Published online Aug 26, 2012. doi: 10.4330/wjc.v4.i8.256
Revised: August 10, 2012
Accepted: August 17, 2012
Published online: August 26, 2012
AIM: To evaluate the effects of eicosapentaenoic acid (EPA) on regional arterial stiffness assessed by strain rate using tissue Doppler imaging.
METHODS: Nineteen eligible patients were prospectively studied (mean age 62 ± 8 years, 68% men). Subjects with large vessel complications and/or diabetes mellitus were excluded. The strain rate of the ascending aorta was measured by tissue Doppler imaging as an index of regional arterial stiffness, and brachial-ankle pulse wave velocity (baPWV) was measured as an index of degree of systemic arteriosclerosis. These indices were compared before and after administration of EPA at 1800 mg/d for one year.
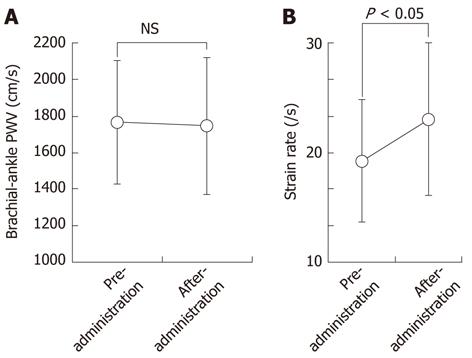
RESULTS: The plasma concentration of EPA increased significantly after EPA administration (3.0% ± 1.1% to 8.5% ± 2.9%, P < 0.001). There were no significant changes in baPWV (1765 ± 335 cm/s to 1745 ± 374 cm/s), low-density lipoprotein cholesterol levels (114 ± 29 mg/dL to 108 ± 28 mg/dL), or systolic blood pressure (131 ± 16 mmHg to 130 ± 13 mmHg) before and after EPA administration. In contrast, the strain rate was significantly increased by administration of EPA (19.2 ± 5.6 s-1, 23.0 ± 6.6 s-1, P < 0.05).
CONCLUSION: One year of administration of EPA resulted in an improvement in regional arterial stiffness which was independent of blood pressure or serum cholesterol levels.
- Citation: Haiden M, Miyasaka Y, Kimura Y, Tsujimoto S, Maeba H, Suwa Y, Iwasaka T, Shiojima I. Effect of eicosapentaenoic acid on regional arterial stiffness: Assessment by tissue Doppler imaging. World J Cardiol 2012; 4(8): 256-259
- URL: https://www.wjgnet.com/1949-8462/full/v4/i8/256.htm
- DOI: https://dx.doi.org/10.4330/wjc.v4.i8.256
Eicosapentaenoic acid (EPA) is derived from fish oil. It was previously reported that EPA administration reduces peripheral arterial stiffness measured by brachial-ankle pulse wave velocity (baPWV)[1]. It was also reported that EPA administration results in a reduction of the incidence of cardiovascular diseases[2]. However, whether EPA has any effect on regional arterial stiffness has remained unclear.
We have previously reported that strain rate of the ascending aorta measured by tissue Doppler imaging is an accurate and blood pressure-independent index of regional arterial stiffness[3]. We therefore examined whether administration of EPA reduces regional arterial stiffness assessed by the strain rate of the ascending aorta.
With approval from the Institutional Review Board, we prospectively enrolled 19 patients (mean age 62 ± 8 years, 68% men) who visited our outpatient clinic for the management of hypertension or dyslipidemia. We excluded patients with diabetes mellitus (HbA1c > 6.0%) and/or large vessel complications including ischemic heart disease, cerebrovascular disease, aortic aneurysm (diameter of aorta ≥ 35 mm), and arteriosclerosis obliterans (ankle-brachial index < 0.8)[4,5], assessed by history taking, physical examination, electrocardiography, transthoracic echocardiography, blood tests, and computed tomography. Blood pressure was less than 140/90 mmHg in all patients, and low-density lipoprotein (LDL) cholesterol levels were less than 140 mg/dL for at least 2 years at the time of their enrollment.
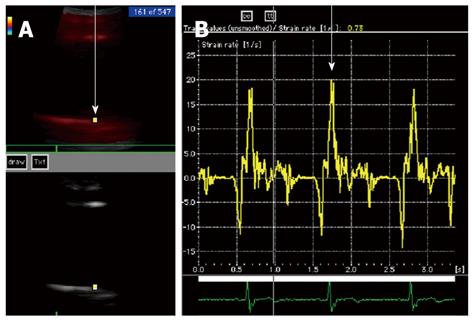
To evaluate the effects of EPA on arterial stiffness, baPWV (as an index of degree of systemic arteriosclerosis[6]) and strain rate (as an index of regional arterial stiffness[3]) were measured simultaneously. Both indices were determined before administration and one year after administration of EPA at a daily dose of 1800 mg. After identifying the long axis of the ascending aortic wall by tissue Doppler imaging, the strain rate was calculated by establishing a region of interest on the ascending aortic wall using Vivid Five (GE, Yokogawa Medical Systems), and the velocity between two points was measured and divided by the distance between these two points[3] (Figure 1). The movement velocity between any two points can be quantitatively assessed by this index, which is known to be unaffected by tissue swing or tethering[7-9]. The same point was assessed before and after EPA administration and the average of three heart beats was evaluated. Comprehensive echocardiography was also performed before and after EPA administration. Blood pressure and baPWV were simultaneously measured using an automatic waveform analyzer (BP-203RPE; AT Company, Colin Medical Technology). Both tests were conducted in a room with calming background music where the temperature was maintained at 20 °C. Each subject was instructed to rest in the supine position for 15 min. Then, blood pressure and baPWV were simultaneously measured, and tissue Doppler imaging was subsequently performed. Written informed consent to participate in the study was obtained from all patients.
The strain rate of the ascending aorta was measured in 10 healthy subjects (mean age 36 ± 19 years, 50% men) to obtain the normal range of the index. Inter-observer variability was assessed from 10 randomly selected images by 2 independent observers, each blinded to the results obtained by the other. Intra-observer variability was assessed by repeated measurements from 10 images by the same observer one week after the first analysis. Inter- and intra-observer variability was calculated as the absolute difference between repeated measurements in percentage of their mean.
The results represent the mean ± SD. The significance of differences between variables was determined by analysis of variance. Student’s t test was used for statistical comparisons and a P value of < 0.05 was considered to be statistically significant.
There was no difference in the proportion of patients with hypertension or dyslipidemia before and after administration of EPA. No appreciable difference was noted in the details of the use of concurrent drugs. There was no significant difference before and after EPA administration in LDL cholesterol levels (114 ± 29 mg/dL to 108 ± 28 mg/dL), triglyceride levels (107 ± 52 mg/dL to 99 ± 24 mg/dL), or systolic blood pressure (131 ± 16 mmHg to 130 ± 13 mmHg). The mean plasma concentration of EPA increased from 3.0% ± 1.1% to 8.5% ± 2.9% at one year after EPA administration (P < 0.001) (Table 1). None of the patients experienced any particular adverse reactions.
| Variables | Pre-administration | After-administration | P value |
| Systemic hypertension | 17 (89%) | 17 (89%) | NS |
| Dyslipidemia | 14 (74%) | 14 (74%) | NS |
| Body mass index (kg/m2) | 23.9 ± 2.3 | 23.9 ± 2.5 | NS |
| Systolic BP (mmHg) | 131 ± 16 | 130 ± 13 | NS |
| Diastolic BP (mmHg) | 83 ± 9 | 80 ± 7 | NS |
| Triglyceride (mg/dL) | 107 ± 52 | 99 ± 24 | NS |
| LDL cholesterol (mg/dL) | 114 ± 29 | 108 ± 28 | NS |
| EPA (%) | 3.0 ± 1.1 | 8.5 ± 2.9 | < 0.001 |
Echocardiographic left ventricular systolic or diastolic parameters were not significantly different before and after administration of EPA (Table 2). baPWV was 1765 ± 335 cm/s before EPA administration and 1745 ± 374 cm/s at one year after EPA administration. The difference was not statistically significant. In contrast, the strain rate of the ascending aorta was significantly increased from 19.2 ± 5.6 s-1 to 23.0 ± 6.6 s-1 by EPA administration (P < 0.05) (Figure 2), indicating that EPA administration for one year improved the regional stiffness of the ascending aorta.
| Variables | Pre-administration | After-administration | P value |
| LA dimension (mm) | 38 ± 5 | 39 ± 4 | NS |
| LVDd (mm) | 48 ± 3 | 48 ± 4 | NS |
| LVDs (mm) | 29 ± 4 | 30 ± 5 | NS |
| Mitral E/A | 0.96 ± 0.28 | 1.01 ± 0.56 | NS |
| Mitral DT (ms) | 218 ± 44 | 232 ± 60 | NS |
| LV ejection fraction (%) | 76 ± 8 | 76 ± 9 | NS |
| Strain rate of aorta (/s) | 19.2 ± 5.6 | 23.0 ± 6.6 | < 0.05 |
The mean value of the strain rate of the ascending aorta in 10 healthy subjects was 31.9 ± 5.0 s-1. Inter-observer and intra-observer variability for strain rate were 12% ± 5% and 11% ± 4%, respectively.
Our data show that administration of EPA for one year resulted in an improvement in regional arterial stiffness as indicated by increased strain rate of the ascending aorta, and that this effect of EPA on regional arterial stiffness is independent of blood pressure or serum cholesterol levels.
The degree of arteriosclerosis represents an important predictive factor for cardiovascular outcome[10-13]. Appropriate management of arteriosclerosis is clinically important, especially in the early stage of cardiovascular diseases. EPA is taken up by vascular smooth muscle cells and considered to maintain the elasticity of arteries. It was previously reported that administration of EPA reduces peripheral arterial stiffness measured by baPWV, and the incidence of cardiovascular disease[2,14,15].
To date, baPWV has been used as a noninvasive index of arterial stiffness or arterial distensibility[6,16]. There are, however, several limitations concerning the evaluation of arterial stiffness by baPWV. Firstly, the actual length of an artery used for measuring PWV is estimated based on an anatomical correction value. Secondly, baPWV is affected by systolic blood pressure and overestimated in hypertensive subjects[17,18]. In contrast, the strain rate measured by tissue Doppler imaging accurately reflects the velocity of tissue movements[7-9], and thus can be used to noninvasively assess regional arterial stiffness. Our previous study demonstrated that the strain rate of the ascending aorta is an accurate and blood pressure-independent index of regional atrial stiffness[3]. Positive results validating tissue Doppler imaging assessment of arterial wall properties have also been reported in the evaluation of abdominal aorta disease[19] and characterization of the common carotid artery[20].
In the present study, one year of administration of EPA significantly improved regional arterial stiffness of the ascending aorta but not baPWV. There are several published reports showing that baPWV was improved by the administration of EPA[2,14,15], in which patients were treated with EPA for at least 2 years. The discrepancy between the present study and previous reports in the effect of EPA on baPWV may be due to the difference in the length of EPA administration. The observation that the effect of EPA on arterial stiffness was not detected by baPWV also suggests that the strain rate of the ascending aorta may be a more sensitive marker of arteriosclerosis than baPWV. Finally, the relatively small number of patients examined and the lack of a control group need to be recognized as limitations of this study.
In conclusion, the strain rate of the ascending aorta was a sensitive and blood pressure-independent marker of arteriosclerosis. Administration of EPA for one year led to an improvement in regional arterial stiffness as indicated by strain rate, even though there was no change in blood pressure or serum cholesterol levels.
Eicosapentaenoic acid (EPA) is derived from fish oil. It was previously reported that administration of EPA reduces the incidence of cardiovascular diseases. However, whether EPA has any effect on regional arterial stiffness has been unclear till now.
This study evaluates the efficacy of EPA on arterial stiffness assessed by the strain rate of the ascending aorta.
This is the first study to report that administration of EPA for one year resulted in an improvement of regional arterial stiffness independently of blood pressure or serum cholesterol levels.
It has been reported that arterial stiffness is associated with an increased incidence of cardiovascular events, and appropriate management of arteriosclerosis is clinically important especially in the early stage of cardiovascular diseases. EPA administration reduces arterial stiffness measured by the strain rate of the ascending aorta and may result in the reduction of the incidence of cardiovascular diseases.
The study is well designed.
Peer reviewer: Gani Bajraktari, Professor, Service of Cardiology, University Clinical Centre of Kosova, Prishtina 10000, Yugoslavia
S- Editor Cheng JX L- Editor Logan S E- Editor Zhang DN
| 1. | Tomiyama H, Takazawa K, Osa S, Hirose K, Hirai A, Iketani T, Monden M, Sanoyama K, Yamashina A. Do eicosapentaenoic acid supplements attenuate age-related increases in arterial stiffness in patients with dyslipidemia?: A preliminary study. Hypertens Res. 2005;28:651-655. |
| 2. | Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747-2757. |
| 3. | Haiden M, Kimura Y, Miyasaka Y, Aota Y, Dote K, Takada A, Iwasaka T. New index of regional arterial stiffness assessed by tissue Doppler imaging. Acta Cardiol. 2008;63:603-608. |
| 4. | Makin AJ, Chung NA, Silverman SH, Lip GY. Vascular endothelial growth factor and tissue factor in patients with established peripheral artery disease: a link between angiogenesis and thrombogenesis? Clin Sci (. Lond). 2003;104:397-404. |
| 5. | Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY, Quyyumi AA. Basic fibroblast growth factor in patients with intermittent claudication: results of a phase I trial. J Am Coll Cardiol. 2000;36:1239-1244. |
| 6. | Wilkinson IB, Cockcroft JR, Webb DJ. Pulse wave analysis and arterial stiffness. J Cardiovasc Pharmacol. 1998;32 Suppl 3:S33-S37. |
| 7. | Voigt JU, Arnold MF, Karlsson M, Hübbert L, Kukulski T, Hatle L, Sutherland GR. Assessment of regional longitudinal myocardial strain rate derived from doppler myocardial imaging indexes in normal and infarcted myocardium. J Am Soc Echocardiogr. 2000;13:588-598. |
| 8. | Heimdal A, Støylen A, Torp H, Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. 1998;11:1013-1019. |
| 9. | Miyasaka Y, Haiden M, Kamihata H, Nishiue T, Iwasaka T. Usefulness of strain rate imaging in detecting ischemic myocardium during dobutamine stress. Int J Cardiol. 2005;102:225-231. |
| 10. | Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236-1241. |
| 11. | Fukuhara M, Matsumura K, Ansai T, Takata Y, Sonoki K, Akifusa S, Wakisaka M, Hamasaki T, Fujisawa K, Yoshida A. Prediction of cognitive function by arterial stiffness in the very elderly. Circ J. 2006;70:756-761. |
| 12. | Hatsuda S, Shoji T, Shinohara K, Kimoto E, Mori K, Fukumoto S, Koyama H, Emoto M, Nishizawa Y. Regional arterial stiffness associated with ischemic heart disease in type 2 diabetes mellitus. J Atheroscler Thromb. 2006;13:114-121. |
| 13. | Lehmann ED, Hopkins KD, Rawesh A, Joseph RC, Kongola K, Coppack SW, Gosling RG. Relation between number of cardiovascular risk factors/events and noninvasive Doppler ultrasound assessments of aortic compliance. Hypertension. 1998;32:565-569. |
| 14. | Mita T, Watada H, Ogihara T, Nomiyama T, Ogawa O, Kinoshita J, Shimizu T, Hirose T, Tanaka Y, Kawamori R. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191:162-167. |
| 15. | Hino A, Adachi H, Toyomasu K, Yoshida N, Enomoto M, Hiratsuka A, Hirai Y, Satoh A, Imaizumi T. Very long chain N-3 fatty acids intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis. 2004;176:145-149. |
| 16. | Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485-490. |
| 17. | Koji Y, Tomiyama H, Ichihashi H, Nagae T, Tanaka N, Takazawa K, Ishimaru S, Yamashina A. Comparison of ankle-brachial pressure index and pulse wave velocity as markers of the presence of coronary artery disease in subjects with a high risk of atherosclerotic cardiovascular disease. Am J Cardiol. 2004;94:868-872. |
| 18. | Yamashina A, Tomiyama H, Arai T, Koji Y, Yambe M, Motobe H, Glunizia Z, Yamamoto Y, Hori S. Nomogram of the relation of brachial-ankle pulse wave velocity with blood pressure. Hypertens Res. 2003;26:801-806. |
| 19. | Harada K, Yasuoka K, Shimada Y. Usefulness of tissue doppler imaging for assessing aortic wall stiffness in children with the Marfan syndrome. Am J Cardiol. 2004;93:1072-1075. |
| 20. | Schmidt-Trucksäss A, Grathwohl D, Schmid A, Boragk R, Upmeier C, Keul J, Huonker M. Assessment of carotid wall motion and stiffness with tissue Doppler imaging. Ultrasound Med Biol. 1998;24:639-646. |