Published online Oct 26, 2013. doi: 10.4330/wjc.v5.i10.375
Revised: August 10, 2013
Accepted: September 13, 2013
Published online: October 26, 2013
Processing time: 169 Days and 23.8 Hours
AIM: To clarify whether the vasoconstrictory response is impaired and to study vascular function in patients with migraine during the headache attack.
METHODS: We studied vascular reactivity in the resistance arteries by using the forearm perfusion technique associated with plethysmography. We measured forearm blood flow by strain-gauge plethysmography during intra-brachial infusion of acetylcholine, sodium nitroprusside or norepinephrine in 11 controls and 13 patients with migraine, 11 of them (M) in the interval between the migraine attacks and 4 during a headache attack (MH). Written informed consent was obtained from patients and healthy controls, and the study was approved by the Ethics Committee of the University Federico II.
RESULTS: Compared to healthy control subjects, in patients with migraine studied during the interictal period, the vasodilating effect of acetylcholine, that acts through the stimulation of endothelial cells and the release of nitric oxide, was markedly reduced, but became normal during the headache attack (P < 0.05 by analysis of variance). The response to nitroprusside, which directly relaxes vascular smooth muscle cells (VSMCs), was depressed in patients with migraine studied during the interictal period, but normal during the headache attack (P < 0.005). During norepinephrine infusion, forearm blood flow decreased in control subjects (-40% ± 5%, P < 0.001). In contrast, in patients with migraine, either when studied during or free of the headache attack forearm blood flow did not change compared to the baseline value (-3% ± 13% and -10.4% ± 15%, P > 0.05).
CONCLUSION: In migrainers, the impaired relaxation of VSMCs is restored during the headache attack. The vasoconstrictory response is impaired and remains unchanged during the migraine attack.
Core tip: Patients with migraine without aura studied in the interictal period are characterized by impaired ability of vascular smooth muscle cells (VSMCs) to relax in response to nitric oxide and to contract in response to norepinephrine. We hypothesize that the two defects compensate for each other and this provides for the maintenance of normal vascular resistance and blood pressure homeostasis. In contrast, during the headache attack, the VSMCs regain their ability to respond to nitric oxide, but remain unresponsive to norepinephrine. Such differential effect of the migraine attack is not surprising, given that nitric oxide and norepinephrine activate different intracellular signaling pathways in VSMCs.
- Citation: Napoli R, Guardasole V, Zarra E, Sena AD, Saccà F, Ruvolo A, Grassi S, Giugliano S, Michele GD, Cittadini A, Carrieri PB, Saccà L. Migraine attack restores the response of vascular smooth muscle cells to nitric oxide but not to norepinephrine. World J Cardiol 2013; 5(10): 375-381
- URL: https://www.wjgnet.com/1949-8462/full/v5/i10/375.htm
- DOI: https://dx.doi.org/10.4330/wjc.v5.i10.375
Migraine is a widely common disease. Two thirds of migraineurs suffer from migraine without aura, whereas a third of patients present with migraine preceded by aura. Migraine has been associated with an increased risk of cardiovascular events, including myocardial infarction and ischemic stroke[1-3]. However, we have recently demonstrated that patients with migraine without aura, studied during the interictal period, do not present peripheral endothelial dysfunction, which is classically associated with a worse cardiovascular risk profile, but rather an abnormal relaxation of the vascular smooth muscle cells (VSMCs), that results in impaired vasodilation[4,5]. However, it is unclear whether the inability of VSMCs to respond to vasodilators is an isolated abnormality or, rather, reflects a more complex hemodynamic alteration, also involving the vasoconstrictory component. Furthermore, the peripheral vascular function in patients with migraine has been studied mainly during the interictal period. Therefore, whether the abnormalities in vascular function observed in patients with migraine are also present during the headache attack is unknown. Elucidation of the vascular response in patients with migraine both free of and during the headache episode would be of great importance to our understanding of the mechanisms involved in the pathogenesis of the disease and to better design appropriate therapeutic approaches.
We studied 13 patients affected by migraine without aura and eleven healthy subjects in whom migraine was excluded, who served as controls (Table 1). The control subjects (C group) were recruited from hospital and laboratory personnel and were matched to the patients with regard to age, body mass index and sex. The diagnosis of migraine was made according to the criteria of the International Headache Society[6,7]. Subjects with hypertension, diabetes, high cholesterol, history of cardiovascular events and cigarette smoking were excluded from the study. None of the patients was taking any medication except those to treat the migraine attack. On the day of study, patients were either headache free for at least five days (11 subjects, M group) or were experiencing a headache attack that had started a few hours earlier (4 patients, MH group). These patients abstained from taking any medication until the end of the study period. Two patients underwent both studies (free of or during the headache attack). Written informed consent was obtained from patients and healthy controls, and the study was approved by the Ethics Committee of the University Federico II. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
| Sex(male/female) | Age(yr) | BMI(kg/m2) | SBP(mmHg) | DBP(mmHg) | HR(beats/min) | |
| Controls (n = 11) | 5/6 | 33 ± 3.4 | 24 ± 0.8 | 127 ± 2.1 | 60 ± 1.8 | 65 ± 2 |
| M (n = 11) | 4/7 | 34 ± 1.9 | 24 ± 1.1 | 125 ± 3.3 | 65 ± 2.6 | 68 ± 3 |
| MH (n = 4) | 0/4 | 28 ± 3.9 | 24 ± 0. | 115 ± 4.2 | 60 ± 1.8 | 68 ± 2 |
We studied vascular reactivity in the resistance arteries by using the forearm perfusion technique associated with plethysmography, as previously described[4,8-11]. Briefly, a plastic cannula (20 G) was inserted into the brachial artery of the nondominant arm under local anesthesia and used for the infusion of the test substances and the monitoring of arterial blood pressure and heart rate. Forearm blood flow (FBF) was measured in both forearms by strain gauge plethysmography, with a calibrated mercury-in-silastic strain gauge applied around the forearm and connected to a plethysmography (Hokanson 045 EC4, PMS. Instruments, Berks, United kingdom) associated with a McLab computer. Each subject underwent the following step-wise infusions into the brachial artery: (1) acetylcholine (Ach) to assess endothelial-mediated vasodilation; and (2) sodium nitroprusside (NP), a nitric oxide (NO) donor that directly stimulates VSMCs, to assess non-endothelial-mediated vasodilation. At least half an hour after the NP infusion and when baseline FBF was restored, each subject received the infusion into the brachial artery of norepinephrine (NE) at the rate of 280 μg/L per minute for 5.5 min to assess the vascular response to sympathetic stimulation. This dose of NE was chosen on the basis of our previous experiments that showed a near half-maximal fall in FBF. The investigators making the measurements of vascular reactivity were blind to the clinical status of the subjects undergoing the experiments.
Based on previously published data[4], we computed the minimum sample size with respect to a two-tailed Student t test, considering: (1) a difference for the slope of the dose response curve to Ach to be detected between controls and migrainers as δ≥ 0.25 mL/(dL·min·μg); (2) a value of SD = 0.156 mL/(dL·min·μg); and (3) a type I error probability = 0.05 and a power = 0.90. This results in a minimum sample size of n = 9 subjects for group. Since no data are available in the literature regarding the response to norepinephrine of FBF in migrainers, we decided to increase the number of subjects to be recruited to 11 per group.
The differences in clinical and metabolic parameters between the three study groups were analyzed by the unpaired Student’s t test with Bonferroni correction for multiple comparisons. Vascular reactivity data are expressed as absolute values of FBF. Comparison between migraine and control subjects was performed by a two-way analysis of variance for repeated measures (General Linear Model, version 13.0, SPSS Inc., Chicago, IL, United States) and Least Significant Difference test was used for post hoc analysis. Comparison between baseline and NE infusion data was performed by the paired Student’s t test. Results are expressed as mean ± SE.
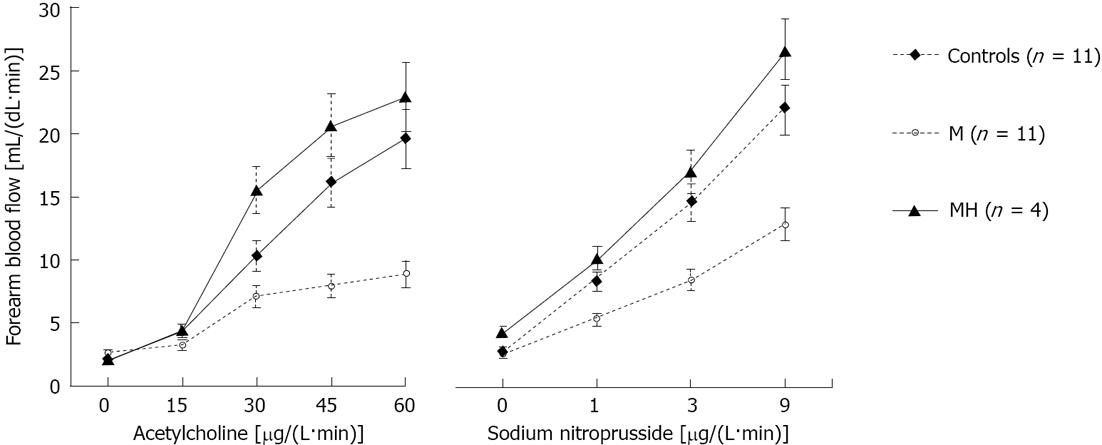
The baseline values of FBF were similar in the three groups (Figure 1). Infusion of ACh, an endothelium-dependent vasodilator, elicited a progressive vasodilatory response in all groups (P < 0.001). However, in patients with migraine studied during the interictal period, FBF response was lower than that of control subjects (P < 0.05). In contrast, patients studied during the headache attack showed a more intense response to Ach infusion (P < 0.02 vs M; Figure 1). In response to the highest dose of Ach, FBF rose to 19.6 ± 3.1, 8.8 ± 2.4, and 22.9 ± 2.2 mL/dL per minute in controls and migraine patients without or with headache attack, respectively (P = 0.036 for M group vs C and P < 0.02 vs MH). The response to ACh was also analyzed using the slope of the dose-response curves. In the patients with migraine without headache the average slope was markedly less steep than in controls (0.11 ± 0.05 and 0.31 ± 0.05 mL/(dL·min·μg), respectively; P = 0.03). In contrast, the slope of the dose response curve to Ach in migraine patients during the headache attack was similar to controls (0.39 ± 0.04 mL/(dL·min·μg), P < 0.02 vs M, P = NS vs C).
The dose-response curve to NP, an NO donor directly acting on VSMCs, is shown in Figure 1. As compared with controls, patients with migraine without headache showed a significantly lower response at all infusion rates (P = 0.004 vs C). In contrast, patients with migraine during the headache attack showed a response to NP similar to controls and markedly increased when compared to migrainers studied during the interictal period (P = NS vs C and P = 0.002 vs M). The maximal response of FBF to NP was 22.2 ± 1.9, 12.8 ± 1.9 and 26.6 ± 3.8 mL/dL per minute in controls and migraine patients without or with headache attack, respectively (P < 0.02 for M group vs C and MH). The response to NP was also analyzed using the slope of the dose-response curves. In the patients with migraine without headache the average slope was markedly less steep than in controls [1.05 ± 0.19 and 1.96 ± 0.20 mL/(dL·min·μg), respectively; P < 0.01]. In contrast, the slope of the dose response curve to NP in migraine patients during the headache attack was similar to controls [2.29 ± 0.29 mL/(dL·min·μg), P < 0.02 vs M, P > 0.05 vs C].
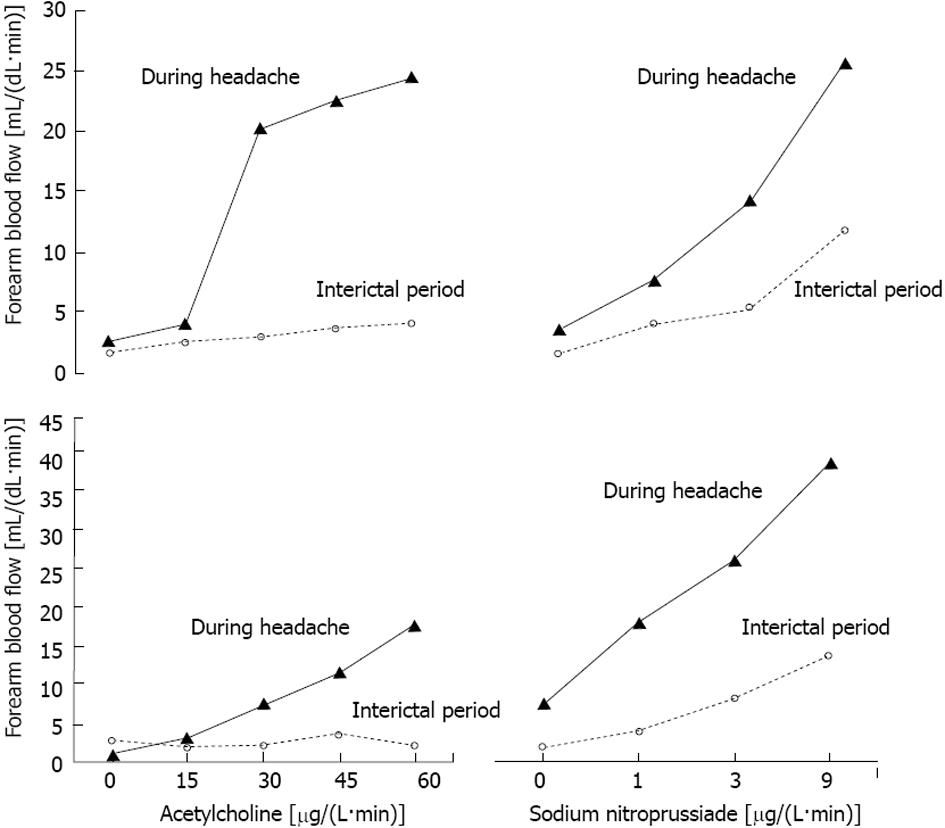
In Figure 2, we report the dose response curves to Ach or NP infusions for the two patients who gave us a unique opportunity to study the phenomenon both during the interictal period and the headache attack. It is striking how potently the response to both Ach and NP was enhanced by the headache attack as compared with the basal response.
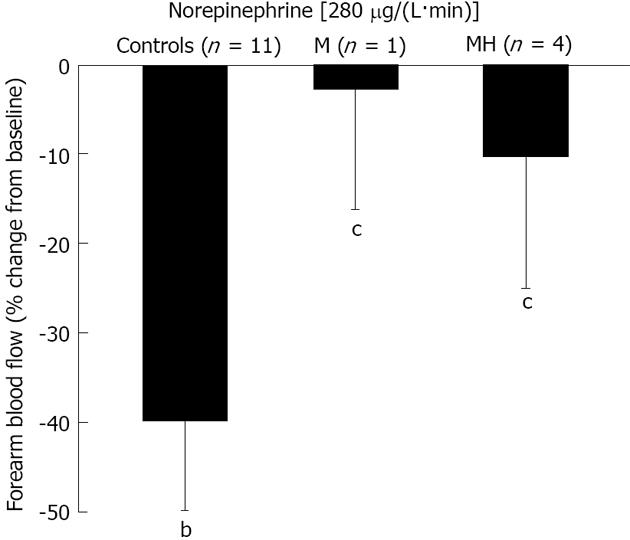
Figure 3 shows the data on the effect of NE infusion. FBF was reduced by 1.19 ± 0.17 mL/dL per minute by NE infusion in C (-40% ± 6%, P = 0.001 vs baseline). In contrast, NE infusion was unable to elicit a vasoconstrictory response in migraine patients either when studied in the headache-free period or during the headache attack (-0.29 ± 0.23 and -0.66 ± 0.69 mL/dL per minute, accounting for a reduction by 3% ± 13% and 10% ± 15% in M and MH, respectively; P > 0.05 vs baseline and P < 0.05 vs C).
In the present study, we measured vascular reactivity in patients with migraine without aura either during the interictal period or during a headache attack. We confirm our previous finding that patients with migraine studied in the interictal period suffer from impaired vasodilation in response to acetylcholine and sodium nitroprusside. Furthermore, we extend our observation to the vasoconstrictory response to an adrenergic agonist and show that in these patients a defect in the response to NE also coexists. In addition, we studied a group of patients with migraine during the headache attack. Under these circumstances, the marked defect in vasodilation completely reverted, as documented by the normal responses to Ach and NP. In contrast, the vasoconstrictory response to the sympathetic agonist NE remained blocked.
Although patients with migraine during the headache-free period have a normal postural increase compared to control subjects, they are also characterized by a 50% reduction of absolute circulating NE levels in both supine and orthostatic position[12-14], suggesting an abnormal regulation of the sympathetic nervous system activity. Because in these patients NE intravenous administration induces more prolonged elevation in blood pressure (BP) than in control subjects, an adrenergic receptor supersensitivity was invoked[12]. In addition, the observation of greater and more prolonged BP response to phenylephrine led to the conclusion that an alpha-adrenergic receptor increased sensitivity was implicated[15]. However, it must be considered that the intravenous administration of NE or phenylephrine does not trigger only the receptors localized in the vessel wall, but can potentially unleash more complex, systemic mechanisms. In addition, indirect data obtained by administering the beta-blocker propranolol to patients with migraine, suggested that beta receptors distribution in the radial artery might be abnormal[16]. To the best of our knowledge, the current study is the only one in which NE is directly infused into the brachial artery in patients with migraine. The agonist was infused locally in very small amounts that were unable to induce systemic perturbations of NE circulating levels, given its very short half-life. This is also supported by the lack of any change in FBF of the contralateral arm in control subjects or in systemic BP (data not shown). Therefore, under the current circumstances, any confounding involvement of indirect sympathetic mechanisms secondary to changes in circulating NE levels can be excluded, and the observed effects only reflect the direct action of NE on the forearm resistance vessels. It must be also stressed that NE stimulates both the alpha-receptors (vasocostrictory response) and the beta-receptors (vasodilatory response). Therefore, the response to NE infusion represents the net balance of two opposite forces. In normal subjects, however, the vasoconstrictory response clearly prevails, whereas in patients with migraine the resistance vessels are unable to respond to the sympathetic agonist. We cannot dissect whether the block of the vasoconstrictory response in migraine patients is due to a relative reduction of the NE effect through the alpha-receptors or an increase of the beta-receptor response or a combination of the two. Unfortunately, no information is available in the literature regarding the adrenergic receptor relative distribution in the cell membranes of peripheral arterial vessels.
Given the inability of VSMCs to relax in response to endothelial NO in the interictal period, were the vasoconstrictory ability of NE intact rather than severely impaired, patients with migraine would experience constantly raised vascular resistance and systemic hypertension. Therefore, the defective NE-induced vasoconstriction observed in patients with migraine might represent a chronic hemodynamic adjustment to compensate for the reduced vasodilatory response to NO by the VSMCs. The hypothesis of a compensatory down-regulation of the vasoconstrictory response of VSMCs would be well in agreement with the generalized reduction of sympathetic nervous system activity previously reported in migraine patients[12].
We have previously demonstrated the presence of impaired vascular reactivity in patients with migraine during the interictal period, entirely attributable to VSMCs dysfunction[4,5]. The impaired vasodilatory response to Ach was associated with normal NO production by endothelial cells. Moreover, the hemodynamic response to NP, a direct stimulator of VSMCs, was markedly impaired. In the current study, we confirm the observation that in patients with migraine studied free from headache the response to Ach and NP is severely impaired. Data in the literature have provided divergent results, either when flow-mediated dilation or forearm perfusion technique associated with plethysmography or other approaches were used[17-23]. In previous studies, migraine patients have not been discriminated with regard to the presence of aura and different vascular beds (micro- vs macro-vascular and intra- vs extra-cranial) have been explored. The possibility exists that the two types of migraine might be characterized by a different vascular reactivity. Accordingly, the cardiovascular risk profile of the two types of migraine appears to be different, suggesting that the intimate mechanism of vascular function diverge and our findings lend support to the hypothesis that migraine without aura is not associated with dysfunction of the endothelial cells potentially triggering atherosclerotic processes[1,2,24-28].
In patients with migraine during the headache attack, basal FBF was similar to that measured off the pain attack and to that of control subjects. In contrast, the impaired vasodilation in response to the infusion of Ach and NP of the interictal period was fully restored. Taken together, our data indicate that the patients with migraine in the interictal period have a reduced sensitivity of their VSMCs to the NO released by the endothelial cells. In contrast, during the headache attack, the response to NO, as suggested by the NP infusion data, becomes similar to that measured in the controls, indicating a restored sensitivity of VSMCs. We have previously demonstrated that during Ach infusion in patients with migraine during the interictal period the release of NO is normal and that endothelial function is intact[4,5]. Interestingly, when in previous studies systemic nitroglycerin, an NO donor, was administered to patients with migraine, an approach used to induce headache in migraine patients or to measure non-endothelial-mediated vasodilation, an increased sensitivity to NO was demonstrated in intra-and extra-cranial vessels[19-25]. Further studies are necessary to clarify the intriguing issue about the mechanisms that come into play during the migraine attack to redirect VSMC sensitivity towards normal.
A potential limitation of the current study is the small sample of patients studied during the headache attack. The forearm perfusion technique requires the cannulation of the brachial artery and, in general, this approach precludes the possibility to study large patients groups. In addition, it is quite hard to perform a forearm study that lasts several hours in patients who during the headache attack abstain from taking analgesics for the potential drug impact on vascular reactivity.
As compared with ultrasonographic techniques, such as the flow mediated dilation, the forearm technique bears much less variability. Indeed, the effects observed in our patients during the headache attack were very clear-cut, providing solid statistics despite the small sample. A final consideration is that we studied patients with spontaneous headache attack. This is a point of great strength of our work, since confounding factors linked to experimental stimuli used to trigger a headache attack were not operative.
In conclusion, patients with migraine without aura studied in the interictal period are characterized by VSMCs impaired ability to relax in response to NO and to contract in response to NE. We hypothesize that the two defects compensate for each other and this provides for the maintenance of normal vascular resistance and blood pressure homeostasis. In contrast, during the headache attack, due to mechanisms still unclear, the VSMCs regain their ability to respond to NO, but remain unresponsive to NE. Such differential effect of the migraine attack is not surprising, given that NO and NE activate different intracellular signaling pathways in VSMCs.
Migraine has been associated with an increased risk of cardiovascular events. However, authors have recently demonstrated that patients with migraine without aura, studied during the interictal period, do not present peripheral endothelial dysfunction, which is classically associated with a worse cardiovascular risk profile, but rather an abnormal relaxation of the vascular smooth muscle cells (VSMCs). It is unclear whether the inability of VSMCs to respond to vasodilators is an isolated abnormality or, rather, reflects a more complex hemodynamic alteration and whether persists during the headache attack.
The demonstration that the vascular abnormality observed in migraine are not due to endothelial dysfunction, but rather to VSMCs impairment might result in novel therapeutic approachs. Furthermore, life style intervention useful to improve endothelial dysfunction might be ineffective to correct the defects in VSMCs dysfunction.
This is the first study to demonstrate that patients with migraine without aura studied in the interictal period are characterized by VSMCs impaired ability to relax in response to nitric oxide (NO) and to contract in response to norepinephrine (NE). Authors hypothesize that the two defects compensate for each other and this provides for the maintenance of normal vascular resistance and blood pressure homeostasis. In contrast, during the headache attack, due to mechanisms still unclear, the VSMCs regain their ability to respond to NO, but remain unresponsive to NE.
Elucidation of the vascular response in patients with migraine both free of and during the headache episode would be of great importance to the authors’ understanding of the mechanisms involved in the pathogenesis of the disease and to better design appropriate therapeutic approaches.
Vascular dysfunction is mainly attributable to endothelial dysfunction. In migraine patients without aura, the inability of VSMCs to respond to nitric oxide can be considered a novel mechanism of vascular dysfunction.
The authors studied peripheral vascular function in patients with migraine without aura. the patients were studied both during and free of the headache attack. Vascular dysfunction in these patients involves both impairment of vasodilation and vasoconstriction, both due to an abnormal functioning of VSMCs. The results are very interesting.
P- Reviewer Bylova N S- Editor Zhai HH L- Editor A E- Editor Wu HL
| 1. | Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296:283-291. [PubMed] |
| 2. | Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 588] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 4. | Napoli R, Guardasole V, Zarra E, Matarazzo M, D’Anna C, Saccà F, Affuso F, Cittadini A, Carrieri PB, Saccà L. Vascular smooth muscle cell dysfunction in patients with migraine. Neurology. 2009;72:2111-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Devor WN, Napoli R, Saccà L. Vascular smooth muscle cell dysfunction in patients with migraine. Neurology. 2010;74:94; author reply 94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9-160. [PubMed] |
| 7. | Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5134] [Cited by in RCA: 5447] [Article Influence: 495.2] [Reference Citation Analysis (0)] |
| 8. | Napoli R, Biondi B, Guardasole V, Matarazzo M, Pardo F, Angelini V, Fazio S, Saccà L. Impact of hyperthyroidism and its correction on vascular reactivity in humans. Circulation. 2001;104:3076-3080. [PubMed] |
| 9. | Napoli R, Cozzolino D, Guardasole V, Angelini V, Zarra E, Matarazzo M, Cittadini A, Saccà L, Torella R. Red wine consumption improves insulin resistance but not endothelial function in type 2 diabetic patients. Metabolism. 2005;54:306-313. [PubMed] |
| 10. | Capaldo B, Napoli R, Guida R, Di Bonito P, Antoniello S, Auletta M, Pardo F, Rendina V, Saccà L. Forearm muscle insulin resistance during hypoglycemia: role of adrenergic mechanisms and hypoglycemia perse. Am J Physiol. 1995;268:E248-E254. [PubMed] |
| 11. | Napoli R, Guardasole V, Angelini V, Zarra E, Terracciano D, D’Anna C, Matarazzo M, Oliviero U, Macchia V, Saccà L. Acute effects of triiodothyronine on endothelial function in human subjects. J Clin Endocrinol Metab. 2007;92:250-254. [PubMed] |
| 12. | Gotoh F, Komatsumoto S, Araki N, Gomi S. Noradrenergic nervous activity in migraine. Arch Neurol. 1984;41:951-955. [PubMed] |
| 13. | Havanka-Kannianinen H, Juujärvi K, Tolonen U, Myllylä VV. Cardiovascular reflexes and plasma noradrenaline levels in migraine patients before and during nimodipine medication. Headache. 1987;27:39-44. [PubMed] |
| 14. | Mikamo K, Takeshima T, Takahashi K. Cardiovascular sympathetic hypofunction in muscle contraction headache and migraine. Headache. 1989;29:86-89. [PubMed] |
| 15. | Boccuni M, Alessandri M, Fusco BM, Cangi F. The pressor hyperresponsiveness to phenylephrine unmasks sympathetic hypofunction in migraine. Cephalalgia. 1989;9:239-245. [PubMed] |
| 16. | Tvedskov JF, Thomsen LL, Thomsen LL, Iversen HK, Williams P, Gibson A, Jenkins K, Peck R, Olesen J. The effect of propranolol on glyceryltrinitrate-induced headache and arterial response. Cephalalgia. 2004;24:1076-1087. [PubMed] |
| 17. | Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology. 2007;68:1563-1570. [PubMed] |
| 18. | de Hoon JN, Smits P, Troost J, Struijker-Boudier HA, Van Bortel LM. Forearm vascular response to nitric oxide and calcitonin gene-related peptide: comparison between migraine patients and control subjects. Cephalalgia. 2006;26:56-63. [PubMed] |
| 19. | Yetkin E, Ozisik H, Ozcan C, Aksoy Y, Turhan H. Decreased endothelium-dependent vasodilatation in patients with migraine: a new aspect to vascular pathophysiology of migraine. Coron Artery Dis. 2006;17:29-33. [PubMed] |
| 20. | Yetkin E, Ozisik H, Ozcan C, Aksoy Y, Turhan H. Increased dilator response to nitrate and decreased flow-mediated dilatation in migraineurs. Headache. 2007;47:104-110. [PubMed] |
| 21. | Silva FA, Rueda-Clausen CF, Silva SY, Zarruk JG, Guzmán JC, Morillo CA, Vesga B, Pradilla G, Flórez M, López-Jaramillo P. Endothelial function in patients with migraine during the interictal period. Headache. 2007;47:45-51. [PubMed] |
| 22. | Edvinsson ML, Edvinsson L. Comparison of CGRP and NO responses in the human peripheral microcirculation of migraine and control subjects. Cephalalgia. 2008;28:563-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Miller D, Waters DD, Warnica W, Szlachcic J, Kreeft J, Théroux P. Is variant angina the coronary manifestation of a generalized vasospastic disorder? N Engl J Med. 1981;304:763-766. [PubMed] |
| 24. | O’Keeffe ST, Tsapatsaris NP, Beetham WP. Increased prevalence of migraine and chest pain in patients with primary Raynaud disease. Ann Intern Med. 1992;116:985-989. [PubMed] |
| 25. | Thomsen LL, Iversen HK, Brinck TA, Olesen J. Arterial supersensitivity to nitric oxide (nitroglycerin) in migraine sufferers. Cephalalgia. 1993;13:395-399; discussion 376. [PubMed] |
| 26. | Kurth T, Chabriat H, Bousser MG. Migraine and stroke: a complex association with clinical implications. Lancet Neurol. 2012;11:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 27. | Katsarava Z, Weimar C. Migraine and stroke. J Neurol Sci. 2010;299:42-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Kurth T, Diener HC. Migraine and stroke: perspectives for stroke physicians. Stroke. 2012;43:3421-3426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |