SEARCH FOR THE "GUILTY" SUBSTRATE BEHIND CORONARY THROMBOTIC EVENTS
Despite widespread adoption of preventive measures and remarkable improvements in medical treatment and revascularization strategies for patients with coronary artery disease (CAD), atherosclerosis and its thrombotic complications continue to be the most deadly and disabling disease in industrialized countries[1]. As cardiologists, we have struggled for decades in accurately, identifying individuals that despite being asymptomatic have a high individual risk of developing thrombotic coronary events. Accordingly, the identification of the so-called “vulnerable” or “high risk plaque” in the coronary tree has been one of the main challenges and pivotal areas of research in modern cardiovascular medicine.
Combined evidence provided by autopsy as well as from invasive studies has shown that rupture-prone plaques have certain characteristics: a thin fibrous cap, a large lipid-rich pool and increased macrophage activity (Figure 1)[2-5]. Currently, there is sound evidence suggesting that these plaques, namely “thin cap fibroatheromas” (TCFAs), are the most common underlying “guilty” substrate in patients suffering acute coronary thrombotic events[3,6]. Moreover, an in-depth analysis of the PROSPECT trial demonstrated that, in spite of significant efforts in secondary prevention, TCFAs remain powerful independent predictors of recurrent adverse cardiovascular events[7].
Figure 1 A 64-year-old male was admitted at our hospital because of progressive angina.
A: Coronary angiogram demonstrated a complex stenosis in the mid segment of the circumflex coronary artery; B-F: Optical coherence tomography revealed a large complex thin-cap fibroatheroma (arrows) proximal and at the site of the stenosis. The fibrous cap covering the lipid rich plaque had a variable thickness and in its thinnest part measured 55 μm (arrows in D). Distally, the same plaque had large lipidic core and an image compatible with a rupture site (*) (E). Please note that residual lining red thrombus with a clear dorsal shadowing was also detected at some sites (+). Finally, at the most severe site (F), a fibrous plaque was noted.
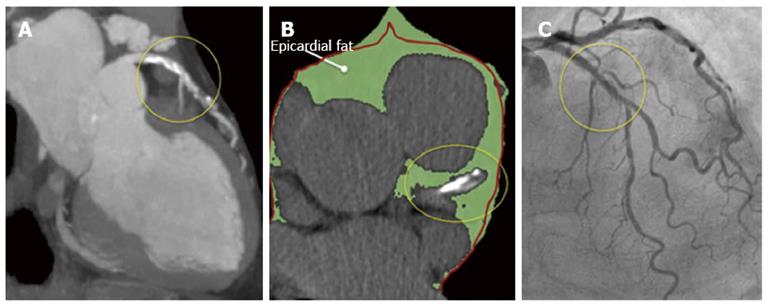
Considering this pathological and clinical background, great efforts have been made in order to identify TCFAs with the ultimate goal of preventing plaque rupture and thereby averting acute coronary syndromes and sudden cardiac death. However, the correct diagnosis of these vulnerable plaques by non-invasive imaging techniques (potentially available and suitable in large patient populations) remains a challenge[8]. Therefore, new diagnostic clues aiming to the identification of these vulnerable plaques, are always received with major enthusiasm by the scientific community. On these grounds, the recently reported association between coronary TCFAs and a specific, readily-accessible, depot of visceral fat called “epicardial adipose tissue” (EAT) (Figure 2) reported by Ito et al[9] deserves to be highlighted as a valuable step-forward in the right direction.
Figure 2 A multislice computed tomography was performed in a 68-year old male because of atypical chest pain.
A: This study revealed a long mixed, calcific and non-calcific plaque in the proximal and mid segments of the left anterior descending artery; B: A large amount of epicardial adipose tissue (EAT) [(159.2 cm3 (green) (figure 3)] was also calculated. Please note that the left anterior descending artery is embedded in EAT (yellow circles). The red line represents the pericardium; C: A coronary angiogram was scheduled and revealed intermediate stenoses in the same arterial segment that was subsequently studied with optical coherence tomography.
EPICARDIAL ADIPOSE TISSUE AND THIN CAP FIBROATHEROMAS: A RECENTLY SUGGESTED ASSOCIATION
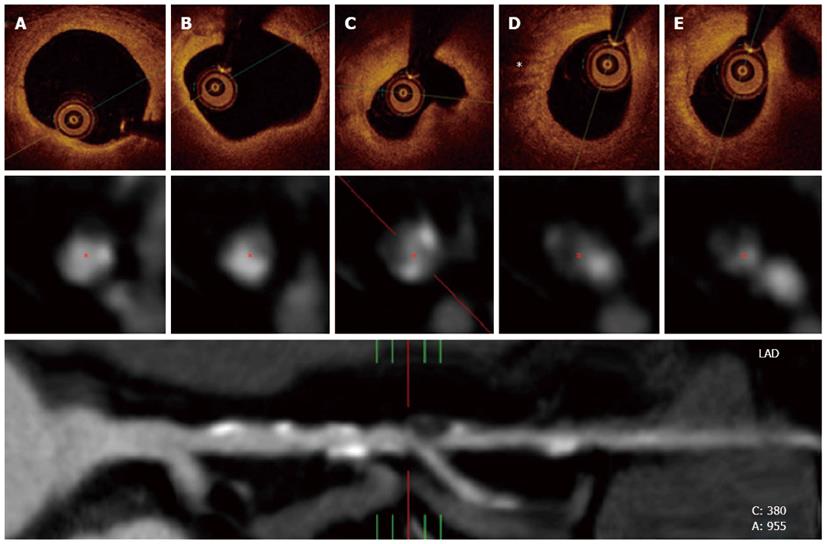
Very recently, Ito et al[9] reported for the first time the relationship between EAT, as quantified with multislice computed tomography (MSCT), and plaque vulnerability, as assessed with intracoronary optical coherence tomography (OCT). This is of remarkable importance because at this moment and notwithstanding well-recognized limitations, MSCT and OCT are the “gold standards” for assessing EAT and plaque vulnerability in vivo, respectively (Figure 3). In their study, Ito et al made a profound examination of a total of 180 vessels form 117 patients with stable angina or acute coronary syndromes that underwent OCT during cardiac catheterization and also a MSCT study within an interval of 9.8 ± 8.8 d. TCFAs were assessed with OCT and defined as plaques with necrotic lipid pools ≥ 2 quadrants and minimum fibrous cap thickness measuring < 65 μm. EAT, coronary plaques, coronary plaques attenuation and the remodeling index which are MSCT findings associated with plaque vulnerability[10,11] were evaluated from MSCT images. Notably, both of these techniques were performed following a rigorous methodology, as demonstrated by their very low inter and intraobserver variability. After categorizing the patients according to EAT tertiles, they found that all of the aforementioned indexes of plaque vulnerability provided by OCT and MSCT, were higher in those within the highest tertile of EAT (high-EAT). Moreover, EAT was significantly correlated with the extension of the necrotic lipid pool and inversely correlated with the thickness of the fibrous cap by OCT (r = -0.400, P < 0.01). Finally, high-EAT was an independent predictor of TCFAs [RR, 2.92 (1.13-7.55)] and of acute coronary syndromes as a clinical presentation [RR, 2.89 (1.14-7.29)].
Figure 3 Optical coherence tomography and multislice computed tomography findings of the same patient in figure 2.
A and B: In the upper panel, optical coherence tomography revealed a complex plaque affecting a long portion of the vessel with calcified and lipidic regions; C: Also, a complex fibroatheroma that included a lipidic core was observed at the site of the most severe stenosis; D: A high light attenuation band with distal shadowing can be attributed to macrophage infiltration (*). The middle panel represents cross-sectional views of the vessel at the same stenosis as visualized by multislice computed tomography (MSCT). Calcified and non-calcified regions including eccentric plaques were found within the diseased segment. The inferior panel shows a MSCT multiplanar reconstruction of the vessel and its anatomical bookmarks.
Therefore, the authors have provided for the first time strong evidence in vivo linking EAT with the vulnerable or high-risk plaques behind coronary thrombotic events and unstable clinical presentations. The authors are to be congratulated on executing this cleverly designed study although a few minor limitations and future challenges are nonetheless worth noting. First, as in all cross sectional studies, causality cannot be assumed and, therefore, these findings should be taken as hypothesis generating and confirmed by prospective studies. Second, the relatively small sample size could be a limitation when drawing conclusions. Third, all of the patients included in the study had already symptoms of CAD so the translation of these findings to an asymptomatic population in terms of primary prevention is unclear. Finally, OCT and MSCT have well-acknowledged limitations in identifying plaque vulnerability and TCFAs but by far, are the best available tools for this purpose in vivo[3,6,10,11]. This study is also an excellent opportunity to briefly review some basic and clinical characteristics attributed to EAT that explains why this particular adipose tissue has been attracting the physician’s attention in the recent years.
ANATOMY AND PHYSIOLOGY OF EPICARDIAL ADIPOSE TISSUE
EAT is an intrathoracic depot of visceral fat that lays over the myocardium and coronary arteries that has received increased attention in the literature in recent years, due to some unique and very interesting characteristics. Embryologically, EAT originates from the splachnopleuric mesoderm and evolves from brown adipose tissue[12]. In the normal human heart, EAT follows the adventitia of coronary arteries, is concentrated in the atrioventricular and interventricular grooves and represents approximately 20% of the heart mass[13]. Remarkably, no structures resembling a fascia (as found on skeletal muscle) separates EAT from the myocardium and coronary vessels and therefore these three tissues share the same circulation and innervation[14]. Because of this anatomical contiguity, it was proposed that EAT could interact locally with the myocardium and coronary arteries through paracrine or vasocaine pathways and a growing amount of evidence is currently supporting this assumption[15].
Besides its embryology and anatomy, the physiology of EAT is also quite special and important differences have been shown in its metabolism when compared with other depots of corporal fat. For example, a higher rate of lipolysis and insulin-induced lipogenesis has been observed in EAT from animal models[12]. Human EAT appears to be richer in saturated fatty acids than subcutaneous fat and, interestingly, the rates of free fatty acids synthesis, incorporation and breakdown are significantly higher in EAT[12,16]. Since 50%-70% of the energy requirements of the heart are supplied by free fatty acids oxidation, it has been proposed that EAT might provide large amounts of energy to the myocardium under non ischemic conditions[15]. Besides its role in the energetic metabolism, other physiological functions of EAT have been proposed. Indeed, EAT may be a source of antiatherogenic and anti-inflammatory adipokines, such as adiponectin and adrenomedullin, may influence vasomotion of coronary arteries and may also provide mechanical and thermogenic cardioprotection to the myocardium and coronary arteries by attenuation of vascular tension and torsion[15,17,18].
Altogether, current evidence supports the hypothesis that under physiological conditions, EAT supplies energy and heat to the coronary arteries and myocardium and may also exert a protective modulation on the coronary vessels[15,17,19]. However, as will be discussed in the next paragraph, a growing body of evidence is associating EAT with pathological states from which atherosclerotic plaque development and instability are of substantial importance.
EPICARDIAL ADIPOSE TISSUE AND ITS IMPACT ON THE DEVELOPMENT OF ATHEROSCLEROTIC PLAQUES
From a clinical perspective, several clinical and epidemiological studies have associated EAT with different cardiometabolic risk factors and with early stages of atherosclerosis and plaque formation[20-24]. Moreover, the presence and severity of CAD and coronary calcification, indicating more advanced states of atherosclerosis, have been also associated with EAT[25-28]. This association has been reinforced by a recent meta-analysis (n = 2872 patients) that found that when compared with patients without CAD, EAT thickness and volume were significantly higher in those with documented CAD[29]. These epidemiological findings have been supported by a large amount of basic evidence pointing at the same direction. Indeed, when compared to subcutaneous fat, EAT shows a more dense inflammatory cell infiltrate, predominantly represented by macrophages[30]. The secretion of some highly atherogenic and inflammatory adipokines such as tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), IL-1b, plasminogen activator inhibitor-1 (PAI-1) and resistin are significantly higher in EAT from patients with CAD[18,31-35]. Interestingly, another supporting evidence comes from a “natural experiment”, since as shown by animal and human studies, increased atherosclerosis is observed in the segments of the coronary arteries proximal to myocardial bridges, where EAT is present, whereas intramyocardial segments of the vessels are free of atherosclerosis[36-38]. Altogether, current evidence supports the hypothesis that under pathological conditions, EAT may become an adverse lipotoxic and proinflammatory organ that could play a significant role in the development of coronary atherosclerosis. Notably, however, it was not until very recently that EAT was linked with different characteristics of coronary atherosclerotic plaques and more importantly, plaque vulnerability.
EPICARDIAL ADIPOSE TISSUE AND PLAQUE VULNERABILITY
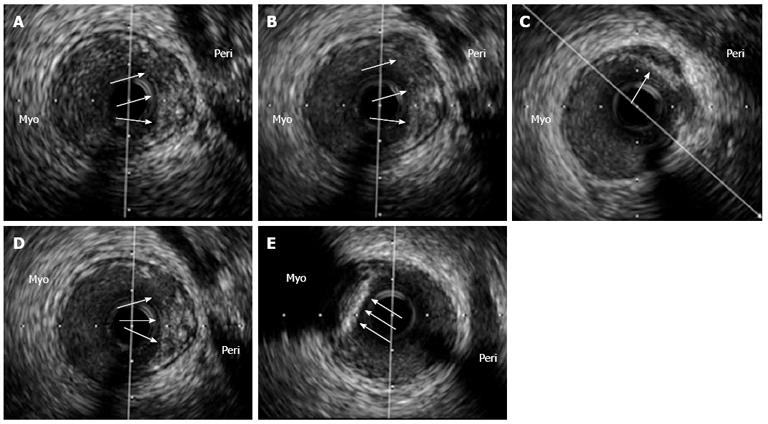
Although the precise mechanisms of plaque rupture are poorly understood, it is widely accepted that the disruption occurs at the site of the fibrous cap, which is usually thin, heavily infiltrated by macrophages and T-lymphocytes and where the underlying necrotic core is also typically large[2]. Various factors and hypothesis have been implicated and proposed in order to explain these phenomena. These include arterial remodeling[39,40], inflammation and apoptosis within the plaque[41,42], impaired collagen synthesis and breakdown in the fibrous cap[43] and local mechanical stress[44]. EAT, interestingly, appears to be related to some of these. For example, some adipokines highly expressed by EAT, such as TNF-α, IL-1 and MCP-1, have been associated with macrophage and smooth muscle cell apoptosis within the plaque, which may contribute to both, the formation of the necrotic core and thinning of the fibrous cap[45]. Moreover, the uptake of fluorodeoxyglucose in the left anterior descending artery (LAD), which is a potential marker of inflammatory activity of the vessel wall, has been significantly correlated with EAT volume in one study[46]. Also, in an intravascular ultrasound analysis of LADs performed by Prati et al[47], most of the plaques with positive remodeling were located towards the epicardial side of the vessel rather than at the myocardial one (Figure 4). Finally, a recent study performed by Alexopoulos et al[48] linked non-invasive characteristics of plaque vulnerability by MSCT with EAT, since it was observed that EAT was an independent predictor of coronary artery calcium [exp(B) = 3.916, P < 0.05], atherosclerotic plaques of any type [exp(B) = 4.532, P < 0.01], non-calcified plaques [exp(B) = 3.849, P < 0.01], and obstructive CAD [exp(B) = 3.824, P < 0.05]. Therefore, the possible mechanisms and pathways through which EAT could be related to plaque vulnerability are many, of significant importance, and a growing body of evidence supporting this association is currently being acquired.
Figure 4 An intravascular ultrasound analysis of the left anterior descending coronary artery in an asymptomatic patient with a previously deployed stent (not shown) that underwent this study as part of an institutional protocol.
A-D: In the pericardial side (Peri) of the vessel, an eccentric and positively remodeled plaque with heterogenic echo-reflection that included a hypoechogenic area, suggestive of a potentially “high risk” plaque, was found (arrows); E: Interestingly, just adjacently and in the opposite vessel side [myocardial side (Myo)], a calcified plaque with intense posterior shadowing, highly suggestive of a stable fibro-calcific plaque (arrows), was also observed. The patient has been asymptomatic during a 3-year clinical follow-up.
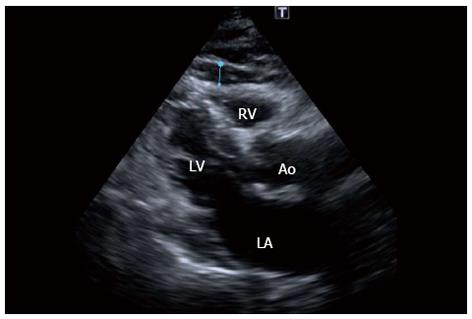
Finally and although at this stage, many basic and cross-sectional studies have shown positive associations between increased EAT and the development and (now) the instability of the atherosclerotic process, these findings should be still interpreted as hypothesis generating and EAT measurement cannot be recommended for clinical practice. However, the scientific basis of the relationship between EAT and the aforementioned phenomena seem to be solid and warrants further studies. Since EAT can be measured by non invasive methods such as transthoracic 2D echocardiography (Figure 5), MSCT and magnetic resonance imaging[49-51], its clinical use for primary prevention purposes could be readily available. A critical aspect in the near future will be the assessment of the incremental predictive value of EAT over established cardiovascular risk factors, if are proved or, more convincingly a cause-effect interaction is established, the introduction of EAT measurement into clinical practice could be taken as one step forward to the “holy grail” for the effective risk stratification[52] and prevention of coronary thrombotic events.
Figure 5 Echocardiogram in the parasternal long-axis view revealing a large amount of epicardial fat (thickness of 6 mm) over the free wall of the right ventricle (blue line) of the patient in figure 1.
RV: Right ventricle, LV: Left ventricle, LA: Left atrium; Ao: Aortic root.
P- Reviewers Said S, Peteiro J, Aronow WS S- Editor Gou SX L- Editor A E- Editor Zhang DN