COMMENTARY ON HOT ARTICLES
We read with great interest the recent article by Toshner et al[1] describing the analysis of pressure decay curve after pulmonary arterial occlusion (between the moment of occlusion and the pulmonary artery occluded pressure, PAOP) to test if the occlusion technique distinguished small from large vessel disease in chronic thromboembolic pulmonary hypertension (CTEPH).
Pulmonary endarterectomy (PEA) of major, lobar, and segmental pulmonary arteries branches is the mainstay of therapy for patients with CTEPH. The best surgical results are achieved with complete endarterectomy and early postoperative reduction of pulmonary vascular resistance (PVR) to < 500 dyn.s.cm-5[2,3]. The cause of residual pulmonary hypertension in most cases is from concomitant small vessel disease, with three possible scenarios: (1) predominant obstructions of small subsegmental elastic pulmonary arteries; (2) classic pulmonary arteriopathy of small muscular arteries and arterioles distal to non-obstructed elastic pulmonary artery; and (3) pulmonary arteriopathy of small muscular arteries and arterioles distal to partially or totally obstructed elastic pulmonary artery[4,5].
The optimal characterization of the contribution of large vessel and small vessel disease to the elevation of afterload and its influence on the hemodynamic severity is crucial for the preoperative assessment and outcome of PEA[6-8].
An approach for the identification of distal vasculopathy in CTEPH is the analysis of pressure decay curve after pulmonary arterial occlusion (between the moment of occlusion and the pulmonary artery occluded pressure, PAOP)[9]. Such curves are made of a first fast component, which corresponds to the reduction of flow through arterial resistance, and a slower component, which corresponds to the emptying of compliant capillaries through a venous resistance. This biexponential fitting of the pressure decay curve allows identification of an inflection point (Poccl), from which one calculates an upstream resistance (Rup), essentially determined by the resistive properties of the large pulmonary arteries, and a downstream resistance determined by the cumulated resistance of small arterioles, capillaries and veinules. Rup is calculated as follows: Rup (%) = 100 × (mPAP-Poccl)/(mPAP-PAOP). In patients with small-vessel arteriopathy the Poccl pressure was higher (a longer time was required for the pressure to reach PAOP), and therefore the Rup was lower. Patients with CTEPH and Rup value < 60% appear to be at highest risk[9].
To test the hypothesis that the occlusion technique is able to discriminate large vessel organized thrombus from distal vasculopathy. Toshner et al[1] performed occlusion pressures on patients with operable CTEPH, distal inoperable CTEPH and post-PEA residual CTEPH. They also undertook measurements in patients with idiopathic or connective tissue associated pulmonary arterial hypertension (PAH), as additional controls, where more diffuse vasculopathy is traditionally accepted. They employed both, the standard flow-directed measurement and the wire-directed approach. The latter involved a wire being passed into an alternative segmental artery and subsequently being floated into a distal artery. The authors found that Rup as measured by the occlusion technique is increased in operable predominantly proximal CTEPH when compared with inoperable CTEPH and idiopathic PAH. However, they obtained a higher Rup cutoff value compared to Kim et al[9]: 79% (sensitivity 100%, specificity 57%) vs 60% (sensitivity and specificity 100%) and they did not explain the differences of the Rups values, including the values of the two operate patients who died (68% and 73%). They recognized that the occlusion technique would not interrogate the correct range of vessel caliber and would mislabel a significant portion of resistance in these small vessels as upstream. This can be supported by the fact that the idiopathic PAH and inoperable CTEPH cohorts had a much higher Rup than would be expected if resistance had been accurately partitioned into clinically relevant small and large vessels[1]. Finally, they proposed the multiple wire-directed measurements in conjunction with the flow-directed one, in order to provide additional information on disease heterogeneity in CTEPH, although they recognize their data does not support the clinical use of this technique in routine assessment.
Beyond the embolic or thrombotic hypothesis of pathogenesis of CTEPH, once vessel obliteration is sufficient to cause increase of pulmonary arterial pressure, a process of pulmonary vascular remodeling like idiopathic PAH lesions is started which self-perpetuates the progression of pulmonary hypertension[10,11]. The presence of large-vessel remodeling process of thrombus organization and small vessel disease might create a wide spectrum of dynamic (steady and pulsatile) afterload in CTEPH patients[12,13]. We proposed the study of Zup, a novel hemodynamic index. Zup is calculated by (mPAP-dPAP) × 100/(mPAP-PAOP), where mPAP and dPAP are mean and diastolic pulmonary arterial pressure, respectively[14]. mPAP is the time-averaged PAP throughout cardiac cycle length and it is accurately described by cardiac output, total PVR and right atrial pressure. Previous studies have established a link between the steady and pulsatile component of PA pressure by estimating mPAP from systolic PAP (sPAP) and dPAP (‘two-pressure model’)[15-17]. The geometric mean of sPAP and dPAP was the most precise estimate of mPAP (mPAP2 = sPAP × dPAP). sPAP and dPAP mainly depend on total PVR and pulmonary artery stiffness and wave reflection. Increasing total PVR results in both sPAP and dPAP increase while increasing pulmonary artery stiffness and wave reflection generate a wider pPAP without significant mPAP change[14,18,19]. A more proximal occlusive site by the fibrotic organized thromboembolic material incorporated into the native vascular intima causes a higher pulmonary artery stiffness. Stiffening of proximal pulmonary arteries could increase characteristic impedance and wave reflection (higher upstream afterload), increasing total PVR but with a lower dPAP, a faster pressure decay profile and Zup increase. Therefore, the balance between mPAP and dPAP provides a rapid tool to describe the functional afterload status of a CTEPH patient, since their absolute contributions on Zup value are higher than PAOP[14].
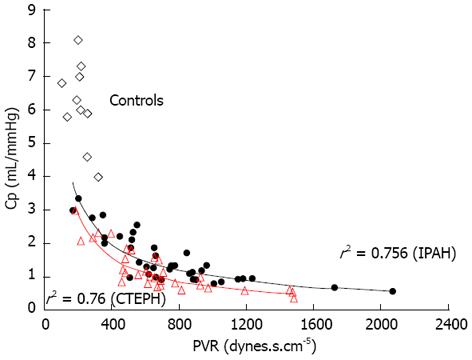
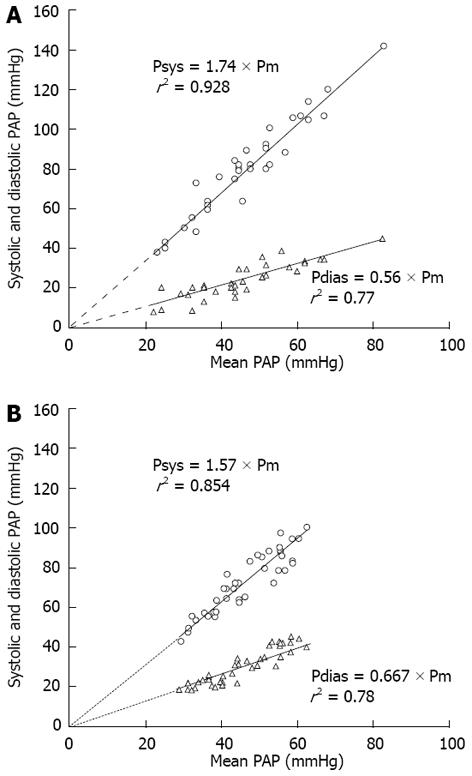
Unlike the partition method described by Kim et al[9] and used by Toshner et al[1], Zup index can be obtained directly from hemodynamic data without assumptions or fitting, and is affected by the extent and localization of anatomic obstruction, vascular remodeling and microvascular disease, setting a wide spectrum of dynamic afterload (steady and pulsatile components)[14]. According to the univariate analysis, we showed that low Zup value (cut-off point < 47%) predicted mortality after PEA with a sensitivity of 100% and a specificity of 78%. The latter increased to 86% when we analyzed the subgroup of 23 patients with higher preoperative PVR (> 9 wood units, median of the cohort), by contrast PVR lost its capacity to predict mortality in this group[14]. In contrast with Toshner et al[1], the Zup value in idiopathic PAH patients was significantly lower than in operable CTEPH patients (43% ± 15% vs 57% ± 15%)[20]. Preoperative operable CTEPH is characterized by a more predominant wave reflection, explaining the lower pulmonary vascular capacitance with a downward and leftward displacement of the PVR-Capacitance curve of the CTEPH patients and a disproportionate increase in sPAP and decrease of dPAP with respect to idiopathic PAH cohort (Figures 1 and 2)[20-22].
Figure 1 Inverse proportional relation between pulmonary vascular resistance and arterial compliance for operable chronic thromboembolic pulmonary hypertension and Idiopathic pulmonary arterial hypertension patients (Hollow diamonds represent ten normal subjects without pulmonary hypertension).
CTEPH: Chronic thromboembolic pulmonary hypertension; IPAH: Idiopathic pulmonary arterial hypertension; PVR: Pulmonary vascular resistance.
Figure 2 Interrelationship of pulmonary arterial pressures for operable chronic thromboembolic pulmonary hypertension and idiopathic pulmonary arterial hypertension patients with similar pulmonary vascular resistance.
A: Chronic thromboembolic pulmonary hypertension; B: Idiopathic pulmonary arterial hypertension.
CTEPH has been recognized as a “dual” pulmonary vascular disorder consisting in a major vessel vascular remodeling process of thrombus organization combined with a small vessel vascular disease[23,24]. PEA is the therapy of choice for patients with surgically accessible CTEPH[25]. The optimal characterization of the reciprocal contribution of large vessel and small vessel disease in the elevation of PVR is crucial for the indication and outcome of PEA[26]. This determination requires the development of diagnostic techniques capable of more objectively partitioning the central surgically correctable component of the PVR from the peripheral component[26]. Although pulmonary arterial occlusion waveform analysis has emerged as a possible way of quantifying the degree of small-vessel disease, it only evaluates the steady component of the afterload and it is possible that this technique inaccurately partitioned resistance into clinically relevant small and large vessels. We proposed a novel hemodynamic index that considers both steady (PVR) and pulsatile (Capacitance) components of the right ventricular afterload simultaneously and could therefore be a complementary tool to improve the risk assessment for PEA in patients with CTEPH.