Published online Oct 26, 2013. doi: 10.4330/wjc.v5.i10.391
Revised: September 9, 2013
Accepted: September 18, 2013
Published online: October 26, 2013
Processing time: 151 Days and 15.3 Hours
Transcatheter closure of the left atrial appendage with the Amplatzer™ cardiac plug device and double antiplatelet treatment for 3 mo has become an alternative treatment for patients with atrial fibrillation at high embolism risk and contraindications for chronic oral anticoagulation. The inadequate implantation of the left atrial appendage closure device and the discontinuation of double antiplatelet therapy are well-known as factors related to device thrombosis. Nevertheless, device thrombosis after adequate implantation requiring surgical treatment or restarting chronic oral anticoagulation has been reported and can reach 15% of patients. The connector pin thrombosis of the Amplatzer™ cardiac plug, despite a good adherence to antiplatelet treatment, has been recently described as a potential mechanism for device thrombosis. Our clinical case reports the management of this condition for the first time, showing that the early detection of thrombotic complications by transesophageal echocardiography permits solving this serious complication with medical treatment only.
Core tip: Percutaneous closure of the left atrial appendage has become an alternative treatment for patients with atrial fibrillation and with contraindications for chronic oral anticoagulation. Recently, the first case of connector pin thrombosis of the Amplatzer™ cardiac plug device for percutaneous left atrial appendage closure was described. Our work describes the management of this serious problem for the first time.
- Citation: Fernández-Rodríguez D, Vannini L, Martín-Yuste V, Brugaletta S, Robles R, Regueiro A, Masotti M, Sabaté M. Medical management of connector pin thrombosis with the Amplatzer cardiac plug left atrial closure device. World J Cardiol 2013; 5(10): 391-393
- URL: https://www.wjgnet.com/1949-8462/full/v5/i10/391.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i10.391
Transcatheter closure of the left atrial appendage (LAA) with the Amplatzer™ cardiac plug (ACP) device has become an alternative treatment for patients with atrial fibrillation (AF) at high embolism risk and with contraindications for chronic oral anticoagulation (OAC)[1,2]. The inadequate implantation of ACP and the discontinuation of double antiplatelet treatment (DAPT) are well-known as factors related to device thrombosis[1,3]. Furthermore, device thrombosis after adequate implantation, requiring surgical treatment or restarting chronic OAC, has been reported[4,5] and can reach 15% of patients[6]. The connector pin thrombosis of the ACP, despite a good adherence to antiplatelet therapy, has been recently described as a potential mechanism for device thrombosis[7]. The aim of this work is to describe the management of this serious complication after ACP device implantation.
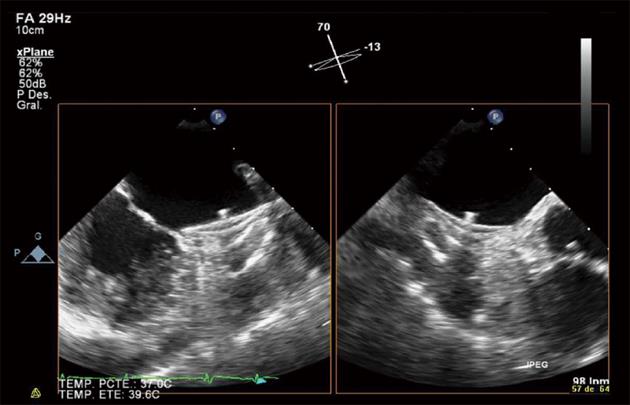
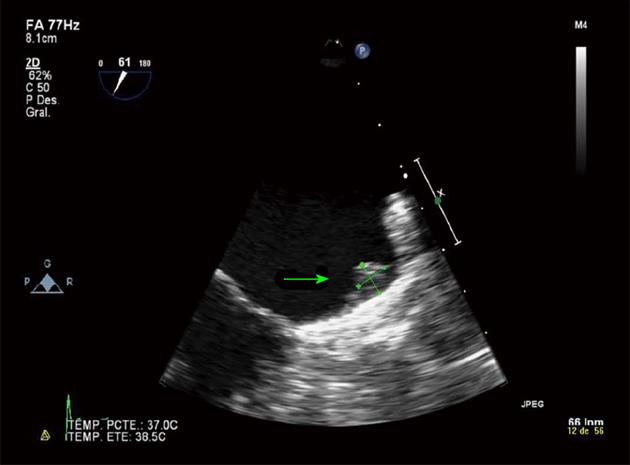
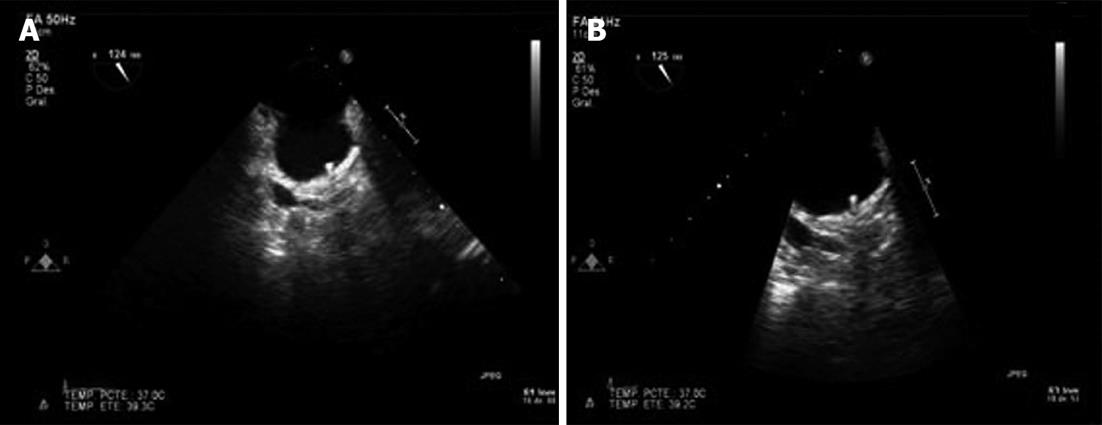
A 79-year-old woman with ischemic heart disease, hypertension and diabetes mellitus presented with paroxysmal AF. The patient was under OAC because of a high embolism risk (CHADS2 score of 4 points) but had multiple admissions because of gastrointestinal bleeding (GIB) of unknown cause, despite an intensive etiological study. To avoid long-term OAC, a percutaneous closure of the LAA with a 26-mm ACP device was performed and the patient was discharged under DAPT (aspirin 100 mg and clopidogrel 75 mg) until the 6th month. The post procedural transesophageal echocardiography (TEE) and the 45 d TEE revealed correct device positioning and the absence of thrombosis of the ACP (Figure 1). The patient remained asymptomatic with the absence of any GIB. The 4 mo transthoracic echocardiography (TTE) demonstrated correct device positioning without thrombotic complications but the 4 mo TEE detected a thrombus over the connector pin of the ACP despite the DAPT (Figure 2). Intravenous anticoagulation with heparin was started and TEE 2 wk later showed thrombosis resolution (Figure 3A). The patient continued with DAPT for two more months. The 6 mo TEE showed the absence of thrombus (Figure 3B), allowing the withdrawal of clopigrel. The 12 mo TTE confirmed the thrombus resolution and the patient remained uneventful, with no GIB or cardioembolic events after 2 years.
Four major points about thrombosis of the ACP could be drawn from our report. Firstly, the incomplete endothelization of the connector pin of the ACP during the initial 6 mo can contribute to the development of thrombosis of the ACP device. Our clinical case is in accordance with the first description of the connector pin thrombosis in correctly implanted ACP devices[7]. For that reason, a second generation of the ACP (ACP 2 or Amulet™) with a modified connector pin has been designed[8]. Secondly, the role of TEE and TTE in detecting device-related complications remains controversial[1]. The correct positioning can be detected with both techniques but TEE is the only method that permits the correct diagnosis of residual or emerging device thrombosis. So, the strict monitoring with TEE is mandatory until the 6th month because of the considerable proportion of device thrombosis. Thirdly, recommended antiplatelet therapy for prevention of thrombotic events varies with the type of device used for transcatheter closure of the LAA. The ACP manufacturer recommends DAPT for 3 mo, based on porcine models[1], but button thrombosis in ACP successfully implanted devices in humans beyond 3 mo suggests the possibility of extending the DAPT until the 6th month with complete exclusion of thrombotic complications by TEE. For these reasons, we recommend a strict echocardiographic follow-up protocol in order to detect any thrombotic complication early (1 d, 45 d, 3 mo and 6 mo TEE and 12 mo TTE). Fourthly, the case illustrates the adequacy of our management of thrombotic complications of ACP. After ACP implantation, DAPT is administered and anticoagulation is stopped due to the contraindication of long-term OAC. If thrombotic complications are detected by a strict TEE monitoring during the follow-up, the early initiation of intravenous anticoagulation can remove the thrombus, preventing serious thrombotic complications if not detected early and avoiding the need of long-term OAC in patients at high risk of complications under OAC treatment.
P- Reviewers Sakabe K, Trohman R S- Editor Wen LL L- Editor Roemmele A E- Editor Wu HL
| 1. | Park JW, Bethencourt A, Sievert H, Santoro G, Meier B, Walsh K, Lopez-Minquez JR, Meerkin D, Valdes M, Ormerod O. Left atrial appendage closure with amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. 2011;77:700-706. [RCA] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 311] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 2. | Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESCGuidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2342] [Cited by in RCA: 2416] [Article Influence: 185.8] [Reference Citation Analysis (0)] |
| 3. | Cruz-Gonzalez I, Martín Moreiras J, García E. Thrombus formation after left atrial appendage exclusion using an Amplatzer cardiac plug device. Catheter Cardiovasc Interv. 2011;78:970-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Cardona L, Ana G, Luísa B, Leal A, António F, Lídia S, Cruz FR. Thrombus formation on a left atrial appendage closure device. Circulation. 2011;124:1595-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Guérios EE, Schmid M, Gloekler S, Khattab AA, Wenaweser PM, Windecker S, Meier B. Left atrial appendage closure with the Amplatzer cardiac plug in patients with atrial fibrillation. Arq Bras Cardiol. 2012;98:528-536. [PubMed] |
| 6. | López-Mínguez JR, Eldoayen-Gragera J, González-Fernández R, Fernández-Vegas C, Fuentes-Cañamero ME, Millán-Nuñez V, Nogales-Asensio JM, Martínez-Naharro A, Sánchez-Giralt S, Doblado-Calatrava M. Immediate and One-year Results in 35 Consecutive Patients After Closure of Left Atrial Appendage With the Amplatzer Cardiac Plug. Rev Esp Cardiol. 2013;66:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Lammers J, Elenbaas T, Meijer A. Thrombus formation on an Amplatzer closure device after left atrial appendage closure. Eur Heart J. 2013;34:741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Freixa X, Chan JL, Tzikas A, Garceau P, Basmadjian A, Ibrahim R. The Amplatzer™ Cardiac Plug 2 for left atrial appendage occlusion: novel features and first-in-man experience. EuroIntervention. 2013;8:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |