INTRODUCTION
Myocardial infarction (MI), known as “heart attack” leads to loss of cardiomyocytes and the injured heart tissue is replaced by scar tissue[1]. When the scar is large enough to interfere with the hearts normal rhythm, heart failure occurs. Nearly 8 million Americans each year experience myocardial infarction[2] and must cope with the consequences of compromised heart muscle function. The myocardial tissue lacks significant intrinsic regenerative capacity to replace the lost cells[3]. Moreover, the relative shortage of organ donors to recipients, and the ineligibility of many heart patients for transplantation revamp the search for new strategies to repair the injured myocardium. The current methods employed include cardiac restraint devices like acorn corcap heart mesh (knitted polyester)[4], marlex mesh (polypropylene)[5], and Merselene mesh (knitted polyester)[6]. Cell transplantation therapies have shown promise for improving heart function after myocardial infarction[7]. However, the cell engraftment efficiency is low due to significant loss of cells from the site of injury following transplantation.
One promising approach is to prevent the increase of heart failure after myocardial infarction is the implantation of engineered cardiac patch at the site of infarction. In addition of having enough elasticity for mechanical support, an ideal cardiac patch material must provide an excellent milieu for cell survival. Furthermore, the ideal biomaterial should be capable of being safely replaced by newly formed tissue and also degrade appropriate time period without producing any toxic products[8]. Naturally derived materials used in experimental or clinical treatment of infarcts include tumor-derived basement membrane matrix gel (matrigel)[9], alginate[10], collagen[11], laminin[12], fibrin[13] and decellularized extracellular matrix (ECM)[14], all of which can enhance cell and tissue function in the myocardial region. They provide a natural substrate for cellular attachment, proliferation, and differentiation in its native state. For the above-mentioned reasons, natural polymers like collagen could be a favourable substrate for tissue engineering applications[15,16]. Furthermore, collagen is the most abundant protein in the human body, and imparts structural integrity and tensile strength to tissues.
Tissue disruption following injury requires collagen for the repair and restoration of structure and function. In addition, collagens have a low antigenicity, being only weakly immunogenic largely due to their homology across species and are biodegradable due to their proteinaceous nature[17]. Using matrigel, a collagen-based multicomponent mixture of ECM proteins and growth factors, Zimmermann et al[18] have established one of the most convincing models of three-dimensional cardiac cell cultures, where differentiation status and functional parameters are similar to that of native myocardium. However, there are concerns about Matrigel’s safety because matrigel and basement membrane matrix are known to enhance tumorigenesis and tumor growth in vivo[19-22]. In 1997, Eschenhagen et al[23] reported for the first time, an artificial heart tissue, which was termed engineered heart tissue (EHT). The embryonic chick cardiomyocytes were mixed with collagen solution and allowed to form gel for EHT. By culturing the cardiomyocytes in the collagen matrix, they produced a spontaneously and coherently contracting 3D heart tissue construct in vitro. However, poor mechanical supportive ability of collagen gels was a major drawback associated with this approach. Hence a combination of poly (glycerol sebacate) (PGS) with collagen was suggested with an objective to overcome this drawback for cardiac tissue engineering (CTE). The elastomer PGS, recently developed for soft tissue engineering[24,25], represents a feasible substrate from the mechanical perspective; Collagen favours enhanced cell adhesion and prevents cells loss at the site of implantation. The conventional electrospinning technique is to dissolve the polymer in a solvent, which evaporates during the spinning process. However, this approach is not pragmatic with PGS. Although, there are solvents available for dissolving PGS[26], its low molecular weight results in such a low solution viscosity that even with a high concentration solution, the electrospinning of PGS fibers cannot occur. Hence, it was necessary to develop a core/shell electrospinning process[27] to produce PGS fibers with a protective shell polymer. The combination of PGS/collagen core/shell fibers with a unique ECM like topography has been suggested to be a potential cardiac patch material for MI. Fabricated core/shell material; the core material is solely responsible for mechanical properties, whereas the shell material is responsible for extrinsic factors like cell adhesion and proliferation. The optimal cell source to create an engineered myocardial patch should be easy to harvest, proliferative, non-immunogenic and has the ability to differentiate into mature, functional cardiomyocytes. Studies have shown that after expansion, stem cells can be directed to differentiate into cardiomyogenic lineages[28,29]. Cardiomyocytes have natural contractile and electrophysiological properties, are difficult to obtain, to expand, and are allogenic. In contrast, bone marrow derived stem cells have the ability to differentiate into any desired cell type in the presence of cues and are non-immunogenic, making them an ideal cell source.
In this study, we hypothesize that a combinatorial approach of PGS/collagen core/shell fibrous patch material and stem cell therapy is of potential interest for the treatment of heart failure rather than either strategy alone. Our approach takes advantage of the ability of an elastomeric biomaterial sheet comprising of PGS/collagen fibers to act as a flexible patch; with this approach: (1) Cells would remain adhered to the nanofibrous patch preventing cell loss and providing a more site-directed repair mechanism. It is increasingly accepted that physical cues play a key role in cell growth and tissue assembly[30,31]. These signals are important in stem cells during self-renewal, proliferation, and differentiation; (2) A softer substrate and the ability to tune the mechanical properties within a given range could be advantageous as cell differentiation was shown to be affected by substrate stiffness[32]; (3) Additionally, it has been estimated that a cell number on the order of one billion would need to be replaced in patients with heart failure[33]. The present study proposed PGS/collagen core/shell fibrous scaffold similar to cardiac ECM like topography, which promotes in situ regeneration and homing of cells; thereby reducing the number of requisite cells, is desirable for cardiac tissue engineering.
MATERIALS AND METHODS
Fabrication of core/shell fibers
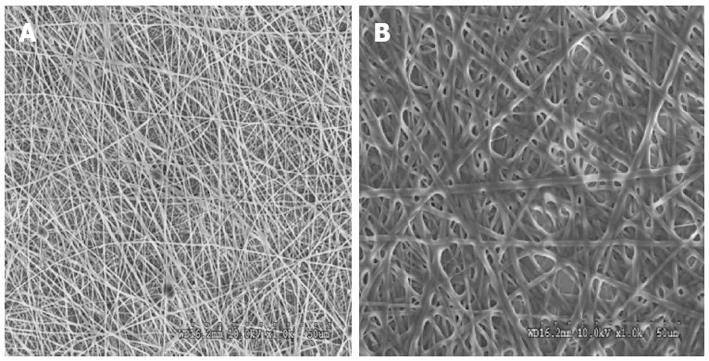
PGS was synthesized by the procedure described by Wang et al[34] a mixture of glycerol and sebacic acid in the ratio of 1:1 was reacted at 120 °C under nitrogen for 24 h. The pressure was then reduced to 40 m Torr and the reaction held at 120 °C for 48 h to synthesise PGS. Collagen type I (8%) was dissolved in 1, 1, 1, 3, 3, 3-hexafluoro-2-propanol (HFP) (Aldrich Chemical Company, Inc., St. Louis, United States) to form shell solution and PGS (15%) was dissolved in same solvent to form the core solution. The coaxial spinneret had an inner diameter of 1 mm and an outer diameter of 2.0 mm was designed such that the fluids were immiscible before exiting the nozzle. Fluid was loaded to the nozzle by two syringe pumps (KD Scientific Inc., MA, United States) that provide a constant-volume flow rate of 0.3 mL/h for core solution and 1.2 mL/h for shell solution. A high voltage electric field (DC high voltage power supply from Gamma High Voltage Research, FL, United States) of 15 kV was applied at the tip of the spinneret. A collector plate was placed at a distance of 15 cm from the tip of the spinneret to collect core/shell fibers. Collagen nanofibers were also fabricated using 8% w/v solution in HFP separately. The electrospinning conditions used were 1.2 mL/h flow rate, 12 cm distance between the needle tip and collector plate and 12 kV voltage supply. The fibers produced were subsequently vacuum dried to remove the residual solvents. The fibers were then cross-linked using 50% glutaraldehyde (Sigma) vapour for 24 h in order to improve its mechanical stability.
Material characterization
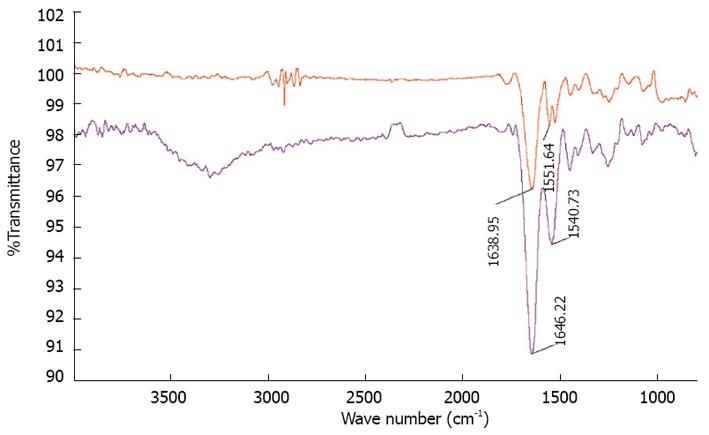
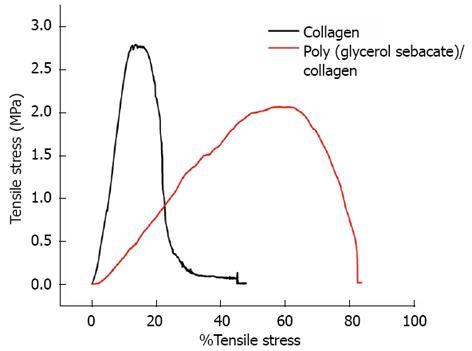
The surface morphology of electrospun nanofibrous scaffolds was studied under scanning electron microscope (JEOL JSM-5600LV) at an accelerating voltage of 10 kV, after gold coating (JEOL JFC-1200 fine coater, Japan). For calculating the fiber diameter of the nanofibers from the SEM images, n = 10 fibers were chosen randomly on each of the scaffolds. For each scaffold material n = 5 samples were chosen for measuring the fiber diameter. The average fiber diameter along with SD was then analyzed from the SEM images using image analysis software (Image J, National Institutes of Health, United States). Functional groups present in the scaffolds were analyzed using Fourier Transform Infrared (FTIR) spectroscopic analysis on avatar 380, (Thermo Nicolet Waltham, MA, United States) over a range of 400-4000 cm-1 at a resolution of 4 cm-1. The hydrophobic or hydrophilic nature of electrospun fibers was measured by sessile drop water contact angle measurement using VCA optima surface analysis system (AST products, Billerica, MA, United States). Tensile properties of electrospun fibrous scaffolds were determined using a tabletop tensile tester (Instron 3345, United States) at 10 mol/L load capacity under dry testing conditions. Rectangular specimens of dimensions 10 mm × 20 mm were used for testing at a rate of 10 mm/min. The data’s were recorded at room conditions 25 °C and 34% humidity. In order to avoid uncertainty of data, only the results of those samples which have failed in the centre; giving a dog bone like appearance have been used for the stress-strain curves calibration. The data’s of those samples which have failed at the grips, where the load was being applied, were not employed for the calculation. The tensile stress-strain curve was drawn using excel sheet.
Cell culture
The electrospun fibers collected on round glass cover slips of 15 mm in diameter, were placed in a 24-well plate with stainless steel rings to prevent lifting on the cover slips. The fibers were sterilized under ultraviolet (UV) light for 2 h, washed thrice with phosphate buffered saline (PBS) for 15 min each, in order to remove any residual solvent and prevent cytotoxicity from glutaraldehyde. The fibers were subsequently immersed in Dulbecco’s modified Eagle’s medium (DMEM) overnight before cell seeding. The rabbit cardiac cells were isolated using collagenase treatment. The rabbit heart was fragmented into tiny pieces and washed thoroughly with 2× and 3× antibiotic solutions made in PBS, thrice for 30 min. Subsequently, it was followed by treatment with 1% collagenase in PBS at 37 °C for 30 min. The fragmented tissues were cultured in DMEM media supplemented with 10% FBS (GIBCO Invitrogen, United States) and 1% antibiotic and antimycotic solutions (Invitrogen Corp, United States) in a 75 cm2 cell culture flask to isolate cardiomyocytes. The culture medium was changed once in every 2 d. MSCs (PT-2501, Lonza, United States) were cultured in low glucose DMEM media supplemented with 10% FBS (GIBCO Invitrogen, United States) and 1% antibiotic and antimycotic solutions (Invitrogen Corp, United States) in a 75 cm2 cell culture flask. Cells were incubated in CO2 incubator at 37 °C at 5% CO2. Before seeding the cells were detached by adding 1 mL of 0.25% trypsin containing 0.1% EDTA. Detached cells were centrifuged, counted by trypan blue assay using a hemocytometer and seeded on the scaffolds. The scaffolds were separated into three groups-the control tissue culture plate (TCP), collagen nanofibrous scaffolds and the PGS/collagen core/shell fibers. These were further segregated into (1) co-culture scaffolds, onto which both MSCs and cardiac cells were seeded in the ratio of 1:1 at a seeding density of 10 000 cells per well (5000 MSCs:5000 cardiac cells); (2) positive control scaffolds, onto which cardiac cells were seeded at the same seeding density of 10 000 cells per well and (3) The final batch comprised of the negative control scaffolds, onto which MSCs were seeded at the same seeding density of 10 000 cells per well.
Cell proliferation
The cell proliferation on different scaffolds was analyzed using MTS assay (CellTiter 96 Aqueous One solution reagent, purchased from Promega, Madison, WI, United States). The rationale behind the MTS assay involves the reduction of yellow tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)-2H-tetrazolium] in MTS to form purple formazan crystals by the dehydrogenase enzymes secreted by mitochondria of metabolically active cells. The formazan dye shows absorbance at 490 nm and the amount of formazan crystals formed is directly proportional to the number of cells. After 5 d of cell seeding, the media was removed from the well plates and the scaffolds were washed in PBS. The scaffolds were then incubated in a 1:5 ratio mixture of MTS assay and serum free DMEM medium for 3 h at 37 °C in a 5% CO2 incubator. After the incubation period, the samples were pipette out into 96 well plates. The absorbance reading was then taken at 490 nm using a microplate reader (Fluostar Optima, BMG Lab Technologies, Germany). The same procedure was repeated for day 10 and day 15 samples.
Cell morphology
The cell morphology was analyzed using SEM. After 15 d of seeding cells on the scaffolds, the media was removed from the wells and the samples were fixed with 3% glutaraldehyde in PBS for 3 h. The scaffolds were then rinsed with distilled water for 15 min and then dehydrated with a series of ethanol gradients starting from 30% to 50%, 75%, 90% and 100% (v/v). Subsequently the samples were treated with Hexamethyldisilazane (HMDS) solution (Sigma) and allowed to air-dry at room temperature in the fume hood. The samples were then gold coated and the cells morphology was analyzed using SEM.
Expression of cardiac marker protein
To observe whether the MSCs co-cultured with cardiac cells have undergone cardiogenic differentiation, immunofluorescent staining of the selected proteins of cardiomyocytes was performed. For confocal analysis, the cells were fixed with 100% ice cold methanol for 15 min. The samples were then washed with PBS once for 15 min and incubated in 0.5% Triton-X solution for 5 min to permeabilize the cell membrane. Non-specific sites were blocked by incubating the cells in 3% BSA (Sigma) for 1 h. Following which primary antibodies α-actinin and troponin-T (Sigma) were added into separate wells, at the dilution of 1:100 and incubated for 90 min at room temperature. This was followed by washing the samples thrice with PBS for 15 min, to remove the excess unbound primary antibodies; followed by incubation for 60 min with Alexa Fluor 488 secondary antibodies (Invitrogen) in the dilution of 1:250 at room temperature. The samples were again washed thrice with PBS for 15 min. Negative controls were also employed in each analysis to delete the disturbance of the primary or secondary antibody. The cell nuclei were stained using 1:5000 dilution of 4,6-diamidino-2-phenylindole hydrochloride (DAPI; Sigma) for 30 min at room temperature. The cells were again washed with PBS thrice to remove any excess staining. The samples were then removed and mounted over glass slides using H-1000 vectashield mounting medium (Vector Laboratories, United States). The edges of the coverslips were sealed using fluoromount. The samples were then viewed using fluorescence microscopy for the cardiac marker protein expression (Olympus FV 1000).
Double immunofluorescent staining was further performed on the co-culture scaffolds to confirm the differentiation of MSCs into cardiomyocytes. The MSC-cardiac cells co-culture cells cultured on TCP, collagen nanofibers and PGS/collagen core/shell fibers were stained with MSC specific marker CD 105 (abcam, United States) in the dilution 1:100 for 90 min. at room temperature; prior to which the non-specific sites were blocked with 3% BSA. This was followed by the addition of secondary antibody Alexa Fluor 488 (green) in the dilution 1:250 for 60 min. at room temperature. The samples were washed with PBS thrice to remove the excess staining. The samples were then treated with cardiac specific marker protein actinin in the dilution 1:100 for 90 min at room temperature. This was followed by the addition of the secondary antibody Alexa Fluor 594 (red) (Invitrogen) in the dilution 1:250 for 60 min. at room temperature. The samples were washed with PBS thrice to remove the excess staining. The samples were then incubated with DAPI in the dilution 1:5000 for 30 min at room temperature. The samples were then removed and mounted over a glass slide using vectashield mounting agent and examined under the fluorescent microscope (Olympus FV 1000).
Statistical analysis
For each experiment n = 5 samples were tested and all the data presented are expressed as mean ± SD and were analyzed using Student’s t-test for the calculation of statistical significance. Differences were considered statistically significant at P≤ 0.05 and P≤ 0.01.
DISCUSSION
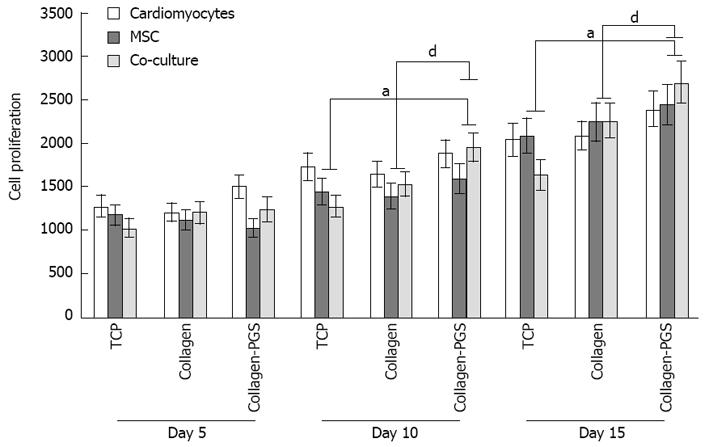
The scaffold properties play an important role in influ-encing the cell responses for cardiac tissue engineering. Cell behaviour such as adhesion and proliferation represent the initial phase of cell-scaffold communication that subsequently influences the cell differentiation[46]. The cell proliferation results as shown in Figure 4, we observed that the MSC and co-culture cells adhesion and proliferation were significantly (P≤ 0.05) higher on day 10 and day 15 on PGS/collagen scaffold compared to the TCP. This was because of the nanofibrous scaffold which resembles the ECM and thereby provides necessary cues, promoting cell proliferation and differentiation as discussed in our previous studies[46-49]. Moreover, studies have reported that cardiomyocyte adhesion and organization into a contractile tissue have been far superior on natural scaffolds compared to synthetic scaffolds[50]. The results observed that the proliferation of co-culture cells was significantly (P≤ 0.01) higher on PGS/collagen core/shell fibers compared to collagen nanofibers on day 10 and day 15 (Figure 4). Additionally, the MSCs proliferation was also significantly higher on day 10 and day 15 on PGS/collagen scaffolds compared to collagen fibers. The percentage increase in the rate of proliferation of co-culture cells from day 5 to day 15 has been calculated to be 87.45% and 118.91% on collagen nanofibers and PGS/collagen core/shell fibers respectively. Enhanced cell population on the co-culture group maybe due to the synergistic effect of cardiac cells and MSCs. The MSCs provide the necessary paracrine signals to prevent apoptosis of the cardiac cells. Recent studies suggest that the transplanted MSCs interact with local tissues in the heart, releasing paracrine factors that support the regenerative process. Thus the beneficial effects of MSCs transplantation are probably mediated primarily through the preservation of cardiac myocytes within the infarction[51]. Additionally, large amount of protective cytokines secreted by MSCs, functioned as the limitation of inflammation, inhibition of apoptosis, and stimulating myoangiogenic differentiation[52]. Stem cell/cardiomyocyte interactions regulate not only cardiac development[53] but also cardiomyocyte function in the adult heart[54]. In MSC-cardiomyocyte co-culture environment, stem cells may promote cardiomyocyte survival[55]. Given the rapid loss of cardiomyocytes after ischemic injury, promoting cardiomyocyte survival is an efficient strategy for preserving viable myocardium[56].
Figure 4 Cell proliferation study for days 5, 10 and 15 on tissue culture plate, poly (glycerol sebacate)/collagen core/shell fibers and collagen nanofibers using cardiomyocytes, mesenchymal stem cells and cardiomyocytes-mesenchymal stem cells co-culture.
Denotes statistical significant difference MSC aP≤ 0.05 vs co-culture cells cultured on TCP and PGS/collagen core/shell fibers; Denotes statistical significant difference MSC dP≤ 0.01 vs co-culture cells cultured on collagen and PGS/collagen core/shell fibers. TCP: Tissue culture plate; PGS: Poly (glycerol sebacate); MSCs: Mesenchymal stem cells.
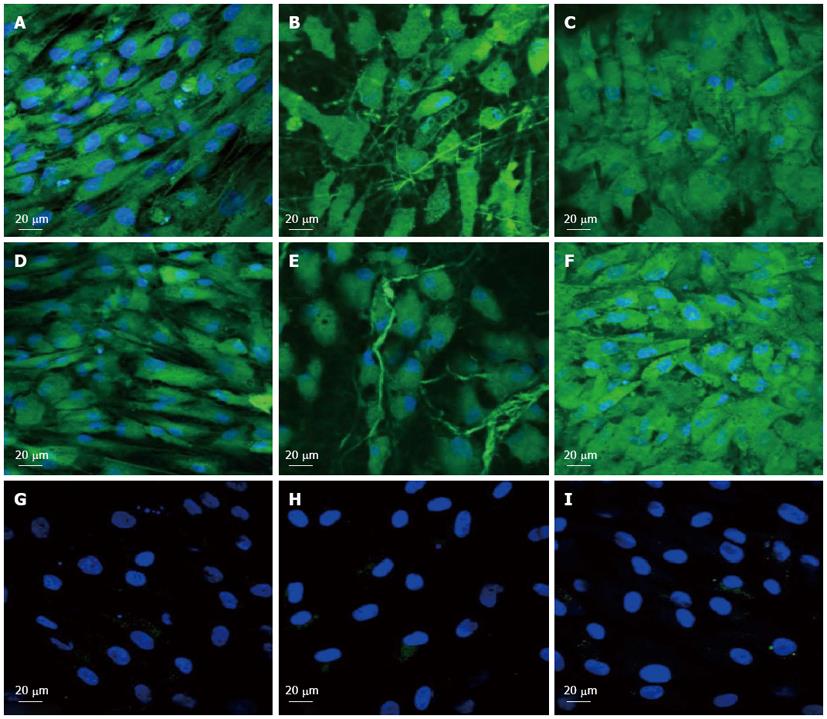
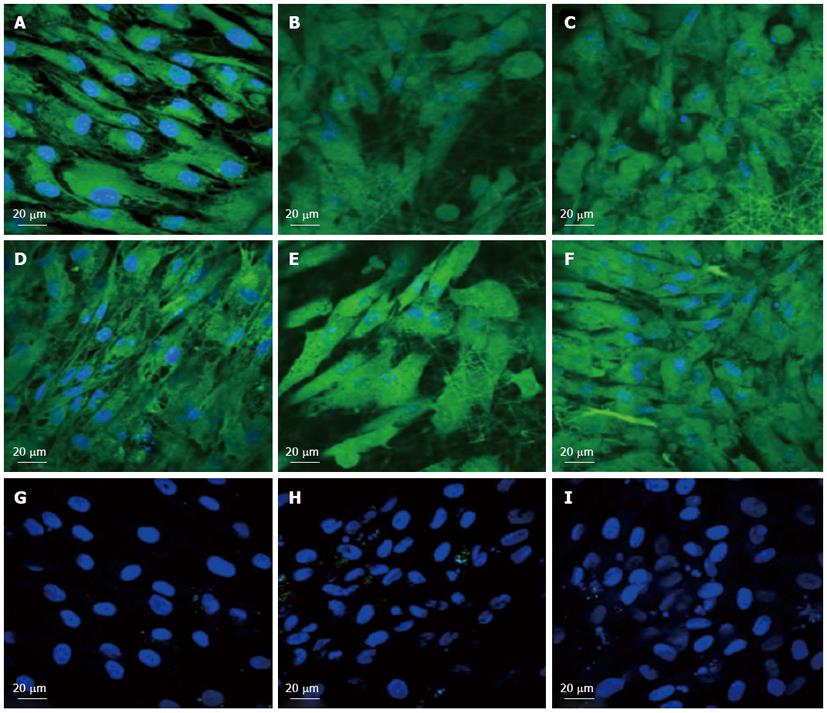
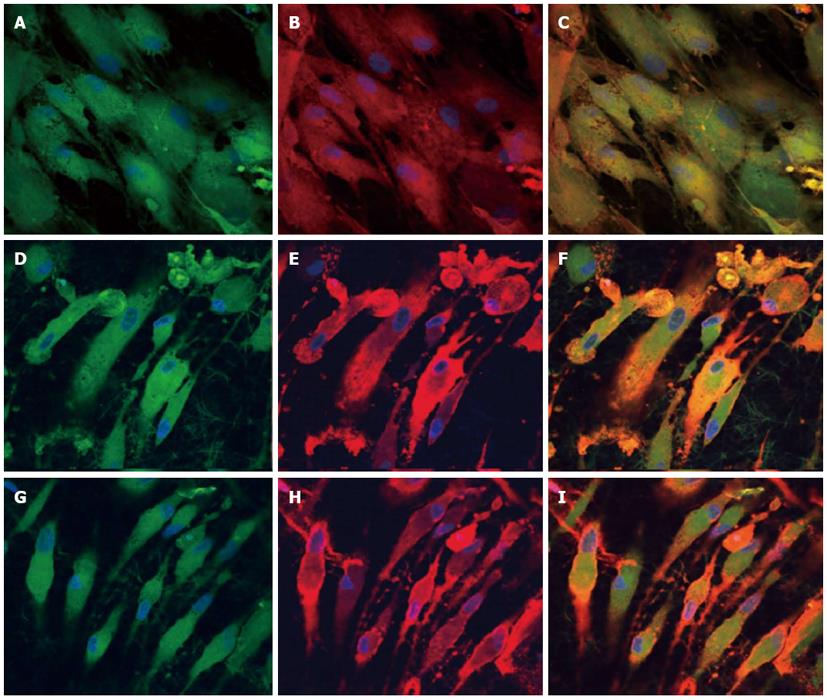
To observe the cellular responses of the patches to the myocardiogenic differentiations of MSCs, the immunofluorescence stains of specific proteins of cardiomyocytes like troponin T and α-actinin were selected[57-59]. Troponin-T is important for effective cardiomyocytes which contain contractile proteins, as it regulates the force and velocity of myocardial contraction[57], and actinin is an important constituent of the contractile apparatus. Many investigators agree that hMSCs also exhibit some cardiogenic potential but the frequency at which cardiac differentiation occurs without any added induction factors is small[60]. Figure 5D-F and Figure 6D-F, demonstrated that the cardiogenic choice can be enhanced in vitro by co-culturing MSCs with cardiac cells. The biological cues secreted by cardiac cells would drive the differentiation of MSCs into cardiac lineage. It has been reported that, in the presence of certain physical and chemical cues, MSCs differentiate into cells that resemble cardiac myocytes and may be applicable for cardiac regeneration[46]. Figure 5 observed that the expression of cardiac proteins actinin (Figure 5D-F) and troponin (Figure 6D-F) are higher in co-culture environment compared to individual cell culture systems of MSCs (Figure 5G-I and Figure 6G-I and cardiac cells (Figure 5A-C and Figure 6A-C). As shown in Figure 5G-I and Figure 6G-I, the MSCs did not express the marker proteins actinin and troponin-T in their undifferentiated state. Hence they express DAPI alone which stains the nucleus of MSCs. It is increasingly accepted that physical cues play a role in cell growth and tissue assembly[30,31]. These signals are important in stem cells (SCs) during self-renewal, proliferation and differentiation. Hence the presence of cardiac cells in the stem cell-cardiac cells co-culture provides the necessary cues to trigger the cardiogenic differentiation of MSCs, in the absence of any other soluble differentiating factors. Furthermore, the cardiac marker expression was higher on the PGS/collagen scaffold compared to the collagen nanofibers. This increase in protein expression is because of the difference in the mechanical property between PGS/collagen and collagen substrates. It has been shown that a softer substrate and the ability to tune the mechanical properties within a given range could be advantageous as cell differentiation was shown to be affected by substrate stiffness[32]. Owing to the favourable mechanical properties of PGS, the cell differentiation was more on PGS/collagen scaffolds compared to collagen scaffolds. There was no protein expression in MSC culture group (Figure 5G-I and Figure 6G-I) proving that in the absence of cues, the cardiogenic differentiation of MSCs may not occur. However in the co-culture environment we found that more cells express the cardiac specific marker proteins, indicating that the MSCs have differentiated into cardiac cells and therefore express troponin T and actinin markers. This cardiogenic differentiation of MSCs was further confirmed by dual immunostaining. Figure 7A, D, G shows the expression of MSC specific marker protein CD 105 by the MSCs cultured in the co-culture environment on TCP, collagen and PGS/collagen core/shell fibers. Figure 7B, E, H shows the expression of cardiac marker protein actinin. The MSCs which have undergone cardiogenic differentiation, express both CD 105 and the cardiac specific marker protein actinin. This result in dual expression of both CD 105 and actinin by the MSCs which have undergone cardiogenic differentiation, as shown in Figure 7C, F, I. We observed that PGS/collagen core/shell fibers (Figure 7I) express higher level of actinin expression in differentiated MSCs compared to collagen scaffolds (Figure 7F). However, the differentiated MSCs did not exhibit any contraction. A similar study was reported[61], where stem cells were delivered to the canine RV on an ECM patch, and tracked with quantum dots, some of these cells were demonstrated to differentiate to mature myocytes. Similar to our study, these cells were considered “cardiogenic”, since they express cardiac markers like troponin T, but do not contract in vitro and have not fully differentiated into functional cardiac myocytes. Moreover, the study also suggested that since the cardiogenic cells did not exhibit the complete cardiac phenotype in vitro, it remained possible that the ‘committed’ cells retained the capacity to proliferate in vitro. Ideally, it has been reported that a full cardiogenic differentiation of stem cells should not occur until the cells are transplanted into the heart, since different regions of the heart have unique ion currents and the expression of these currents may be affected by the local environment[62].
Figure 5 Immunocytochemical analysis for the expression of cardiac marker protein actinin at 60 × magnification on the tissue culture plate (A, D, G), collagen nanofibers (B, E, H) and poly (glycerol sebacate)/collagen core/shell fibers (C, F, I) comprising of cardiomyocytes (A-C), mesenchymal stem cells-cardiomyocytes co-culture group (D-F) and mesenchymal stem cells (G-I).
Nucleus stained with 4,6-diamidino-2-phenylindole hydrochloride.
Figure 6 Immunocytochemical analysis for the expression of the cardiac marker protein Troponin at 60 × magnification on the tissue culture plate (A, D, G), collagen nanofibers (B, E, H) and poly (glycerol sebacate)/collagen core/shell fibers (C, F, I) comprising of cardiomyocytes (A-C), mesenchymal stem cells-cardiomyocytes co-culture group (D-F) and mesenchymal stem cells (G-I).
Nucleus stained with 4,6-diamidino-2-phenylindole hydrochloride.
Figure 7 Dual immunocytochemical analysis for the expression of mesenchymal stem cells marker protein CD 105 (A, D, G) and cardiac marker protein Actinin (B, E, H) in the co-culture samples and the merged image showing the dual expression of both CD 105 and Actinin (C, F, I); on the tissue culture plate (A, B, C), collagen nanofibers (D, E, F) and poly (glycerol sebacate)/collagen core/shell fibers (G, H, I) at 60 × magnification.
Nucleus stained with 4,6-diamidino-2-phenylindole hydrochloride.
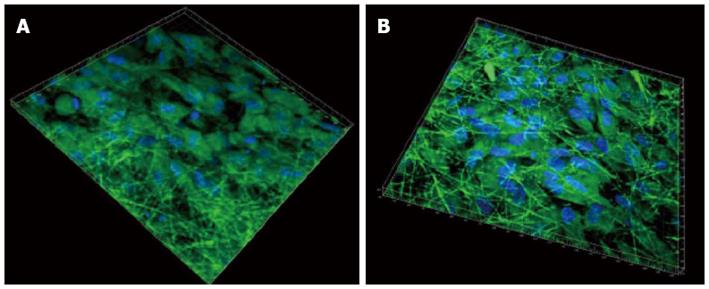
The ability of seeded cells to adhere, survive and migrate within a scaffold is crucial when trying to regenerate a tissue in vivo. Previous reports of cardiac tissue engineering noted that even if the surface layers of the construct were filled with cells, its interior was frequently devoid of tissue regeneration[63]. Since the fibrous scaffolds are highly porous, it favours the cells to penetrate deep within the scaffold. This was further confirmed by 3D confocal images, as shown in Figure 8, processed using Imaris software. It was observed that the cells penetrated more profoundly within the PGS/collagen fibers (542 nm) when compared to the collagen scaffolds (475 nm). This is because of the favourable mechanical property and flexibility of PGS, which favours easier cell migration, causing the cells to crawl inside, towards the interior of the scaffold.
Figure 8 3D image using Imaris software of cardiomyocytes-mesenchymal stem cells co-culture group stained with cardiac specific marker protein troponin at 60 × magnification on (A) collagen fibers (B) poly (glycerol sebacate)/collagen core/shell fibers.
Nucleus stained with 4,6-diamidino-2-phenylindole hydrochloride.
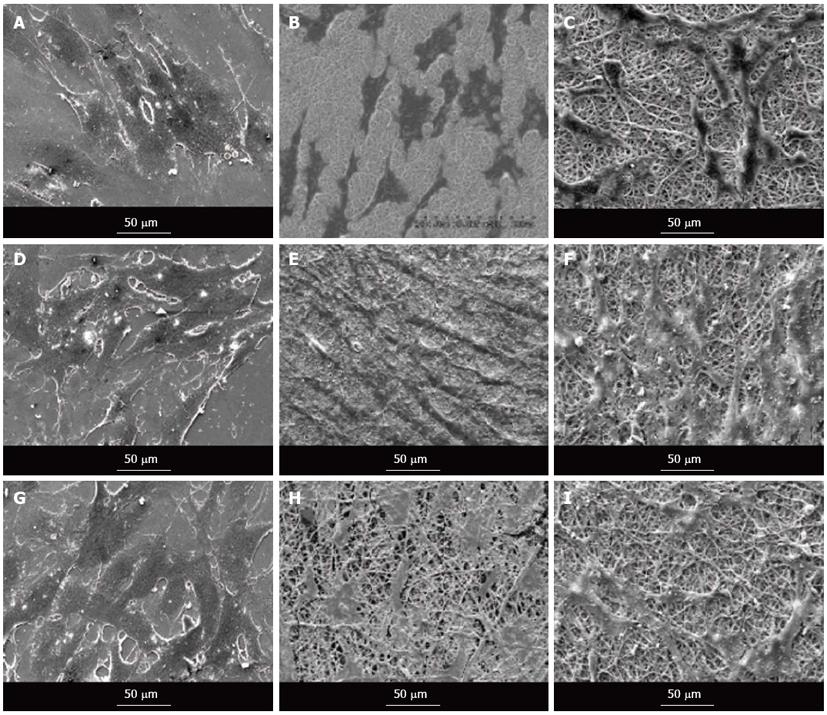
Quantification of the number of cells present on nanofibrous scaffolds and cellular behaviour is also another pivotal indicator to determine the potential application of a material construct for any tissue engineering application. The cell morphology was studied using SEM images as shown in Figure 9. It showed that the nanofiber contour allowed the cardiac cells to make extensive use of available cues for isotropic or anisotropic growth, and to some degree even to crawl inside and pull the fibers, as evidenced in Figure 9A-C. The results showed that the cell-to-cell interaction between the MSC and cardiac cells group, leading to cell fusion, was observed in co-culture group (Figure 9D-F). This cell-to-cell interaction favoured the MSCs to undergo cardiogenic differentiation. The differentiated MSCs in the co-culture system acquired the cardiomyocyte phenotype as revealed in Figure 9D-F. MSCs also showed favourable growth on the nanofibers with the greater cell-to-cell contact and extension of filopodia as evidenced in Figure 9G-I.
Figure 9 Scanning electron microscope images showing the cell morphology of cardiomyocytes (A-C), cardiomyocytes-mesenchymal stem cells co-culture cells (D-F) and mesenchymal stem cells (G-I) grown on tissue culture plate (A, D, G), collagen nanofibers (B, E, H) and poly (glycerol sebacate)/collagen core/shell fibers (C, F, I) on day 15 at 500 × magnification.
We have employed the use of co-culture system of stem cells with scaffold microenvironments engineered to improve tissue survival and enhance differentiation. Transplanted MSCs may differentiate in situ into cells of cardiomyogenic lineage, promoted by the local microenvironment. These in turn could integrate into the myocardium and help to regenerate damaged tissue. We have reported that direct cell-to-cell contact between MSC and adult cardiac cells is necessary for the differentiation of MSC into cardiac cells. This integrative approach of PGS/collagen core/shell nanofibrous cardiac patch material, to prevent cell loss, and stem cell therapy, would maximize the capacity of myocardial tissue regeneration.
In conclusion, even though the implanted stem cells survived and regenerated the infarcted myocardium, the site of MI is a poor environment for cell growth. To increase cell viability, some factors to improve such an infertile environment are desirable. We aimed at improving the quality of the local microenvironment by trapping the cells within the nanofiber mesh and then transplanting to the infarcted site, which may in turn improve survival of the cells and facilitate the biological behaviour of implanted cells. Electrospun fibrous scaffolds provide both flexibility and guidance for cardiac cells growth and MSC differentiation into cardiac lineage and thus can be successfully applied to obtain structurally and functionally competent cardiac tissue constructs. There have been several studies of different scaffolds for cardiac tissue engineering, but there has been few research focused on elastomeric scaffolds like PGS for CTE. In fact, no research has been done so far on the PGS/collagen scaffold material for MI. New solutions including the recruitment, in vitro proliferation and homing of the patients own stem cells, combined with biocompatible and bio-mimicking materials will open new doors in the field of cardiac tissue engineering for MI. The biomaterial employed should be able to interact on the molecular level, with the cells in a precise and controlled manner, similar to the natural interactions existing between cells and the native ECM. We have shown that the direct cell-to-cell contact between MSCs and adult cardiac cells, governed the differentiation of MSCs into cardiac cells. This novel combinatorial paradigm of a PGS/collagen mechanical construct with MSC-cardiac cells co-culture environment might ultimately bring cardiac tissue engineering into clinical application.