Revised: December 11, 2012
Accepted: December 21, 2012
Published online: February 26, 2013
Processing time: 197 Days and 23.8 Hours
We report the case of a 14-year-old boy with ventricular preexcitation. A standard, fluoroscopy guided, ablation procedure was successfully performed in a postero-midseptal region with a total fluoroscopy time of about 45 min (2430 cGy.cm2). A few hours after the procedure, preexcitation reappeared. A second ablation procedure was scheduled using the EnSite NavX™ mapping system. During mapping along the tricuspid groove, preexcitation suddenly disappeared due to mechanical “bumping” of the accessory pathway and it did not recover over the next 30 min. As per our routine practice, the phase of geometry reconstruction has been continuously recorded by the system; thus, an off-line analysis allowed to pinpoint the site of earliest activation and the site of mechanical bumping, where radiofrequency obtained the accessory pathway ablation. The second procedure was performed without using fluoroscopy at all. Thanks to the geometry reconstruction, the procedure was completely successful thus avoiding a further rehospitalization.
- Citation: Casella M, Dello Russo A, Fassini G, Andreini D, De Iuliis P, Mushtaq S, Bartoletti S, Riva S, Tondo C. Manifold benefits of choosing a minimally fluoroscopic catheter ablation approach. World J Cardiol 2013; 5(2): 8-11
- URL: https://www.wjgnet.com/1949-8462/full/v5/i2/8.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i2.8
In the last few years a growing number of papers and case-reports have been published showing the feasibility and safety of a minimally fluoroscopic approach in supraventricular tachycardias ablation[1].
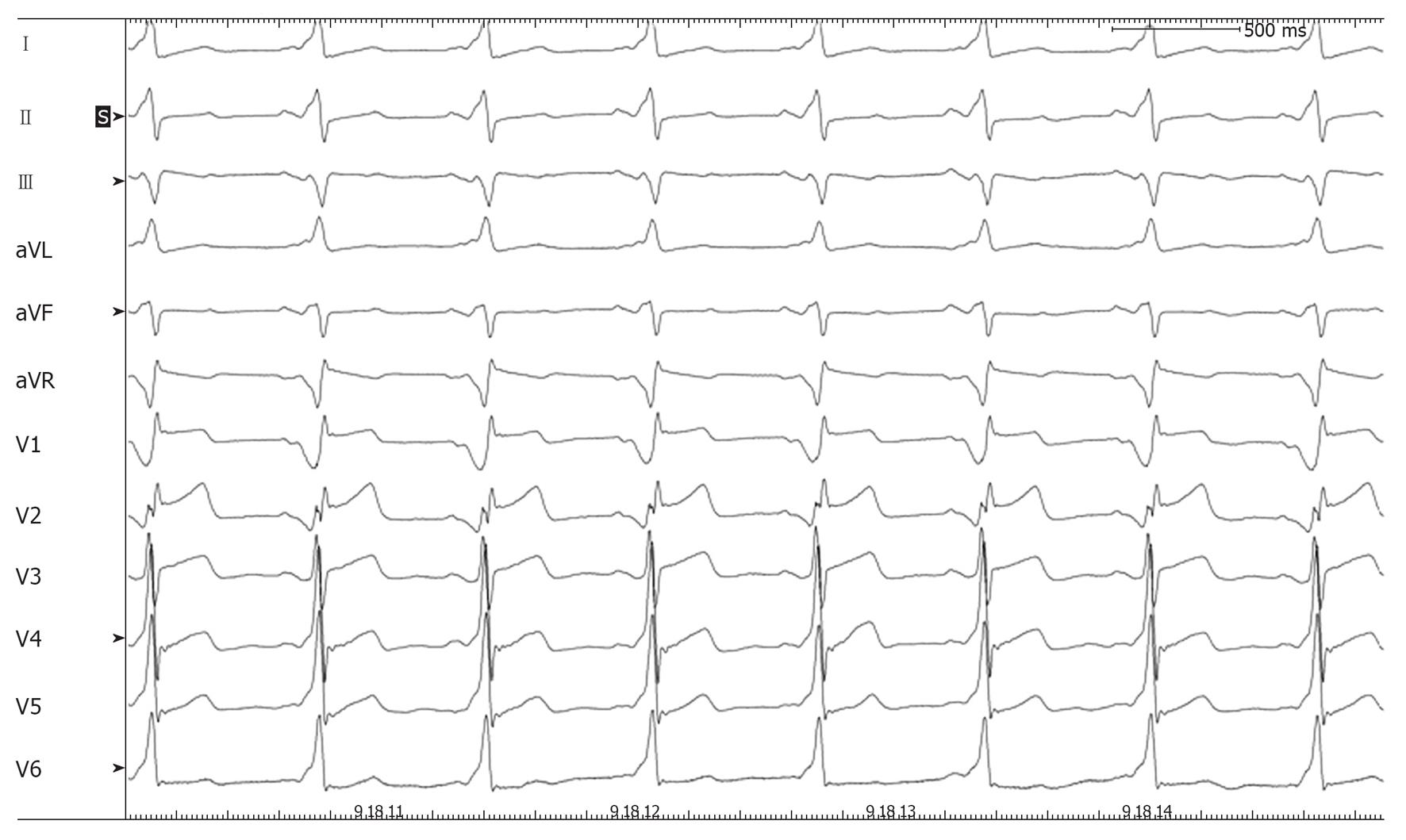
We report the case of a 14-year-old boy with asymptomatic ventricular preexcitation noticed during a standard visit for competitive sports qualification (soccer). The patient underwent a transesophageal electrophysiological study, which revealed that the accessory pathway had a short refractory period (220 ms) and that preexcited atrial fibrillation could be easily induced by atrial stimulation. The patient was denied eligibility for competitive sports and was then referred to our institution to perform catheter ablation of the accessory pathway. With the parents’ consent, the procedure was performed under general anesthesia with endotracheal intubation. Mapping along the tricuspid groove was performed with an irrigated-tip ablation catheter (Thermocool Biosense) showing fused atrioventricular potential near the roof of the coronary sinus ostium. Radiofrequency (RF) pulses delivered at that site were ineffective. Access to the left atrium was then obtained through both retrograde aortic and transseptal approach in order to map the mitral groove and three further RF pulses were delivered in the left postero-septal region, again without suppressing the preexcitation. Mapping along the tricuspid groove was performed again and a fused atrioventricular potential was observed preceding the surface delta wave by 30 ms in a location slightly higher than before, in a postero-midseptal region. A single RF pulse at this site obtained immediate disappearance of the preexcitation and elicited a junctional rhythm with 1:1 retrograde conduction; three consolidation pulses (15 W) were delivered at the same site (Figures 1 and 2). The procedure was concluded after a 30-min monitoring period followed by ventricular stimulation (which documented retrograde conduction only through the atrioventricular node) and adenosine injection (which documented transient complete atrioventricular block). The total fluoroscopy time amounted to 44 min and 53 s (2430, 41 cGy.cm2), corresponding to 4 mSV, the same radiation dose of 40-50 chest X-rays[2]. Thus, this procedure carried, to our patient, a lifetime attributable risk of malignancy of about 5/10 000, as calculated using Table 12D-1 of the BEIR VII report[3].
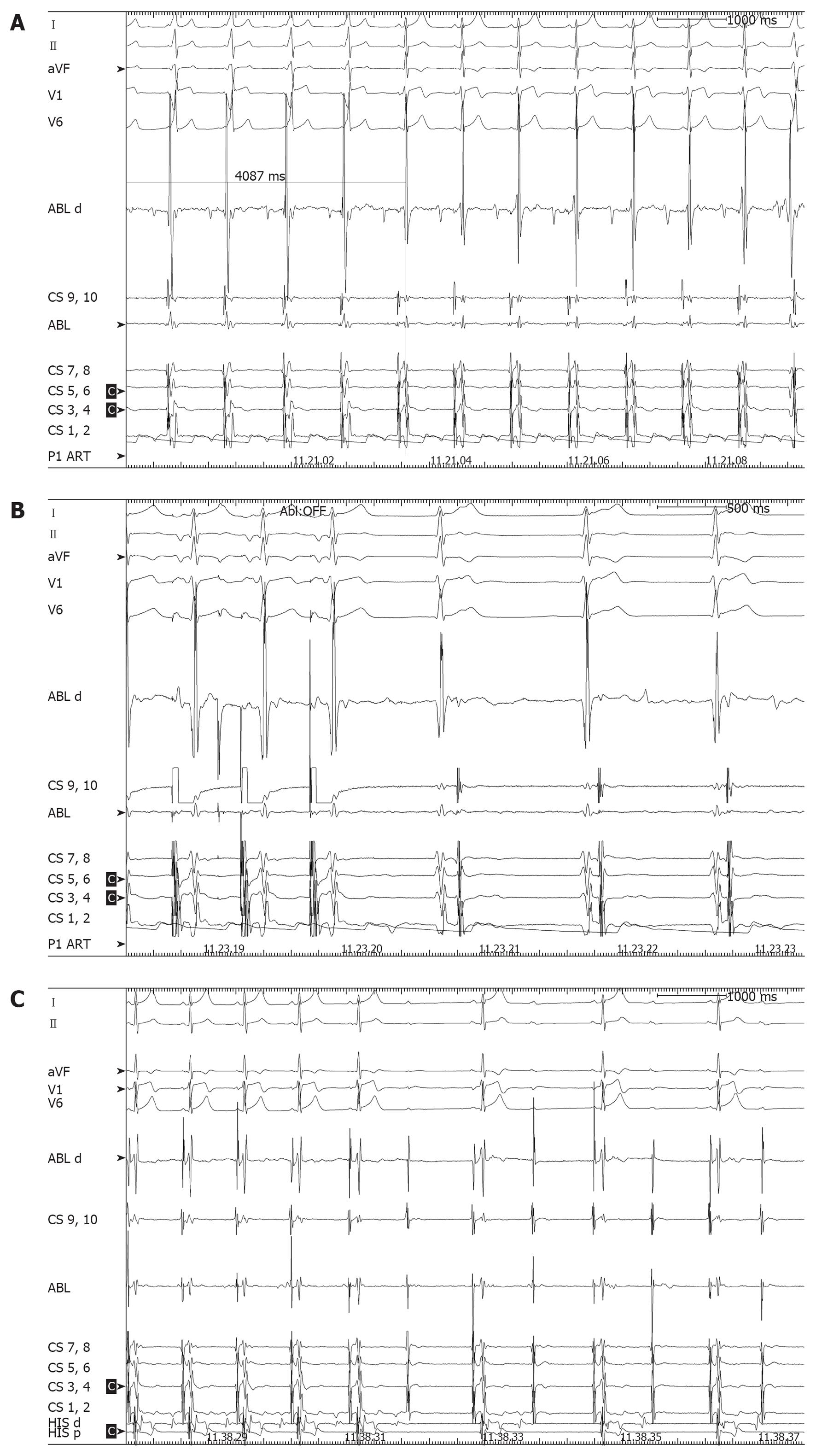
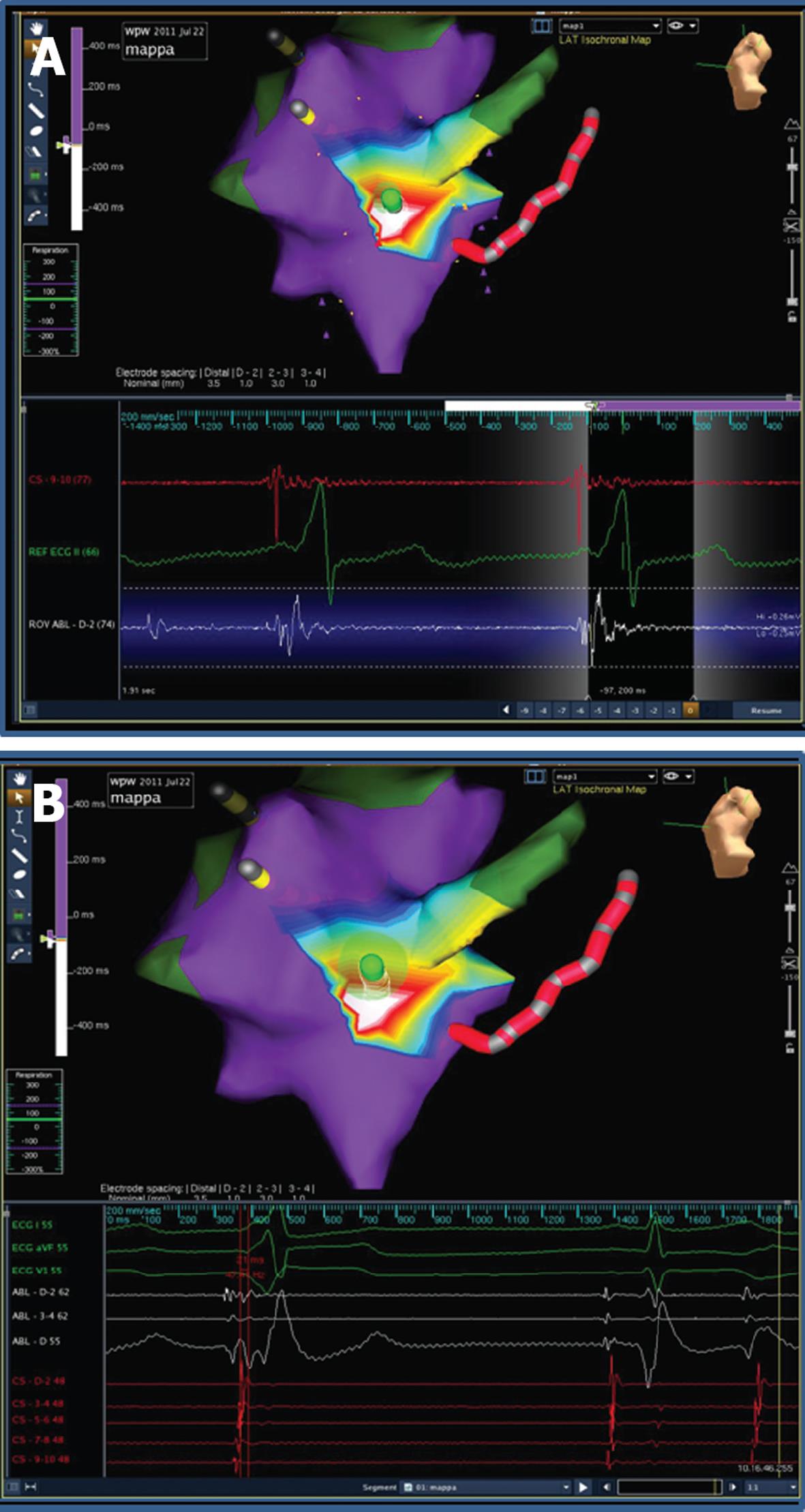
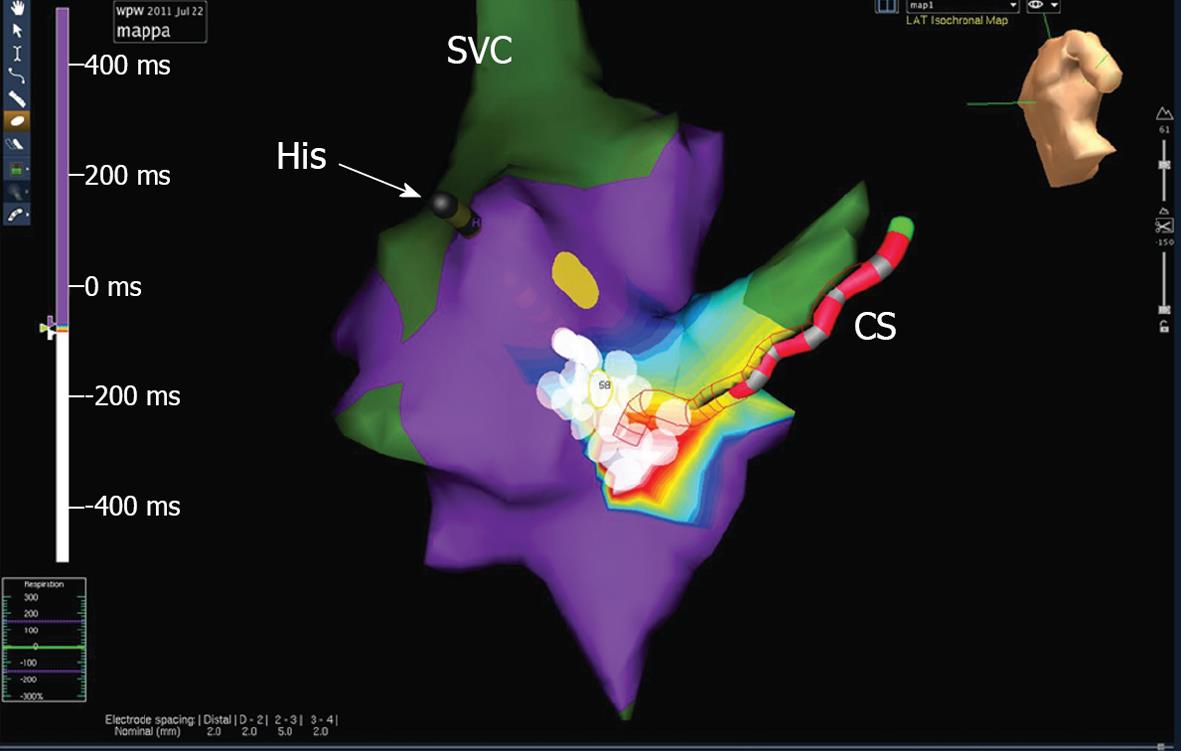
A few hours after the procedure, preexcitation reappeared on electrocardiograms (ECG) with the same morphology. In view of the patient’s strong motivation and after discussing the case with his parents, a second ablation procedure was scheduled for the next day, but in view of the large radiation exposure from the previous procedure, it was decided to use the EnSite NavX™ electroanatomical mapping system as a navigation tool[1]. Ablation was again performed under general anesthesia. The phase of geometry reconstruction was continuously recorded by the system, as per our routine practice. During mapping along the tricuspid groove, preexcitation suddenly disappeared due to mechanical “bumping” of the accessory pathway and it did not recover over the next 30 min. Thus, an off-line analysis of the electroanatomical mapping phase[4] was performed and the activation map obtained allowed to pinpoint the site of earliest activation and the site of mechanical bumping, where seven RF pulses (up to 30 W) were delivered (Figures 3 and 4). The procedure was concluded after a 40-min monitoring period followed by atrial and ventricular stimulation, isoprenaline infusion and adenosine injection, with no evidence of either preexcitation or atrioventricular reentrant tachycardia. The second procedure was performed without using fluoroscopy at all. The patient was discharged after 2 d, with a normal ECG. On a follow-up visit 3 mo later, he remained free of preexcitation.
This issue is of particular interest in pediatric and young patients, as in our case, because they are more vulnerable to the effects of radiation and have a longer life expectancy than adults. In our case, the first procedure was performed with conventional fluoroscopic guidance, according to the operator’s discretion, as to date no guidelines or recommendations are available on this specific regard. The fluoroscopic procedure provided our patient with a non-negligible lifetime attributable risk of malignancy[3], while the second procedure was associated to no ionizing radiation exposure and, as a consequence, it carried no radiological risk.
As an additional peculiarity, in our case the mapping system was useful not only for non-fluoroscopic navigation but also for arrhythmia mapping. As usual in accessory pathway or complex arrhythmia ablations, we record on the system the complete phase of geometry reconstruction, a routine habit that has proved to be particularly helpful. After a lasting mechanical “bumping”, in a conventional fluoroscopy-guided procedure, the study should be stopped without ablation. In our case instead, an off-line analysis of the geometry reconstruction phase allowed to obtain an activation map where the sites of bumping, earliest activation and atrioventricular node were pinpointed. The ablation guided by the off-line activation map proved effective during the subsequent follow-up. Thus the mapping system allowed successful ablation, despite the absence of any preexcitation to be mapped, and ensured safety from procedural complications (i.e., atrioventricular node lesion) with no increase in life-term radiological risk.
We thank Dr. Viviana Biagioli for editorial assistance.
P- Reviewer Lin SL S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Casella M, Pelargonio G, Dello Russo A, Riva S, Bartoletti S, Santangeli P, Scarà A, Sanna T, Proietti R, Di Biase L. “Near-zero” fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavX™ mapping system: personal experience and review of the literature. J Interv Card Electrophysiol. 2011;31:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Bushong SC, Morin RL. Radiation safety. J Am Coll Radiol. 2004;1:144-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Nuclear and Radiation Studies Board, Division on Earth and Life Studies, National Research Council of the National Academies. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press 2006; . |
| 4. | Casella M, Perna F, Dello Russo A, Pelargonio G, Bartoletti S, Ricco A, Sanna T, Pieroni M, Forleo G, Pappalardo A. Right ventricular substrate mapping using the Ensite Navx system: Accuracy of high-density voltage map obtained by automatic point acquisition during geometry reconstruction. Heart Rhythm. 2009;6:1598-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |