Published online Oct 26, 2013. doi: 10.4330/wjc.v5.i10.387
Revised: August 17, 2013
Accepted: September 18, 2013
Published online: October 26, 2013
Processing time: 112 Days and 7.2 Hours
Left ventricular myxomas account for 2.5% of all cardiac myxoma cases. There are very few case reports on left ventricular myxoma (LVM) presented after complete surgical resection of left atrial myxoma. Here we report a case of a 58-year-old male presented to the hospital for transient limb weakness, numbness and dysarthria. Magnetic resonance image of the brain revealed multiple thromboembolic cerebrovascular accidents. Transthoracic echocardiogram (TTE) revealed a left atrial myxoma. It was resected completely with good surgical margins. After one and half year he started having dizziness, and transient right sided weakness. Computer tomography scan of the head revealed a progression of thromboembolic disease. TTE revealed a LVM that was confirmed by transesophageal echocardiogram. It was resected with good surgical margins 3 wk after recurrent cerebrovascular accident.
Core tip: Left ventricular myxoma (LVM) after surgical resection of left atrial myxoma is very rare. Etiologies for recurrent LVM after left atrial myxoma resection are incomplete surgical resection, metastasis, totipotent multicentricity and missed. Here we are describing a case that was probably a metastatic LVM as it is uncommon statistically for it to be a recurrent myxoma in the left ventricle after complete resection from left atrium. If there is a progression of the cerebral hemorrhagic lesions it would confirm our diagnosis of the metastatic process.
-
Citation: Seethala S. Left ventricular myxoma: Missed
vs metastatic. World J Cardiol 2013; 5(10): 387-390 - URL: https://www.wjgnet.com/1949-8462/full/v5/i10/387.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i10.387
Left ventricular myxomas account for 2.5% of all cardiac myxoma cases. There are very few case reports on left ventricular myxoma (LVM) presented after complete surgical resection of left atrial myxoma.
A 58-year-old male with a past medical history of hypertension and diabetes went to see a primary care physician with complaints of multiple episodes of transient limb weakness, numbness and dysarthria lasting less than 1 h. A magnetic resonance image (MRI) of the brain was obtained revealing multiple bilateral, supra, infratentorial, cortical and sub-cortical infarctions in watershed areas consistent with multiple thromboembolic strokes. Upon admission to the hospital, routine lab work (complete blood count, complete metabolic profile, lipid panel, thyroid function tests, coagulation studies), and carotid doppler failed to reveal any significant abnormalities other than poorly controlled diabetes, and a serum cholesterol of 113 mg/dL. A transthoracic echocardiogram (TTE) demonstrated a 3.5 cm homogenous mass in the left atrium with mild dilation and a normal left ventricle (LV). A pre-operative coronary angiogram failed to reveal any significant coronary artery disease. The left atrial mass was subsequently resected with good surgical margins and a small incidental patent foramen was successfully closed. Final pathology of the mass confirmed it to be a transparent myxoma. Patient was discharged home in stable condition and did well for few months without any major symptoms other than generalized weakness.
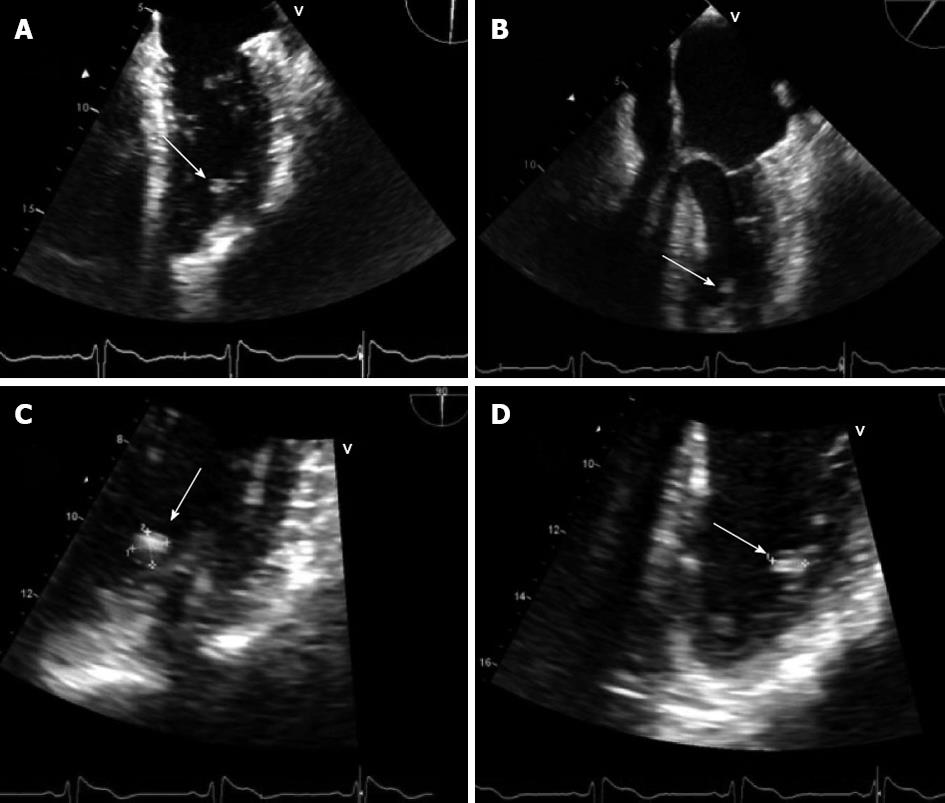
Nearly one year later the patient was diagnosed with generalized partial seizures following an episode of right-side weakness and was started on antiepileptic medication, which he refused to take. Over the next 2-3 wk he experienced two more episodes of right sided weakness associated with dizziness and admitted for non-adherence with medication. He then presented to our institution with near syncope and atypical chest pain. Routine cardiac evaluation was negative and he was discharged after 2 d. Later, he again presented to the emergency department, this time for intermittent right-sided weakness and transient dizziness. A computer tomography (CT) scan of the head revealed interval progression of thromboembolic disease. An MRI confirmed the CT scan findings; multiple small hemorrhagic lesions were subsequently identified and later confirmed by cerebral angiography. Multiple mycotic aneurysms were ruled out by blood cultures. TTE was performed and it revealed a 0.94 cm × 0.74 cm mass attached to lateral wall of LV. A transesophageal echo (TEE) then confirmed the presence of a mobile, round homogenous mass attached to the anterolateral wall of the LV (Figure 1). Three weeks after the recurrent cerebrovascular accident (CVA) the mass was resected. Initially, a left atrial and then aortic approach was attempted to locate and isolate the mass. Both approaches were unsuccessful. Eventually, an anterolateral approach located the mass buried in trabecular muscles of the posteroapical area without any valvular attachment. Excision was done without a difficulty. Pathology confirmed a LVM and the patient was discharged 1 wk later.
Primary cardiac tumors are rare and have an average incidence of 0.02%[1,2]. Of these, cardiac myxomas account for 88% of cases and are primarily benign in nature[3]. Myxomas constitute 0.23% of all the open heart surgical procedures[2]. The most common location for myxoma is the left atrium followed by the right atrium. A biatrial location is occasionally seen but all other locations are quite uncommon[3]. Myxomas found in the left ventricle account for only 2.5% of cases[3,4]. Myxomas are primarily sporadic while familial cases constitute up to 10%[2]. Familial myxomas have unusual locations and recurrences, and some are associated with Carney’s Complex (myxomas of the heart, and skin, spotty skin pigmentation, blue nevi, and endocrine over activity)[3].
Most myxomas are either asymptomatic or produce non-specific symptoms such as malaise, fatigue or heart failure symptoms. Embolic events are one of the major clinical presentations of myxomas. The risk of embolization is mostly determined by the morphology of the myxoma rather than its size. As in this case, semi-transparent polyploid myxomas carry a high risk for embolization compared to round myxomas[2]. Valvular myxomas carry high risk of embolization[2,5]. This variation in prevalence of emboli seen in published series can be explained by the fact that valvular myxomas carry a high risk of embolization compared to myxomas located elsewhere. Even though the final embolic destination is commonly the cerebrovascular territory other arterial territories such as the pulmonary or coronary circulation can be involved[1-4] .
The other common mechanisms of symptom production with myxomas are mechanical obstruction and arrhythmias when cardiac conducting system is involved[1-4].
Most myxomas are diagnosed with TTE, but are often missed when located in unusual places. In this case, locating the LV myxoma was difficult both on TTE and intraoperatively was difficult due to its concealment by trabecular muscle. TEE and MRI are best studies for localizing and characterizing myxoma. In all cases of suspected myxomas TEE should be performed[3].
Surgical resection is the treatment of choice for myxomas and should be performed as early as possible as there is a risk of embolization. In the presence of a recent CVA, surgical resection may be delayed for up to 4 wk and should be performed on pump with systemic heparinization[6]. Even though surgical technique is changing constantly, resection should include clean surgical margins to reduce the likely of recurrence[7,8]. There are very few cases myxoma has recurred in the LV after the resection of the tumor in LA[7]. In this case the myxoma recurred in the LV one year after the initial resection. The index TTE and the initial surgery did not give reason to suspect a LV myxoma. Possible mechanisms for the LVM in this case are recurrence (incomplete surgical resection or new growth of reserve cell or implantation from original tumor), or missed during initial evaluation, or metastasis. During the index diagnosis, neither MRI nor TEE was performed thus raising the possibility of LV myxoma was initially missed. However, though limited, neither TTE nor cardiac cauterizations have identified any LVM.
Recurrent myxomas often grow faster than primary tumors and can occur in 3% of sporadic cases and 20% of familial cases[9]. Incomplete surgical margins is one of the major reason to have recurrences[7]. The tumor recurs near the original resection site in 85% of cases with an atrial location in 97%[7]. In this case initial sporadic myxoma had good surgical margins during the index surgical resection. The site of recurrence however, was LV making the recurrence secondary to incomplete resection much less likely. Metastatic seeding of myxoma cells is well described in the literature. The malignant nature of myxomas is defined based on growth rate behavior rather than histological features. Malignant myxoma may be identified by high interleukin 6 levels, presence of constitutional symptoms, elevated gamma globulins, and a high erythrocyte sedimentation rate (ESR) after complete resection of the tumor[9]. In this case, the patient reported some constitutional symptoms malaise and generalized weakness but lack of specificity of these symptoms and the failure to obtain a post-operative ESR make supporting malignant potential of the tumor problematic. Multiple cerebral hemorrhagic lesions (probably secondary to small aneurysms) were noted in this patient and may support the idea of metastatic process. A malignant nature may be confirmed in future if the tumor is subsequently found at other distant sites. We excluded the probability of familial disease by taking a good family history, and there were no signs or symptoms of Carneys Complex[9]. At this time, it is believed that this recurrent LV myxoma case is most likely due to a metastatic process. Careful follow up has been planned for this patient to monitor for recurrence of myxoma as well as any worsening of neurological symptoms.
Follow up echocardiography is required to evaluate for recurrence. It is highly crucial in familial cases and in those cases where good surgical margins cannot be achieved[6].
P- Reviewer Artunc F S- Editor Zhai HH L- Editor A E- Editor Liu XM
| 1. | Sarjeant JM, Butany J, Cusimano RJ. Cancer of the heart: epidemiology and management of primary tumors and metastases. Am J Cardiovasc Drugs. 2003;3:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Cetin G, Gursoy M, Ugurlucan M, Uzunhasan I, Hatemi AC, Tireli E, Kucukoglu S, Kansiz E. Single-institutional 22 years experience on cardiac myxomas. Angiology. 2010;61:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Korkmaz AA, Tamtekin B, Onan B, Demir AS, Guden M, Uckurt Y. Combination of right atrial and left ventricular myxoma. Ann Thorac Surg. 2010;89:e33-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Meller J, Teichholz LE, Pichard AD, Matta R, Litwak R, Herman MV, Massie KF. Left ventricular myxoma: echocardiographic diagnosis and review of the literature. Am J Med. 1977;63:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore). 2001;80:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 581] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 6. | Arruda MV, Braile DM, Joaquim MR, Soares MJ, Alves RH. Resection of left ventricular myxoma after embolic stroke. Rev Bras Cir Cardiovasc. 2008;23:578-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Shinfeld A, Katsumata T, Westaby S. Recurrent cardiac myxoma: seeding or multifocal disease? Ann Thorac Surg. 1998;66:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Gao C, Yang M, Wang G, Wang J, Xiao C, Wu Y, Li J. Excision of atrial myxoma using robotic technology. J Thorac Cardiovasc Surg. 2010;139:1282-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Amano J, Kono T, Wada Y, Zhang T, Koide N, Fujimori M, Ito K. Cardiac myxoma: its origin and tumor characteristics. Ann Thorac Cardiovasc Surg. 2003;9:215-221. [PubMed] |