INVITED COMMENTARY ON HOT ARTICLES
Atherosclerosis still remains as the major etiological factor for the world’s largest killing disorder, i.e., coronary artery disease. Despite several novel advances at the therapeutic front, the treatment of atherosclerosis still remains elusive. In the recent past, the focus has shifted to the prevention of its occurrence rather that its cure. Universally acknowledged as a lifestyle disorder, studies in animal models show that calorie restriction is anti-atherogenic[1]. The direct cellular response for calorie restriction or fasting has been elucidated as “autophagy”. Autophagy (literally meaning “self-eating”) is a lysosomal pathway of cellular digestion of damaged or excess organelles and misfolded protein aggregates as well as invading micro-organisms[2]. Depending upon the mode of cargo delivery to lysosomes, three different forms of autophagy have been described: macroautophagy, microautophagy and chaperone-mediated autophagy (CMA). While in microautophagy and CMA, cargo is delivered directly into the lysosomes through invaginations in the lysosomal membrane and a membrane translocation complex respectively, macroautophagy on the other hand involves sequestration of cytoplasmic components in a double membrane vesicle called the autophagosome, which subsequently fuses with the lysosomes to form an autophagolysosome resulting in the degradation of components via lysosomal hydrolases. Macroautophagy (referred to as “autophagy” hereafter) is an evolutionary conserved, highly regulated and dynamic pathway regulated by autophagy-related (ATG) genes and ATG null mice are used as in vivo model systems to study autophagic functions and regulation[2]. Although autophagy has been implicated in the pathogenesis of neurodegenerative disorders, cancers and heart failure[3], the role of autophagy in atherosclerosis is a burgeoning area of research. Autophagy has been observed in macrophages, smooth muscle cells and vascular endothelial cells and it protects the cells against oxidative stress by degrading the damaged intracellular material[4]. A special kind of autophagy, termed as “lipophagy”, also helps in cholesterol egress from lipid-laden cells to high density lipoprotein (HDL) via lysosomal lipases[5].
Recently, two papers published in the April 4, 2012 issue of Cell Metabolism add new dimensions to our understanding of the role that autophagy plays in regulating atherosclerotic plaque development. Although it is well established that crosstalk between lipid metabolism and inflammation orchestrates the development of atherosclerotic lesions[6], Razani et al[7] provide yet another link between the two processes by elucidating that autophagy prevents cholesterol crystal-induced inflammasome activation. On the other hand, Liao et al[8] provide evidence that autophagy prevents macrophage apoptosis and defective efferocytosis, both of which characterize advanced plaques and are associated with necrotic cores. Using various animal models, the authors unambiguously establish the anti-inflammatory and anti-apoptotic role of autophagy in atherosclerosis.
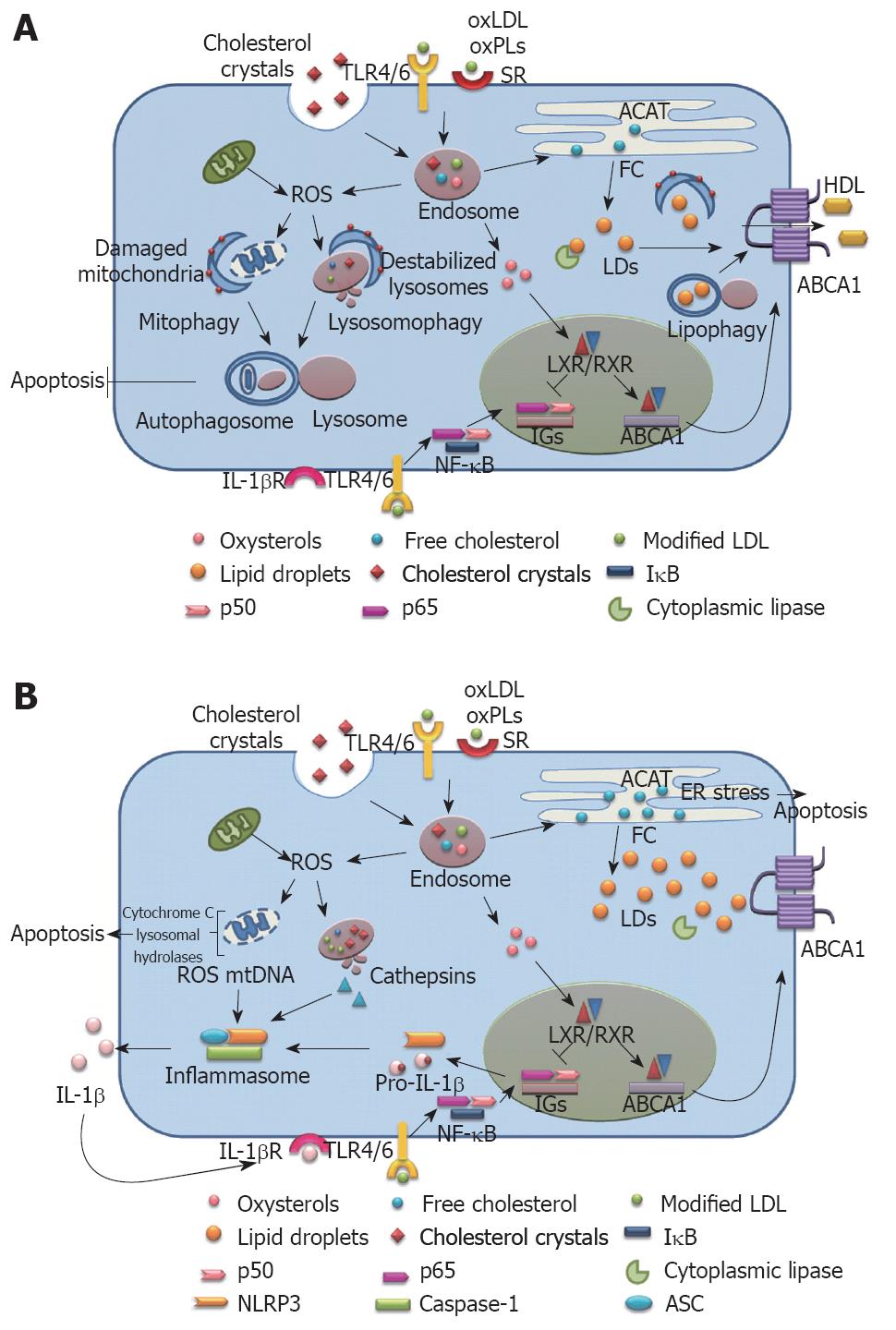
Both these authors, utilizing animal models with different genetic backgrounds and taking p62/SQSTM1 as a marker, have shown that autophagy becomes dysfunctional as the lesion development progresses. Razani et al[7] fed ApoE-null mice and their wild type counterparts a western diet (WD) for 2 mo and observed a dramatic increase in the p62 levels in atherosclerotic aortas, which increased with increasing age of the ApoE-/- mice and was consistent with the findings of Liao et al[8] who also found increasing levels of p62 in macrophage-rich areas of lesion in WD-fed LDLR-/- mice with increasing age. However, this increase in p62 expression levels was not due to an increase in p62 mRNA levels[7,8]. Considering the protective role of autophagy in cell survival and maintenance of cellular homeostasis, complete autophagic deficiency would be incompatible with life and Razani et al[7] show that haplo-insufficiency of autophagy is inadequate to accelerate atheroma progression. This implies that initially autophagy is functional and becomes severely compromised with the plaque progression. This is also reflected by the similar protein levels of Beclin-1 (which is involved in autophagosome nucleation) in WD-fed ApoE null and wild type mice[7]. It is important in this context to mention here that high fat diet or chronic lipid challenge decreases the fusion between autophagosomes and lysosomes[9], which might explain why, despite the initiation of autophagy and formation of autophagosomes, the autophagic flux through lysosomes becomes defective as the plaque progresses and lipid content increases. Further, Singh et al[5] have shown that autophagy also plays a significant role in the reverse cholesterol transport by hydrolysis of intracellular lipid droplets of foam cells via lysosomal lipases (lipophagy) and promoting their egress through the ATP-binding cassette A1 to HDL. Thus, defective lipophagy increases intracellular lipid content and progressively leads to dysfunctional autophagy forming a vicious circle.
What are the consequences of this defective autophagy? As we have seen from the cytoprotective role of basal autophagy in various cells, it is apparent that in the absence of autophagy, apoptosis would increase. Liao et al[8] have also shown that ATG5 null mice show increased apoptosis upon exposure to oxidative and endoplasmic reticulum stress. Furthermore, they have shown that inhibition of autophagy results in increased activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity resulting in increased reactive oxygen species (ROS) production, which may damage mitochondria and other organelles including lysosomal membrane destabilization[8]. Due to a defect in the autophagy, damaged mitochondria release more ROS and cytochrome c, and the spillage of lysosomal hydrolases due to lysosomal rupture would ultimately result in apoptosis of macrophages. Further worsening the situation is the observation that autophagy inhibition also reduces phagocytic clearance of apoptotic macrophages, i.e., efferocytosis, ultimately resulting in a necrotic core[8].
Another consequence of this increased ROS, release of mitochondrial DNA and cathepsins, is activation of inflammasomes. Inflammasomes are multimeric protein complexes involving pattern recognition receptors (PRRs) that participate in inflammatory signaling. The nucleotide-binding domain leucine-rich repeat containing (NLR) family, pyrin domain containing 3 (NLRP3) inflammasome is the best-characterized inflammasome of all, and is responsible for caspase-1 mediated cleavage and secretion of pro-inflammatory cytokines interleukin (IL)-1β and IL-18[10]. The activation of the NLRP3 inflammasome is a two-step process. The first step, also called the “priming step”, involves activation of nuclear factor kappa B (NF-κB) by PRRs or by cytokine receptors resulting in the increased expression of pro-IL-1β and NLRP3 itself[11]. The second step involves the assembly and activation of the NLRP3 inflammasome by various exogenous and endogenous stimuli such as pore-forming toxins[12], crystalline substances (silica, asbestos and monosodium urate crystals)[13,14], extracellular ATP[12] (released by dying cells), peptide aggregates and various other microbial stimuli[15]. The activators of both these steps are present in atherosclerotic plaques. Whereas the priming signal can be provided by modified low density lipoprotein (LDL) which activates NF-κB via toll-like receptor (TLR)4/6 and various scavenger receptors (CD36 and SR-A), cholesterol crystals can activate the NLRP3 inflammasome. Moreover, oxidized LDL (oxLDL) found in early atherosclerotic lesions can provide both the signals. However, since none of the activators of the NLRP3 inflammasome has been shown to interact directly with NLRP3, this suggests that a common upstream signaling event is involved in its activation[10]. A number of hypotheses have been put forward to explain the assembly and activation of the NLRP3 inflammasome. One of the suggested mechanisms is that NLRP3 senses intracellular ROS. Earlier NADPH oxidases were thought to be responsible for NLRP3 activation but recently ROS produced in mitochondria have also been implicated in NLRP3 activation[13,16,17]. Cathepsins B and L released upon lysosomal membrane destabilization due to cholesterol crystals or ROS have also been shown to activate NLRP3[18]. In fact, cathepsin B- and L-deficient mouse macrophages display reduced secretion of IL-1β when exposed to cholesterol crystals[19] and increased levels of cathepsins B and L co-localizing with macrophage markers are found in atherosclerotic plaques as compared to normal healthy tissue[20,21]. NLRP3 activation through any of the above stated pathways ultimately leads to an increase in pro-inflammatory IL-1β secretion that promotes secretion of many other chemokines and cytokines and also induces expression of endothelial adhesion molecules[22] resulting in infiltration of neutrophils which not only themselves result in chronic inflammation and increased risk of thrombosis, but also trigger intimal recruitment of monocytes[23].
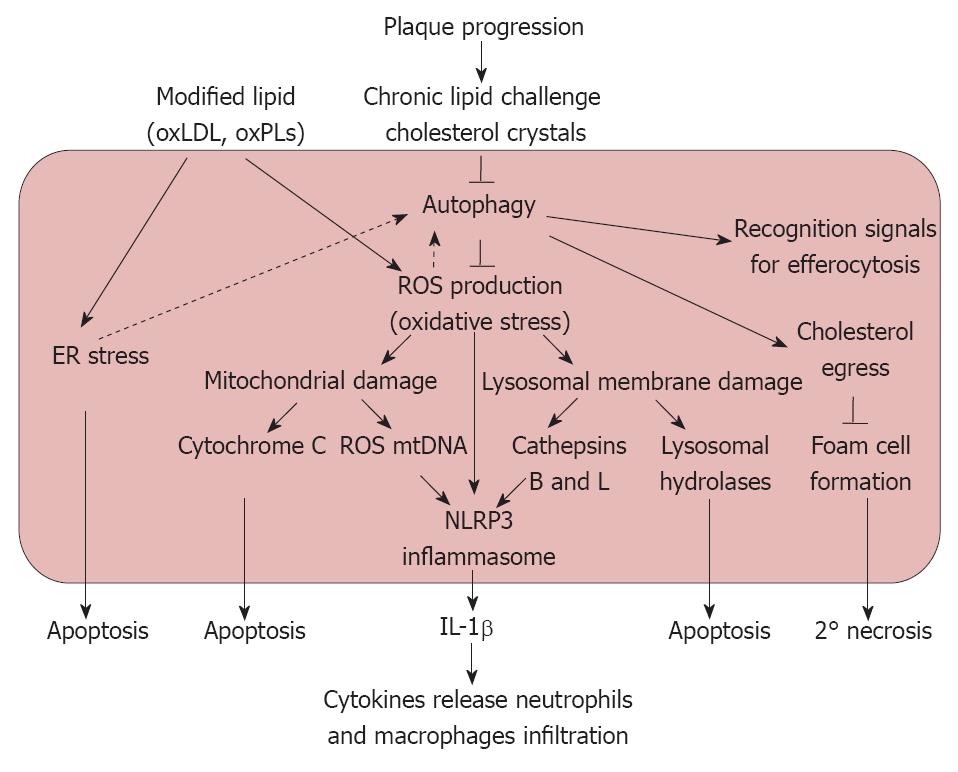
We agree with the authors in proposing a pathway for the observed protective effects of autophagy in atherosclerosis (Figures 1 and 2), noting the following points: (1) oxLDL results in oxidative and endoplasmic reticulum stress through the activation of CD36 as well as the TLR2/6 pathway[23]; (2) This oxidative stress has the inherent capacity to induce cellular autophagy; (3) As the atherosclerotic lesion progresses, the increased intracellular lipid accumulation results in dysfunctional autophagy; (4) Inhibition of autophagy results in the increased activity of NADPH oxidase leading to the production of ROS that results in mitochondrial damage and lysosomal membrane rupture; (5) In the absence of autophagic rescue, the accumulation of damaged mitochondria and lysosomes initiates an inflammatory response by activating the NLRP3 inflammasome as the lesion progresses into an advanced stage; (6) The co-operation between macrophage apoptosis induced by ROS overproduction and defective efferocytosis (due to deficient autophagy) results in atherosclerotic plaque necrosis, which in turn can precipitate acute athero-thrombotic events; and (7) Although Razani et al[7] and Liao et al[8] have made appreciable efforts towards unraveling the role of autophagy in atherosclerosis, the role of this phenomenon in plaque development is far from clear. Consequently, there is a need to explore the mechanism that governs the crosstalk between autophagy and apoptosis within the cells of the arterial wall and how this crosstalk is responsible for the initiation and regression of atherosclerotic lesions.
Figure 1 Proposed pathways elucidating the possible role of autophagy in atherosclerosis.
ER: Endoplasmic reticulum; IL-1β: Interleukin-1β;oxLDL: Oxidized low density lipoprotein; oxPLs: Oxidized phospholipids; ROS: Reactive oxygen species; NLRP3: Nucleotide-binding domain leucine-rich repeat containing (NLR) family, pyrin domain containing 3.
Figure 2 Schematic depiction of the relationship between autophagy, cholesterol metabolism, inflammasome activation and atherogenesis in (A) early lesions when autophagy is functional, and (B) advanced lesion where autophagy is compromised.
ABCA1: ATP-binding cassette A1; ACAT: Acylcoenzyme A cholesterol acyltransferase; ASC: Apoptosis-associated speck-like protein; ER: Endoplasmic reticulum; FC: Free cholesterol; HDL: High density lipoprotein; IGs: Inflammatory genes; IL-1β: Interleukin-1β; IL-1βR: Interleukin-1β receptor; LD: Lipid droplets; LXR: Liver X receptor; NF-κB: Nuclear factor kappa B; NLRP3: Nucleotide-binding domain leucine-rich repeat containing (NLR) family, pyrin domain containing 3; oxLDL: Oxidized low density lipoprotein; oxPLs: Oxidized phospholipids; ROS: Reactive oxygen species; RXR: Retinoic X receptor; SR: Scavenger receptor; TLR: Toll-like receptor.