a bioactive lipid signaling molecule formed when either one of two isoforms of the enzyme SphK1 or 2 catalyzes the addition of a phosphate group to sphingosine. S1P exerts a wide variety of biological activities in many eukaryotic cell types[36-38]. It was initially proposed to act as an intracellular second messenger, based on the ability of extracellular growth factors to activate SphK and increase intracellular S1P levels. The discovery and cloning of five G protein-coupled receptors (S1P1, S1P2, S1P3, S1P4, S1P5) expressed on the cell membrane has stimulated the notion that S1P is an extracellular signaling ligand, regulating a host of cellular functions such as proliferation, survival, immunomodulation, apoptosis, migration, cytoskeletal organization, and differentiation/morphogenesis[39]. Basal plasma and serum concentration levels of S1P are generally low ranging within 200-900 nmol/L, but can increase rapidly and transiently when cells are exposed to various agonists[40,41]. The concentration of S1P is controlled by two enzyme, SphK and S1P lyase. While SphK activity can be upregulated by a variety of growth factors, S1P lyase activity in other hand is constantly at the high level, and this makes the intracellular S1P level very low in most tissues. However, erythrocytes and platelets have low S1P lyase activity resulting in high S1P concentration in blood plasma[38,42]. This concentration gradient is presumed to provide the basis for the integral role for the bioactivity of S1P involved in lymphocyte trafficking[43].
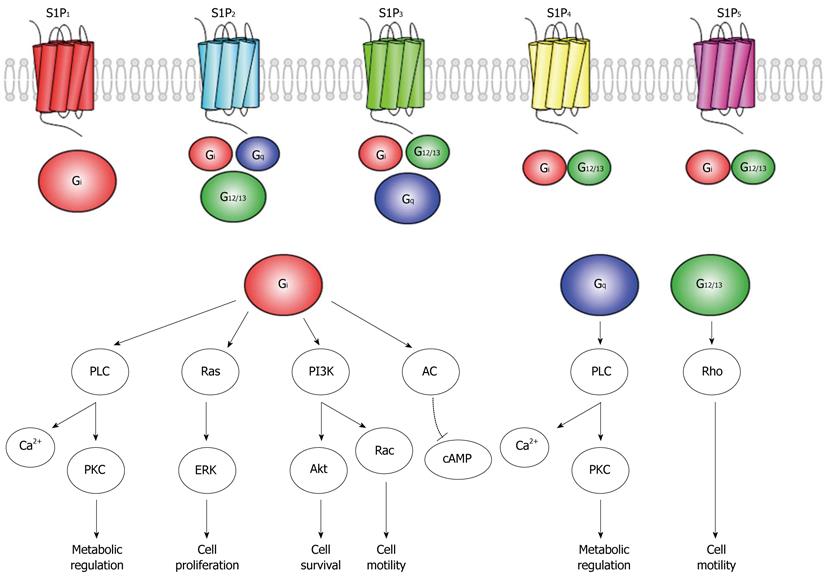
After the discovery of S1P receptors, there has been extensive work aimed at understanding the role of S1P as extracellular ligands. A schematic of the S1P receptors are shown in Figure 2. S1P mediates its effects through binding to G protein-coupled receptors (S1P1-5) which activates a variety of signaling via transduction of G proteins isoforms (Gs, Gi, Gq, and G12/13). The prosurvival phosphatidylinositol-3-kinase (PI3K)/Akt have been shown to be downstream molecules regulated by the S1P1 receptor signaling, Akt activation is a principal factor in the prevention of apoptosis[44,45]. S1P also stimulates cell growth and proliferation via activation of mitogen-activated protein kinase extracellular signal-regulated kinases (ERK)[46]. It is believed that elevated ERK phosphorylation plays a role in cell survival and proliferation in the penumbra, and ERK activity may block apoptosis by enhancing the level of the antiapoptotic protein Bcl-2 through cAMP responsive element binding protein activation[44]. S1P is also assumed to prevent necrosis mediated by the PKCε pathway[47].
The synthesis of S1P is catalyzed by SphK which is responsible for linking a phosphate group to sphingosine. There are two isozymes of SphK designated as SphK1 and SphK2. SphK1 and SphK2 show different subcellular localizations and enzymatic properties as well as different expression in various tissues. Mouse and human SphK1 exhibit substantial homology and SphK2 is highly homologous to SphK1 except for 240 additional amino acids located at the N terminus and in the center of the enzyme. The genes encoding these isozymes are localized on different chromosomes[48]. Genetic deletion of both isozymes results in fetal death from severe bleeding, inadequate vasculogenesis, and incomplete neural tube closure[48,49]. In contrast, mice null for either the SphK1 or the SphK2 isozyme exhibit normal development and are otherwise unremarkable in the basal state[49]. It is presumed that these isozymes have the complementary functions. The regulation of SphK activity is complex. It is stimulated by G-protein coupled receptor agonists (muscarinic receptor agonists[50], formyl peptide[51], nucleotides, bradykinin[52], lysophosphatidic acid[53], and S1P[54]), agonists at receptor tyrosine kinases (platelet-derived growth factor[55], endothelial growth factor[56], nerve growth factor[57], fibroblast growth factor[57], vascular endothelial growth factor[58]), immunoglobulin receptor crosslinking[59], monoganglioside (GM1)[60], estrogen[61] and activators of PKCε[62]. Both TNF-α and phorbol ester, which stimulates PKC, phosphorylate and thus activate SphK1 at serine225 mediated by ERK1/2[63,64]. The tumor necrosis factor (TNF) α response requires binding by TNF receptor-associated factor-2 (TRAF2). Other interacting proteins that stimulate SphK include delta-catenin/NPRAP (neural plakophilin-related armadillo repeat protein), aminoacylase 1, and eukaryotic elongation factor 1A (EEFA1)[65]. Reported inhibitory interacting proteins are SKIP (SK1-interacting protein), PECAM-1 (platelet endothelial adhesion molecule-1), and FHL-2 suppressed VEGF-induced PI-3 kinase/Akt activation via interactions with SphK1[66-69].
Despite the structural similarities and even though it catalyses the production of S1P, SphK2 has shown to have opposing actions to SphK1[70]. Thus, SphK2 inhibits cell growth and enhances apoptosis, in part by regulating ceramide levels[70]. Downregulation of SphK2 reduced conversion of sphingosine to ceramide, while downregulation of SphK1 increased it. The pathway of sphingosine into pro-apoptotic ceramide is dependent on SphK2, but not SphK1, acting in concert with S1P phosphohydrolase 1[71]. And also interestingly, there are organ specific deviations of SphK. While SphK1 is the more abundant isozyme in lung, spleen, kidney, heart, renal proximal tubules and cardiomyocytes, SphK2 isozyme predominates in the brain[72-74]. The intracellular locations of two enzymes are also different. SphK1 is localized predominantly in the cytoplasm while, SphK2 is mainly localized in the nucleus[75]. The reason of this deviation difference is that SphK1 has two functional nuclear export signal (NES) sequences which positively direct SphK1 to an extranuclear location. On the other hand, a nuclear localization signal (NLS) sequence has been found in SphK2 which keeps SphK2 in the nucleus and NES shuttles SphK2 between the cytoplasm and the nucleus according to demand[75]. The underlying reasons for this and its functions have yet to be elucidated.
The cardioprotective effect of S1P was first reported in 2001[76]. In neonatal rat cardiac myocytes, exogenously applied S1P enhanced cardiac myocyte survival during hypoxia[76]. Subsequent studies were undertaken using cultured adult mouse cardiac myocytes subjected to hypoxia in vitro that mimics ischemia in vivo during coronary artery occlusion. This system permitted measurements of S1P effects on myocyte viability during stress and activation of cell signaling from plasma membrane to mitochondria. There were three major findings that advanced understanding of S1P prosurvival effects during hypoxia[77]. First, it was found that S1P1 receptors are abundantly expressed by adult mouse cardiac myocytes[78]. Second, exogenously applied S1P enhanced survival during prolonged in vitro hypoxia through mechanisms that required S1P1 receptor function and G protein Gi-independent activation of the prosurvival kinase Akt/PKB. Finally, Akt-mediated phosphorylation of myocyte substrates that interact with mitochondria, such as GSK-3 and BAD, contributed to cardioprotection. In these studies the selective S1P1 receptor agonist SEW2871 and the S1P analog FTY720 were as effective as S1P in preserving myocytes viability during hypoxia[77]. In contrast, Means et al[79] were unable to demonstrate prosurvival signaling mediated by the S1P1 receptor. The divergent observations surrounding the cardioprotective effects of S1P1 agonism may result from methodologic differences. Even though, these data strongly suggest that the S1P1 receptor, which is the most abundant S1P receptor subtype in cardiac myocytes, is at least partially responsible for S1P-mediated prosurvival signaling and for maintaining myocytes viability during hypoxia[77], and during hypoxia/reoxygenation[37]. In a study of the other receptors, it was shown that combined deletion of S1P2 and S1P3 receptors augmented infarct size in mice subjected to ischemia/reperfusion injury[80]. In these hearts, activation of Akt was markedly attenuated compared to wildtype mice, but the absence of either receptor subtype alone affected neither infarct size nor Akt activation after ischemia/reperfusion injury. S1P augmented Akt activity in control murine myocytes, but was not effective in the double knockout cells[80]. Thus, these observations suggest that the less abundant cardiac myocyte S1P receptors (S1P2 and S1P3) may also be necessary for cell survival during ischemia/reperfusion injury.
In SphK1 null ventricular myocytes subjected to in vitro hypoxia, cell death and cytochrome c release were greater than in wild-type controls[36]. Exogenous S1P enhanced survival of both wild-type and SphK1 null cells. GM-1 treatment, which activates PKC domain and subsequently upregulates SphK to produce S1P, induced cytoprotection in wild-type cardiac myocytes but not in SphK1 null cells. These observations indicate that GM-1 activates SphK1, presumably via PKCε-mediated phosphorylation. Interestingly, the beneficial effects of GM-1 on wild-type cardiac myocytes were abolished by pretreatment with either an S1P1 receptor antagonist or pertussis toxin, which ADP-ribosylates and thereby inactivates Gi, suggesting that endogenous S1P was transported to the extracellular space for activation of its cognate G-protein coupled receptors[36]. A potential mechanism for extrusion of S1P is via ABC transporters, which have been demonstrated in a variety of cell types[81,82], as well as in murine and human hearts[83,84]. Recently, a specific S1P transporter, SPN2, which also transports the phophorylated form of FTY720, has also been described[85]. Knapp et al[86] also mentioned the importance of the S1P/ceramide levels ratio which could be responsible for increased apoptosis in the myocardial infarction in the rat.
As noted above, GM-1 enhanced the survival of cardiac fibroblasts subjected either to PKC inhibition or to C2-ceramide (N-acetyl-sphingoid bases) treatment[62]. GM-1 also increased S1P levels, an effect abrogated by the SphK inhibitor, DMS[62]. Using isolated adult mouse hearts, exogenous S1P and GM-1 separately induced substantial resistance to ischemia-reperfusion injury in wild-type mice[87]. Similar experiments were reported by Lecour et al[88] in isolated rat heart. The importance of the prosurvival kinase, PKCε, was emphasized by experiments in which GM-1 proved to be ineffective in PKCε-null hearts. In addition, GM-1, but not exogenous S1P, stimulated translocation of activated PKCε to myocyte particulate fractions[87]. Nevertheless, exogenously administered S1P was effective both in isolated PKCε-null hearts subjected to ischemia/reperfusion injury[87] and in isolated cardiac myocytes from these hearts subjected to hypoxia[36]. Thus, S1P acting at cell surface receptors or activation of intracellular SphK confers cardioprotection during acute ischemia/reperfusion injury. Consistent with this hypothesis, it was shown that PKCε activation is essential for cardioprotection induced by ischemic preconditioning (IPC)[89]. PKCε peptide agonists mimicked preconditioning effects on contractile recovery and tissue viability in wild-type hearts after prolonged ischemia-reperfusion injury[90]. In contrast, inducible cardioprotection was blocked by PKC peptide antagonists and targeted deletion of the PKCε gene[91]. A subsequent series of experiments directly tested the hypothesis that SphK activation mediates IPC in isolated mouse hearts[90]. It was determined that IPC sufficient to reduce infarction size in wild-type hearts increased SphK localization and activity in tissue membrane fractions. Interestingly, IPC triggered SphK translocation to tissue membrane fractions in PKCε-null hearts but did not enhance enzymatic activity or decrease infarction size after ischemia-reperfusion injury[90]. As noted above, DMS, the endogenous sphingolipid generated by N-methylation of sphingosine, inhibited tissue SphK activity, while 10 μmol/L of DMS pretreatment abolished IPC-induced cardioprotection in wild-type hearts[90]. Subsequent experiments elucidated unpredicted effects of low DMS concentrations on SphK[92]. In contrast to moderate dose DMS (10 μmol/L), low-dose DMS stimulated translocation of activated PKCε to tissue particulate fractions and reduced cardiac ischemia-reperfusion injury. Importantly, low-dose DMS effects were abolished in PKCε-null hearts, and SphK1 was found to co-immunoprecipitate with activated PKC phosphorylated at serine729. Low-dose DMS induced translocation of total Akt from Triton-insoluble fractions to cytosol and increased activated Akt phosphorylated at serine473[92].
When tested with the classic SphK inhibitor, DMS, the activity of SphK2 was unaffected by concentrations as high as 20 μmol/L. Consistent with this observation, DMS was only a partial inhibitor of total cytosolic SphK activity[93]. Also SphK2 was not inhibited by the sphingosine analogue, FTY720. As noted earlier, SphK1 was efficiently inhibited by both DMS and FTY720. Furthermore, when the cytosolic fraction from SphK1 knockout mouse hearts was tested, residual activity due to SphK2 was not inhibited by DMS or FTY720[93]. These observations confirmed the specificity of SphK1 inhibition and indicated the lack of inhibition of SphK2 was not an artifact of purification. SphK2 from rat liver and spleen was also not inhibited by DMS. In contrast, l-sphingosine was an effective inhibitor of both forms[93]. Taken together, along with data obtained in SphK1-null hearts, these observations indicated that DMS inhibits only the SK1 form in the heart. Thus, prior experiments in other cells and tissues in which DMS was used as inhibitor of SphK may require reinterpretation.
The time course of SphK activity in adult rat hearts subjected to ischemia/reperfusion injury and preconditioning has been reported[94]. Cytosolic SphK activity declined by 61% during ischemia and did not recover upon reperfusion, paralleling the effects on left ventricular developed pressure (LVDP). IPC reduced the decrease in enzyme activity during ischemia by half and, upon reperfusion activity, returned to normal. LVDP recovered to 79% of control values, and infarct size was reduced. The low baseline-specific activity of SphK declined by 67% after 45 min of ischemia and remained at that level during reperfusion. IPC restored SphK activity almost to normal during reperfusion. Parallel effects were observed in mitochondria from the same hearts[94]. In these experiments[94], total S1P in cardiac tissue was quantified by liquid chromatography followed by tandem mass spectrometry[38]. In non-preconditioned hearts, S1P content declined from base line after both ischemia and reperfusion. Preconditioned hearts had higher S1P levels after ischemia/reperfusion relative to control hearts. Treatment of non-preconditioned hearts at reperfusion (pharmacologic postconditioning) with 100 nmol/L of S1P improved recovery of LVDP. Thus, maintenance of SphK activity resulting from higher S1P levels is critical for recovery from ischemia/reperfusion injury. In this connection, the activity of S1P phosphatases and lyase has not been reported during experiments involving ischemia/reperfusion injury in the heart.
Despite compelling evidence that DMS modulates resistance to injury by inhibiting SphK1, however, this drug also has been shown to alters other kinases such as PKC activity[92]. Accordingly, SphK1 knockout mice have been employed in a series of subsequent studies[95,96]. SphK2 expression increased in hearts after SphK1 gene disruption, resulting in total SphK activity half that of wild type. Although SphK1-null hearts exhibited normal hemodynamic performance under baseline conditions, contractile abnormalities and infarction were more severe after ischemia/reperfusion than in wild-type hearts[95]. As predicted, targeted disruption of the SphK1 gene abolished IPC-induced cardioprotection[95]. Importantly, when the index ischemia time was reduced from 50 to 40 min, infarct size in the SK1 knockout hearts declined to the level seen in the wild type hearts subjected to ischemia/reperfusion injury. At this reduced level of injury, IPC was still ineffective in producing cardioprotection in the knockout hearts. However, exogenous S1P retained the ability to induce cardioprotection in these SphK1-null hearts. Despite an increase in SphK2 expression in the SphK1-null hearts, infusion of DMS did not affect infarct size, confirming prior in vitro experiments and suggesting that the absence of SphK1 rather than the increased presence of SphK2 was critical to the loss of cardioprotection in myocardium null for SphK1[95]. However, Vessey et al[97] recently demonstrated that myocardial damage is enhanced after ischemia/reperfusion in mice null for SphK2 and that the cardioprotective intervention of preconditioning is abolished by deletion in the SphK2 gene. These observations are contrary to prior suggestions derived from in vitro models that SphK1 and SphK2 drive opposing functions that regulate cell fate[97].
In another recent study, it was reported that previous adenoviral gene transfer of SphK1 protected against hemodynamic deterioration and reduced creatine kinase release and arrhythmias during acute ischemia/reperfusion injury in isolated rat hearts[96]. When gene transfer was performed at the time of acute left anterior descending coronary artery ligation, studies 2 wk later revealed improved left ventricular function in the treated mice, reduced infarct size, more neovascularization, and reduced collagen content[96].
Like IPC, ischemic postconditioning is cardioprotective[98], and this observation has recently been extended to patients undergoing percutaneous coronary interventions[99]. To ascertain whether the SphK/S1P pathway is a determinant of successful postconditioning, isolated wild type and SphK1-null mouse hearts were subjected to ischemia/reperfusion injury[100]. At the onset of reperfusion, hearts selected for treatment underwent 3 brief cycles of postconditioning (5 s of ischemia followed by 5 s of reperfusion). Results were similar to the preconditioning studies cited above: hemodynamics were improved and infarction size reduced compared with untreated hearts[100]. Phospho-Akt and phospho-ERK were enhanced. None of these findings were present in SphK1-null hearts. Thus, SphK1 is also critical for successful ischemic postconditioning. In this connection, it has recently been found that a ramped ischemic postconditioning protocol combined with low-dose sphingosine + S1P given at the time of reperfusion can rescue isolated hearts from as much as 90 min of ischemia[101].
While S1P signaling has long been known to mediate protection in peripheral and cardiac ischemia, only recently has this bioactive lipid pathway drawn attention in cerebral ischemia.
S1P has shown many neuroprotective mechanisms in both in vitro and in vivo. S1P is presumed to protect central nervous system through many different ways[102,103]. In addition to the above mentioned prosurvival effect of S1P, S1P may also protect the brain vasculature by reducing leukocyte adhesion secondary to altering endothelial adhesion molecule expression and preventing endothelial apoptosis through Bcl-2 activation. There is also evidence that S1P may act as a proximal trigger of cerebroprotection (both neuronal and vascular) through activation of signaling molecules such as endothelial nitric oxide synthase[102].
In models of stroke, Kimura et al[104] found that S1P concentrations in the brain were significantly decreased 3 d after ischemia. However, S1P in the brain was increased thereafter and reached a maximum 14 d after the insult. Upregulation of S1P was observed at the infarct border zone and at the infarct core, and mostly colocalized to microglia and some astrocytes, indicating that microglia may be the main source of S1P production in ischemic brain[104].
Moreover, the S1P regulating enzymes SphKs show differential tissue expression patterns and different subcellular localization[72]. Although SphK1 has greater expression and activity than SphK2 in many organs such as lung and spleen, SphK2 expression levels are greater than SphK1 in the brain, suggesting a more prominent physiological role for SphK2 in the brain and brain vasculature[73,74]. Among brain resident cells, primary glial cells express more SphK2 mRNA than primary neurons, and the highest mRNA concentrations were found in cortex, while mRNA was least abundant in striatum[74]. Increased SphK2 was observed in response to cerebral ischemia both in vitro and in vivo[74]. As mentioned earlier, SphK2 could promote apoptosis, instead of cell survival as SphK1 shows at the non-central nervous system[70], there is also accumulated evidence that SphK2 could play an important role as a prosurvival factor in the central nervous system[73,105,106].
A widely used anesthetic agent isoflurane is now considered to be one of the promising therapeutic strategies for many neurological diseases including ischemic stroke[107]. Zhou et al[107] reported that isoflurane given post injury attenuated brain damage after subarachnoid hemorrhage, and that the neuroprotective effect was associated with decreased neuronal apoptosis partly through the antiapoptotic effect of sphingosine-related pathway activation including SphK1 and S1P1,3 receptors. Isoflurane-mediated neuroprotection has also been examined during neonatal hypoxia ischemia through the S1P/PI3K/Akt signaling. The PI3K/Akt signaling cascade has been shown to play a key role in preventing apoptosis under hypoxic or ischemic conditions. Hypoxic preconditioning (HPC) has also been investigated in the contest of cerebral ischemia[105,108]. With respect to elucidating the molecular basis of preconditioning-induced tolerance, Yung et al[106] showed that hypoxic preconditioning significantly reduced infarct volume and improved neurological outcome in wild-type and SphK1-/-, but not in SphK2-/- mice. Wacker et al[105,108] also documented HPC-induced ischemic tolerance and the concomitant protection of the blood-brain-barrier depended on SphK2 signaling. SphK2-generated S1P participates in both the normal maintenance of occlusion at cytoskeletally linked cell junctions, as well as the mediation of HPC-induced increases in the expression of claudin-5 and VE-cadherin at these junctions, which may be compulsory for induction of the vasculoprotective phenotype by HPC. The present data demonstrates that SK2 is a universal mediator of isoflurane- and hypoxia-induced preconditioning.
FTY720 (Fingolimod) is a novel immunomodulatory agent, which in its phosphorylated form acts as a high affinity agonist of S1P receptors[109,110]. It became the first oral drug to be FDA-approved for clinical use in the treatment of multiple sclerosis. FTY720 readily crosses the blood-brain barrier and exerts a number of direct effects in the central nervous system. FTY720 is phosphorylated by SphK, mainly by SphK2[73,111], into the active compound phospho-FTY720, which then acts on 4 of the 5 known S1P receptor subtypes (S1P1, S1P3, S1P4, S1P5), and shows neuroprotective effect against many central nervous system disease including cerebral ischemia[73,112-115]. Mechanisms include regulation of myelination and microglial activation following injury, proliferation and migration of neural precursor cells toward injury sites, and potentiation of growth-factor regulated neuronal differentiation, survival, and process extension, and also antiapoptotic and anti-inflammatory pathways[104,113,115-119]. FTY720 also exerts immunomodulatory actions by affecting lymphocyte production, trafficking, and apoptosis through S1P receptors which induces a depletion of circulating lymphocytes by preventing the egress of lymphocytes from the lymph nodes. Mechanistically, this is due to a downregulation of the S1P type 1 receptor (S1P1). Expression levels of endothelial adhesion molecules such as E-selectin, P-selectin, intracellular adhesion molecule-1 or vascular cell adhesion molecule-1 were shown to be induced by FTY720 treatment, and therefore might contribute to the prevention of early infiltration of neurotrophils and activation of microglia/macrophages. These findings suggest that anti-inflammatory mechanisms, and possibly vasculoprotection, rather than direct effects on neurons, underlie the beneficial effects of fingolimod after stroke. Most of the past reports have shown beneficial effect of S1P in the field of ischemia, but by contrast, Liesz et al[120] showed opposite results. These authors found that S1P treatment did show a reduction of lymphocyte brain invasion but could not achieve a significant reduction of infarct volumes and behavior dysfunction[120]. Liu et al[121] recently published a systematic meta-analysis of the efficacy of FTY720 in animal model of stroke. In this study, they concluded that FTY720 reduced infarct volume and improve functional outcome. However, the authors also indicated that more experimental studies should be performed to evaluate the safety of FTY720 in the future. Thus, taken this recent scientific highlights together, it is obvious that S1P receptor pathways and sphingolipids regulating enzymes are a highly promising target in stroke treatment.
During the past few years, a plethora of new information identifying the importance of sphingolipid signaling pathways in the cardiovascular and cerebrovascular diseases has accumulated. The potential for the development of new therapeutic agents based on this understanding is high, but this is clearly a new area of investigation that is still in its infancy.
P- Reviewers Lazou A, Pérez-Castrillon J, Liu T, Di Bella G S- Editor Huang XZ L- Editor A E- Editor Zhang DN
| 1. | Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 2. | Nagatsuka Y, Hara-Yokoyama M, Kasama T, Takekoshi M, Maeda F, Ihara S, Fujiwara S, Ohshima E, Ishii K, Kobayashi T. Carbohydrate-dependent signaling from the phosphatidylglucoside-based microdomain induces granulocytic differentiation of HL60 cells. Proc Natl Acad Sci USA. 2003;100:7454-7459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Kolesnick RN, Hemer MR. Characterization of a ceramide kinase activity from human leukemia (HL-60) cells. Separation from diacylglycerol kinase activity. J Biol Chem. 1990;265:18803-18808. [PubMed] |
| 4. | Serra M, Saba JD. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv Enzyme Regul. 2010;50:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121:4222-4230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Dressler KA, Mathias S, Kolesnick RN. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255:1715-1718. [PubMed] |
| 7. | Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, Kolesnick RN. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525-535. [PubMed] |
| 8. | Kolesnick RN, Haimovitz-Friedman A, Fuks Z. The sphingomyelin signal transduction pathway mediates apoptosis for tumor necrosis factor, Fas, and ionizing radiation. Biochem Cell Biol. 1994;72:471-474. [PubMed] |
| 9. | Okazaki T, Bielawska A, Bell RM, Hannun YA. Role of ceramide as a lipid mediator of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990;265:15823-15831. [PubMed] |
| 10. | Kim MY, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991;266:484-489. [PubMed] |
| 11. | Turinsky J, Bayly BP, O’Sullivan DM. 1,2-Diacylglycerol and ceramide levels in rat liver and skeletal muscle in vivo. Am J Physiol. 1991;261:E620-E627. [PubMed] |
| 12. | Mazière C, Conte MA, Leborgne L, Levade T, Hornebeck W, Santus R, Mazière JC. UVA radiation stimulates ceramide production: relationship to oxidative stress and potential role in ERK, JNK, and p38 activation. Biochem Biophys Res Commun. 2001;281:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Chang Y, Abe A, Shayman JA. Ceramide formation during heat shock: a potential mediator of alpha B-crystallin transcription. Proc Natl Acad Sci USA. 1995;92:12275-12279. [PubMed] |
| 14. | Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 541] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 15. | Taniguchi M, Kitatani K, Kondo T, Hashimoto-Nishimura M, Asano S, Hayashi A, Mitsutake S, Igarashi Y, Umehara H, Takeya H. Regulation of autophagy and its associated cell death by “sphingolipid rheostat”: reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J Biol Chem. 2012;287:39898-39910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769-1771. [PubMed] |
| 17. | Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, Ghidoni R, Codogno P. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004;279:18384-18391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 357] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Wakita H, Tokura Y, Yagi H, Nishimura K, Furukawa F, Takigawa M. Keratinocyte differentiation is induced by cell-permeant ceramides and its proliferation is promoted by sphingosine. Arch Dermatol Res. 1994;286:350-354. [PubMed] |
| 19. | Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457-5464. [PubMed] |
| 20. | Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25-31. [PubMed] |
| 21. | Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608-36615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 22. | Arana L, Gangoiti P, Ouro A, Trueba M, Gómez-Muñoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010;9:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Gangoiti P, Granado MH, Wang SW, Kong JY, Steinbrecher UP, Gómez-Muñoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell Signal. 2008;20:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Gangoiti P, Granado MH, Arana L, Ouro A, Gomez-Muñoz A. Activation of protein kinase C-alpha is essential for stimulation of cell proliferation by ceramide 1-phosphate. FEBS Lett. 2010;584:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Suzuki E, Handa K, Toledo MS, Hakomori S. Sphingosine-dependent apoptosis: a unified concept based on multiple mechanisms operating in concert. Proc Natl Acad Sci USA. 2004;101:14788-14793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Hannun YA, Loomis CR, Merrill AH, Bell RM. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986;261:12604-12609. [PubMed] |
| 27. | Ohta H, Sweeney EA, Masamune A, Yatomi Y, Hakomori S, Igarashi Y. Induction of apoptosis by sphingosine in human leukemic HL-60 cells: a possible endogenous modulator of apoptotic DNA fragmentation occurring during phorbol ester-induced differentiation. Cancer Res. 1995;55:691-697. [PubMed] |
| 28. | McDonough PM, Yasui K, Betto R, Salviati G, Glembotski CC, Palade PT, Sabbadini RA. Control of cardiac Ca2+ levels. Inhibitory actions of sphingosine on Ca2+ transients and L-type Ca2+ channel conductance. Circ Res. 1994;75:981-989. [PubMed] |
| 29. | Smith ER, Jones PL, Boss JM, Merrill AH. Changing J774A.1 cells to new medium perturbs multiple signaling pathways, including the modulation of protein kinase C by endogenous sphingoid bases. J Biol Chem. 1997;272:5640-5646. [PubMed] |
| 30. | Gomez-Muñoz A, Hamza EH, Brindley DN. Effects of sphingosine, albumin and unsaturated fatty acids on the activation and translocation of phosphatidate phosphohydrolases in rat hepatocytes. Biochim Biophys Acta. 1992;1127:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Jamal Z, Martin A, Gomez-Muñoz A, Brindley DN. Plasma membrane fractions from rat liver contain a phosphatidate phosphohydrolase distinct from that in the endoplasmic reticulum and cytosol. J Biol Chem. 1991;266:2988-2996. [PubMed] |
| 32. | Natarajan V, Jayaram HN, Scribner WM, Garcia JG. Activation of endothelial cell phospholipase D by sphingosine and sphingosine-1-phosphate. Am J Respir Cell Mol Biol. 1994;11:221-229. [PubMed] |
| 33. | Sakane F, Yamada K, Kanoh H. Different effects of sphingosine, R59022 and anionic amphiphiles on two diacylglycerol kinase isozymes purified from porcine thymus cytosol. FEBS Lett. 1989;255:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Yamada K, Sakane F, Imai S, Takemura H. Sphingosine activates cellular diacylglycerol kinase in intact Jurkat cells, a human T-cell line. Biochim Biophys Acta. 1993;1169:217-224. [PubMed] |
| 35. | Vessey DA, Li L, Kelley M, Zhang J, Karliner JS. Sphingosine can pre- and post-condition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Mol Toxicol. 2008;22:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Tao R, Zhang J, Vessey DA, Honbo N, Karliner JS. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res. 2007;74:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Tao R, Hoover HE, Zhang J, Honbo N, Alano CC, Karliner JS. Cardiomyocyte S1P1 receptor-mediated extracellular signal-related kinase signaling and desensitization. J Cardiovasc Pharmacol. 2009;53:486-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 721] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 39. | Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265-269. [PubMed] |
| 40. | Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ui M, Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352 Pt 3:809-815. [PubMed] |
| 41. | Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 42. | Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193-202. [PubMed] |
| 43. | Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 650] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 44. | Limaye V, Li X, Hahn C, Xia P, Berndt MC, Vadas MA, Gamble JR. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood. 2005;105:3169-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 45. | Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta. 2002;1582:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 250] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 46. | Pébay A, Toutant M, Prémont J, Calvo CF, Venance L, Cordier J, Glowinski J, Tencé M. Sphingosine-1-phosphate induces proliferation of astrocytes: regulation by intracellular signalling cascades. Eur J Neurosci. 2001;13:2067-2076. [PubMed] |
| 47. | Agudo-López A, Miguel BG, Fernández I, Martínez AM. Involvement of mitochondria on neuroprotective effect of sphingosine-1-phosphate in cell death in an in vitro model of brain ischemia. Neurosci Lett. 2010;470:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722-23728. [PubMed] |
| 49. | Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113-11121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 586] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 50. | Meyer zu Heringdorf D, Lass H, Alemany R, Laser KT, Neumann E, Zhang C, Schmidt M, Rauen U, Jakobs KH, van Koppen CJ. Sphingosine kinase-mediated Ca2+ signalling by G-protein-coupled receptors. EMBO J. 1998;17:2830-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Alemany R, Meyer zu Heringdorf D, van Koppen CJ, Jakobs KH. Formyl peptide receptor signaling in HL-60 cells through sphingosine kinase. J Biol Chem. 1999;274:3994-3999. [PubMed] |
| 52. | Blaukat A, Dikic I. Activation of sphingosine kinase by the bradykinin B2 receptor and its implication in regulation of the ERK/MAP kinase pathway. Biol Chem. 2001;382:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Young KW, Bootman MD, Channing DR, Lipp P, Maycox PR, Meakin J, Challiss RA, Nahorski SR. Lysophosphatidic acid-induced Ca2+ mobilization requires intracellular sphingosine 1-phosphate production. Potential involvement of endogenous EDG-4 receptors. J Biol Chem. 2000;275:38532-38539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Meyer zu Heringdorf D, Lass H, Kuchar I, Lipinski M, Alemany R, Rümenapp U, Jakobs KH. Stimulation of intracellular sphingosine-1-phosphate production by G-protein-coupled sphingosine-1-phosphate receptors. Eur J Pharmacol. 2001;414:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 732] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 56. | Meyer zu Heringdorf D, Lass H, Kuchar I, Alemany R, Guo Y, Schmidt M, Jakobs KH. Role of sphingosine kinase in Ca(2+) signalling by epidermal growth factor receptor. FEBS Lett. 1999;461:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Rius RA, Edsall LC, Spiegel S. Activation of sphingosine kinase in pheochromocytoma PC12 neuronal cells in response to trophic factors. FEBS Lett. 1997;417:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758-7768. [PubMed] |
| 59. | Melendez A, Floto RA, Gillooly DJ, Harnett MM, Allen JM. FcgammaRI coupling to phospholipase D initiates sphingosine kinase-mediated calcium mobilization and vesicular trafficking. J Biol Chem. 1998;273:9393-9402. [PubMed] |
| 60. | Wang F, Buckley NE, Olivera A, Goodemote KA, Su Y, Spiegel S. Involvement of sphingolipids metabolites in cellular proliferation modulated by ganglioside GM1. Glycoconj J. 1996;13:937-945. [PubMed] |
| 61. | Sukocheva OA, Wang L, Albanese N, Pitson SM, Vadas MA, Xia P. Sphingosine kinase transmits estrogen signaling in human breast cancer cells. Mol Endocrinol. 2003;17:2002-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Cavallini L, Venerando R, Miotto G, Alexandre A. Ganglioside GM1 protection from apoptosis of rat heart fibroblasts. Arch Biochem Biophys. 1999;370:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491-5500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 456] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 64. | Pitson SM, Moretti PA, Zebol JR, Xia P, Gamble JR, Vadas MA, D’Andrea RJ, Wattenberg BW. Expression of a catalytically inactive sphingosine kinase mutant blocks agonist-induced sphingosine kinase activation. A dominant-negative sphingosine kinase. J Biol Chem. 2000;275:33945-33950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 65. | Leclercq TM, Moretti PA, Vadas MA, Pitson SM. Eukaryotic elongation factor 1A interacts with sphingosine kinase and directly enhances its catalytic activity. J Biol Chem. 2008;283:9606-9614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Kovanich D, van der Heyden MA, Aye TT, van Veen TA, Heck AJ, Scholten A. Sphingosine kinase interacting protein is an A-kinase anchoring protein specific for type I cAMP-dependent protein kinase. Chembiochem. 2010;11:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Lacaná E, Maceyka M, Milstien S, Spiegel S. Cloning and characterization of a protein kinase A anchoring protein (AKAP)-related protein that interacts with and regulates sphingosine kinase 1 activity. J Biol Chem. 2002;277:32947-32953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Fukuda Y, Aoyama Y, Wada A, Igarashi Y. Identification of PECAM-1 association with sphingosine kinase 1 and its regulation by agonist-induced phosphorylation. Biochim Biophys Acta. 2004;1636:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Hayashi H, Nakagami H, Takami Y, Koriyama H, Mori M, Tamai K, Sun J, Nagao K, Morishita R, Kaneda Y. FHL-2 suppresses VEGF-induced phosphatidylinositol 3-kinase/Akt activation via interaction with sphingosine kinase-1. Arterioscler Thromb Vasc Biol. 2009;29:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118-37129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 502] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 71. | Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J Cardiovasc Pharmacol. 2009;53:189-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832-46839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 333] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 73. | Pfeilschifter W, Czech-Zechmeister B, Sujak M, Mirceska A, Koch A, Rami A, Steinmetz H, Foerch C, Huwiler A, Pfeilschifter J. Activation of sphingosine kinase 2 is an endogenous protective mechanism in cerebral ischemia. Biochem Biophys Res Commun. 2011;413:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Blondeau N, Lai Y, Tyndall S, Popolo M, Topalkara K, Pru JK, Zhang L, Kim H, Liao JK, Ding K. Distribution of sphingosine kinase activity and mRNA in rodent brain. J Neurochem. 2007;103:509-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 75. | Inagaki Y, Li PY, Wada A, Mitsutake S, Igarashi Y. Identification of functional nuclear export sequences in human sphingosine kinase 1. Biochem Biophys Res Commun. 2003;311:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:1713-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150-H3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 78. | Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833-9841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 79. | Means CK, Miyamoto S, Chun J, Brown JH. S1P1 receptor localization confers selectivity for Gi-mediated cAMP and contractile responses. J Biol Chem. 2008;283:11954-11963. [PubMed] |
| 80. | Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944-H2951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 81. | Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667-6675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 230] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 82. | Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC). Prostaglandins Other Lipid Mediat. 2007;84:154-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 83. | Mungrue IN, Zhao P, Yao Y, Meng H, Rau C, Havel JV, Gorgels TG, Bergen AA, MacLellan WR, Drake TA. Abcc6 deficiency causes increased infarct size and apoptosis in a mouse cardiac ischemia-reperfusion model. Arterioscler Thromb Vasc Biol. 2011;31:2806-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Solbach TF, Paulus B, Weyand M, Eschenhagen T, Zolk O, Fromm MF. ATP-binding cassette transporters in human heart failure. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J Biol Chem. 2011;286:1758-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 86. | Knapp M, Zendzian-Piotrowska M, Kurek K, Błachnio-Zabielska A. Myocardial infarction changes sphingolipid metabolism in the uninfarcted ventricular wall of the rat. Lipids. 2012;47:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970-H1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 89. | Saurin AT, Pennington DJ, Raat NJ, Latchman DS, Owen MJ, Marber MS. Targeted disruption of the protein kinase C epsilon gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovasc Res. 2002;55:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 90. | Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 91. | Gray MO, Zhou HZ, Schafhalter-Zoppoth I, Zhu P, Mochly-Rosen D, Messing RO. Preservation of base-line hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase C epsilon. J Biol Chem. 2004;279:3596-3604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Jin ZQ, Karliner JS. Low dose N, N-dimethylsphingosine is cardioprotective and activates cytosolic sphingosine kinase by a PKCepsilon dependent mechanism. Cardiovasc Res. 2006;71:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Vessey DA, Kelley M, Li L, Huang Y, Zhou HZ, Zhu BQ, Karliner JS. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit. 2006;12:BR318-BR324. [PubMed] |
| 95. | Jin ZQ, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 96. | Nishino Y, Webb I, Marber MS. Sphingosine kinase isoforms and cardiac protection. Cardiovasc Res. 2007;76:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 97. | Vessey DA, Li L, Jin ZQ, Kelley M, Honbo N, Zhang J, Karliner JS. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:961059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579-H588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1468] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 99. | Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF. Long-term benefit of postconditioning. Circulation. 2008;117:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 100. | Jin ZQ, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, Waeber C. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol. 2008;153:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 103. | Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1913] [Cited by in RCA: 2055] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 104. | Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, Shimazaki K, Hoshino Y, Yatomi Y, Sakata Y. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008;39:3411-3417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 105. | Wacker BK, Freie AB, Perfater JL, Gidday JM. Junctional protein regulation by sphingosine kinase 2 contributes to blood-brain barrier protection in hypoxic preconditioning-induced cerebral ischemic tolerance. J Cereb Blood Flow Metab. 2012;32:1014-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 106. | Yung LM, Wei Y, Qin T, Wang Y, Smith CD, Waeber C. Sphingosine kinase 2 mediates cerebral preconditioning and protects the mouse brain against ischemic injury. Stroke. 2012;43:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, Zhang JH. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41:1521-1527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 108. | Wacker BK, Park TS, Gidday JM. Hypoxic preconditioning-induced cerebral ischemic tolerance: role of microvascular sphingosine kinase 2. Stroke. 2009;40:3342-3348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 109. | Baumruker T, Billich A, Brinkmann V. FTY720, an immunomodulatory sphingolipid mimetic: translation of a novel mechanism into clinical benefit in multiple sclerosis. Expert Opin Investig Drugs. 2007;16:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012;366:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 111. | Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408-47415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 377] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 112. | Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453-21457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1232] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 113. | Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 114. | Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, Rami A, Huwiler A, Pfeilschifter J. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem Biophys Res Commun. 2009;389:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 115. | Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, Guo S, Qin T, Alsharif N, Brinkmann V. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69:119-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 116. | Brinkmann V, Pinschewer DD, Feng L, Chen S. FTY720: altered lymphocyte traffic results in allograft protection. Transplantation. 2001;72:764-769. [PubMed] |
| 117. | Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA, Antel JP, Soliven B. Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia. 2007;55:1656-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 118. | Stessin AM, Gursel DB, Schwartz A, Parashar B, Kulidzhanov FG, Sabbas AM, Boockvar J, Nori D, Wernicke AG. FTY720, sphingosine 1-phosphate receptor modulator, selectively radioprotects hippocampal neural stem cells. Neurosci Lett. 2012;516:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 119. | Yagi H, Kamba R, Chiba K, Soga H, Yaguchi K, Nakamura M, Itoh T. Immunosuppressant FTY720 inhibits thymocyte emigration. Eur J Immunol. 2000;30:1435-1444. [PubMed] |
| 120. | Liesz A, Sun L, Zhou W, Schwarting S, Mracsko E, Zorn M, Bauer H, Sommer C, Veltkamp R. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS One. 2011;6:e21312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 121. | Liu J, Zhang C, Tao W, Liu M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. Int J Neurosci. 2013;123:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |