Published online May 26, 2012. doi: 10.4330/wjc.v4.i5.188
Revised: April 27, 2012
Accepted: May 4, 2012
Published online: May 26, 2012
AIM: To investigate the luminal esophageal temperature (LET) at the time of delivery of energy for pulmonary vein isolation (PVI).
METHODS: This study included a total of 110 patients with atrial fibrillation who underwent their first PVI procedure in our laboratory between March 2010 and February 2011. The LET was monitored in all patients. We measured the number of times that LET reached the cut-off temperature, the time when LET reached the cut-off temperature, the maximum temperature (T max) of the LET, and the time to return to the original pre-energy delivery temperature once the delivery of energy was stopped.
RESULTS: Seventy-eight patients reached the cut-off temperature. It took 6 s at the shortest time for the LET to reach the cut-off temperature, and 216.5 ± 102.9 s for the temperature to return to the level before the delivery of energy. Some patients experienced a transient drop in the LET (TDLET) just before energy delivery. Ablation at these sites always produced a rise to the LET cut-off temperature. TDLET was not observed at sites where the LET did not rise. Thus, the TDLET before the energy delivery was useful to distinguish a high risk of esophageal injury before delivery of energy.
CONCLUSION: Sites with a TDLET before energy delivery should be ablated with great caution or, perhaps, not at all.
- Citation: Sato D, Teramoto K, Kitajima H, Nishina N, Kida Y, Mani H, Esato M, Chun YH, Iwasaka T. Measuring luminal esophageal temperature during pulmonary vein isolation of atrial fibrillation. World J Cardiol 2012; 4(5): 188-194
- URL: https://www.wjgnet.com/1949-8462/full/v4/i5/188.htm
- DOI: https://dx.doi.org/10.4330/wjc.v4.i5.188
Pulmonary vein isolation (PVI) by radiofrequency (RF) catheter ablation (CA) has become an established strategy for the management of patients with drug refractory, symptomatic atrial fibrillation (AF)[1-6]. However, in comparison to the CA of the other arrhythmias, there are many and varied complications[7,8]. Specifically, a left atrial esophageal fistula due to thermal injury of the esophagus is a rare but devastating complication after PVI[9,10]. Likewise, acute pyloric spasms and gastric hypomotility resulting from thermal damage to the periesophageal vagal nerves caused by CA for AF can be devastating as well[11].
A report exists that illustrates the potential for esophageal damage during the application of RF energy, and the ability to monitor luminal esophageal temperature (LET) by inserting a temperature probe in the esophagus[12,13]. However, there have been no reports on the detailed examination of the progress of the LET in PVI for AF. Therefore, we investigated progress of the LET at the time of the delivery of energy for PVI of AF, by real-time LET monitoring.
This study included a total of 110 patients with drug-refractory AF (82 paroxysmal and 28 persistent) who underwent first PVI by CA in our laboratory between March 2010 and February 2011. Patient characteristics are shown in Table 1. All patients gave their written informed consent and antiarrhythmic drugs (AADs) were discontinued 7 d before the ablation session. All patients were effectively anticoagulated for > 4 wk, and a 64-slice multidetector computed tomography (MDCT) scan of the left atrium (LA) and/or transesophageal echocardiography was performed before the CA to exclude any atrial thrombi.
| Age (yr) | 63.8 ± 11.2 |
| Male sex, n (%) | 79 (71.8) |
| BMI (kg/m2) | 24.1 ± 3.4 |
| Paroxysmal/persistent AF | 82/28 |
| Ineffective drugs, n | 1.9 ± 1.1 |
| LA diameter (mm) | 43.1 ± 6.7 |
| LA volume (cm3) | 124.8 ± 43.3 |
| LA volume index (cm3/m2) | 73.5 ± 25.6 |
| Ejection fraction (%) | 65.4 ± 11.9 |
| LSPV diameter (mm) | 20.7 ± 4.7 |
| LIPV diameter (mm) | 16.3 ± 2.4 |
| RSPV diameter (mm) | 20.4 ± 4.7 |
| RIPV diameter (mm) | 17.1 ± 3.5 |
Transthoracic echocardiography was performed on all patients to measure LA diameter. MDCT was performed for electroanatomical mapping integration, pulmonary vein anatomy delineation, LA thrombi exclusion, measuring the PV ostium diameter, and LA volume estimation.
The procedures were performed during deep sedation/analgesia with the administration of propofol and dexmedetomidine with preservation of spontaneous breathing and continuous monitoring of the systemic arterial pressure and oxygen saturation.
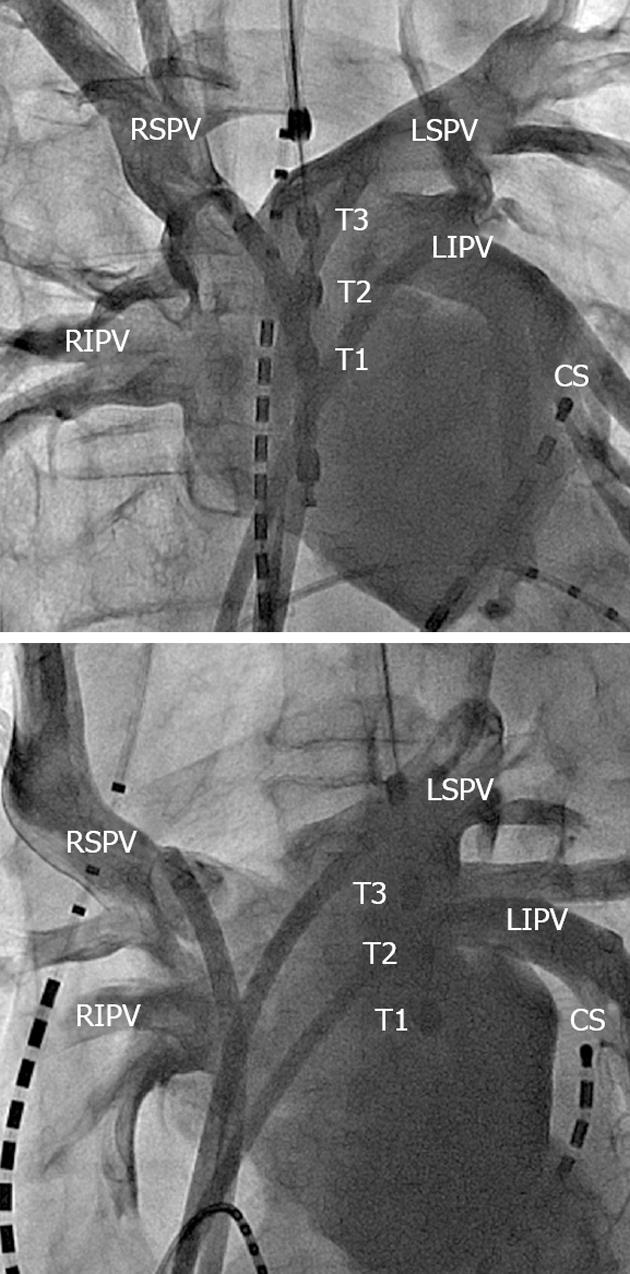
A 6 Fr duo-decapolar three-site mapping catheter (EPstar SAC, Japan Lifeline Inc., Tokyo, Japan) was positioned in the coronary sinus for pacing, recording and internal cardioversion. Four venous sheaths (5, 8, 8 and 8.5 Fr) and one arterial sheath (4 Fr) were placed in the femoral veins/arteries. A 5 Fr catheter was introduced into the right ventricle. A single trans-septal puncture was performed. After the trans-septal puncture, two non-steerable long sheaths (LAMP 45; St Jude Medical, St Paul, MN, United States; Preface; Biosense-Webster, Diamond Bar, CA, United States) and a steerable introducer (Agilis NxT Steerable Introducer Small Curl; St Jude Medical) were introduced into the left superior pulmonary vein, left inferior pulmonary vein (LIPV) and right superior pulmonary vein, respectively. Left arteriography by injection of contrast medium via the three long sheaths was simultaneously performed to obtain the anatomical relationship between the area around the PV ostium and the esophagus (Figure 1).
A 5000-U intravenous bolus of heparin was administered after the successful trans-septal puncture. The activated clotting time (ACT) was measured every 30 min, and the heparin dose was adjusted to maintain a target ACT of 300 s.
Two circular mapping catheters (EPstar Libero; Japan Lifeline Inc.) were placed in the superior and inferior pulmonary veins, respectively, and the left- and right-sided ipsilateral PVs were circumferentially and extensively ablated, respectively, with use of an electromagnetic mapping system (CARTO, Biosense Webster, United States) and electrophysiologic guidance. The LA posterior wall, at a distance of 1 to 3 cm from the left- or right-sided ostia of the PVs, was anatomically ablated. The distal edges of the anterior aspect of the PVs with early PV potentials or continuous PV and LA potentials were targeted for ablation. Isolation of the left sided PVs was performed during distal coronary sinus pacing and isolation of the right-sided PVs during sinus rhythm.
Ablation was performed with an open irrigated tip catheter (ThermoCool; Biosense Webster). A generator was used to deliver 25 W of RF energy to the catheter tip and finished delivery in 20-25 s at all sites. The irrigation flow rate was set to 17 mL/min. For safety, the generator was set to reduce the power if the temperature of the catheter exceeded 43 °C. The ablation catheter was irrigated for 2 s just before and just after delivering the energy. A cut-off LET of 42 °C was defined for the termination of the energy delivery. Even if an ablation catheter was near to the temperature probe in fluoroscopy, we delivered energy if the LET did not rise. After the LET normalized, the ablation was continued with less power and/or an alternative ablation course was chosen to prevent further temperature rises. The endpoint of the ablation was the elimination of all PV potentials and pacing maneuvers performed inside the PVs to test for any remaining PV conduction or complete PVI with bidirectional block[14].
After completing the PVI, the cavotricuspid isthmus was also ablated to create bidirectional conduction block[15]. Following the PVI and cavotricuspid isthmus ablation, decremental pacing was performed from the coronary sinus or LA appendage starting at a cycle length of 300 ms and ending with loss of 1:1 atrial capture, which was repeated two times. If burst pacing from the coronary sinus or LA appendage was able to induce sustained AF lasting > 5 min, the LA roof line and the mitral isthmus between the LIPV and mitral annulus were also ablated to achieve bidirectional conduction block. Finally, in the presence of sustained AF induced by burst pacing, ablation-targeting complex fractionated atrial electrography (CFAE) was performed within the LA including the coronary sinus.
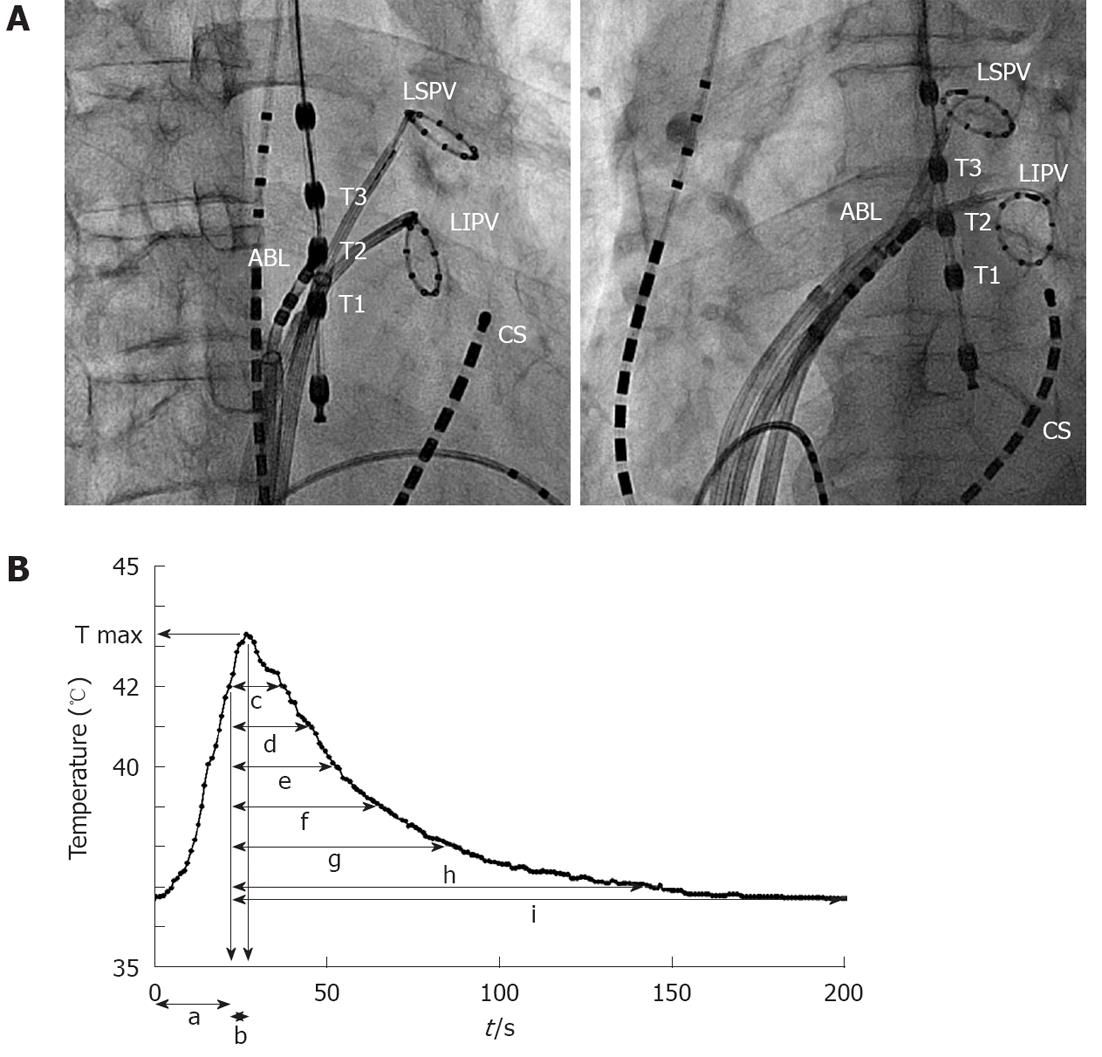
In all patients, an LET monitoring probe (Esotherm; FIAB SpA, Florence, Italy) with three olive-shaped metal thermocouple electrodes (distance 10 mm) was placed within the esophagus under fluoroscopic guidance directly posterior to the LA. The temperature probe was adjusted to equal heights of the PV ostium after left arteriography and selective cannulation of the PVs with the ablation catheter. The temperature probe was connected to three precision thermometers allowing continuous measurement, recording and display of the LET.
We measured the number of times the LET reached the cut-off temperature, the time when the LET reached the cut-off temperature, the maximum temperature (T max) of the LET, the time to reach T max after the LET reached the cut-off temperature, and the time for the temperature to decrease to the following values after stopping delivery of energy: 42, 41, 40, 39, 38 and 37 °C and the temperature before the delivery of energy. We also measured the temperature before the delivery of energy at the site where the LET reached the cut-off temperature (Figure 2).
After 3-6 mo, in the absence of any AF recurrence, anticoagulant treatment was discontinued unless other major risk factors were present. AADs were not prescribed for patients with paroxysmal AF, but were prescribed for 3 mo following the ablation in patients with persistent AF. All patients were scheduled to visit our clinic at 1, 3 and 6 mo after discharge. In the case of nonappearance at the follow-up dates, patients were contacted by telephone. A recurrence of AF was defined according to the patient’s symptoms and electrocardiogram. All patients took proton pump inhibitors (PPIs) after ablation for at least 2 wk.
Continuous variables are expressed as the mean ± SD except for the count and time variables. Statistical significance was assessed using the unpaired Student’s t test or Mann-Whitney test if necessary. Categorical variables, expressed as numbers or percentages, were analyzed with the χ2 test or Fisher’s exact test. All tests were two-tailed and a P value < 0.05 was considered statistically significant.
The study was approved by the Institutional Review Board of Takeda Hospital (Kyoto, Japan).
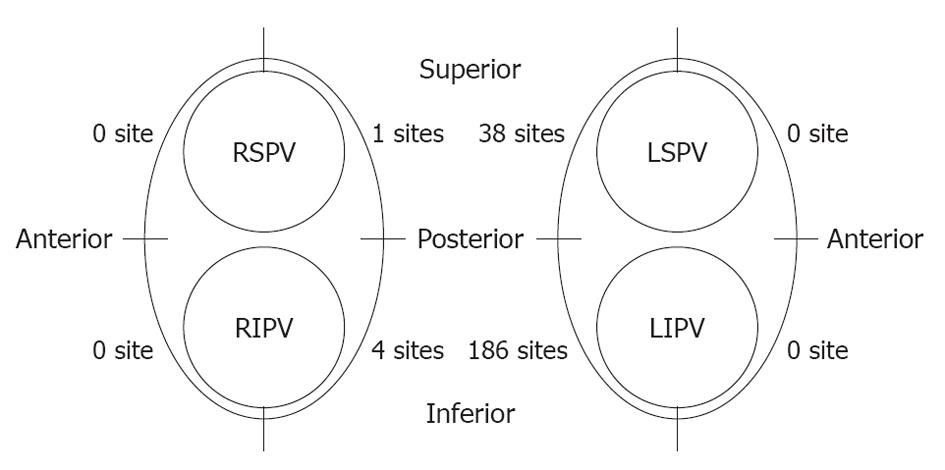
A total of 229 RF energy deliveries (in 78 out of 110 patients) reached the cut-off temperature. Most of these sites were located on the LA posterior wall near the left PVs, and in particular around the LIPV ostium. The LET also increased on the left side of the right PVs, whereas there were only five sites with an LET > 42 °C (Figure 3).
There was no atrio-esophageal fistula formation. There was no significant difference in the age, sex, body mass index, LA diameter, LA volume, LA volume index, and diameter of the PVs between the LET increase and non-LET increase groups (Table 2).
| Non-LET increase(n = 32) | LET increase(n = 78) | P value | |
| Age (yr) | 65.3 ± 11.9 | 63.2 ± 10.9 | 0.395 |
| Male sex, n (%) | 21 (65.6) | 58 (74.4) | 0.355 |
| BMI (kg/m2) | 24.3 ± 3.6 | 24.0 ± 3.3 | 0.619 |
| Paroxysmal/persistent AF | 24/8 | 58/20 | 0.944 |
| LA diameter (mm) | 43.7 ± 6.1 | 42.9 ± 6.9 | 0.582 |
| LA volume (cm3) | 127.7 ± 42.7 | 123.2 ± 43.3 | 0.658 |
| LA volume index (cm3/m2) | 77.5 ± 27.0 | 71.7 ± 24.8 | 0.334 |
| Ejection fraction (%) | 62.9 ± 12.3 | 66.5 ± 11.7 | 0.155 |
| LSPV diameter (mm) | 21.2 ± 5.1 | 20.5 ± 4.5 | 0.972 |
| LIPV diameter (mm) | 15.9 ± 2.5 | 16.5 ± 2.3 | 0.295 |
| RSPV diameter (mm) | 20.8 ± 5.6 | 20.2 ± 4.4 | 0.548 |
| RIPV diameter (mm) | 17.0 ± 4.5 | 17.1 ± 3.1 | 0.972 |
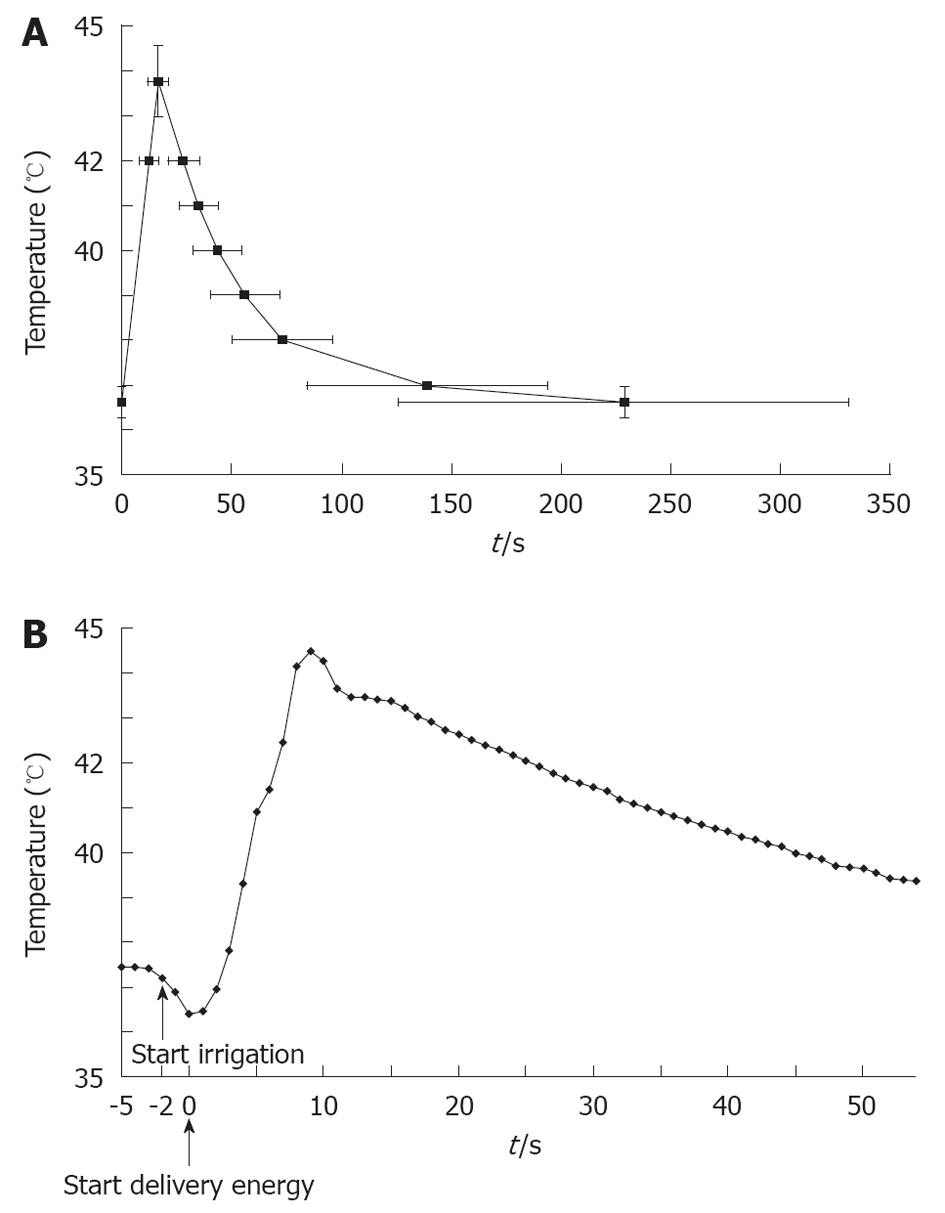
The time for the LET to reach the cut-off temperature was 12.2 ± 4.5 s (280.8 ± 103.7 J). The LET reached the cut-off temperature within 10 s in 32 of 110 (29.1%) patients. The shortest time was 6 s. It was 4.1 ± 1.9 s to reach T max after the LET reached the cut-off temperature. The time for the temperature to decrease to the following values after stopping delivery of energy: 42, 41, 40, 39, 38 and 37 °C, and the temperature before the delivery of energy was 16.4 ± 6.3, 23.3 ± 8.3, 31.9 ± 9.9, 45.0 ± 15.8, 61.3 ± 22.9, 126.7 ± 54.5 and 216.5 ± 102.9 s (Figure 4A).
LET often overshot the cut-off temperature after cessation of energy delivery. A temperature overshoot was observed in all patients. The average T max was 43.8 ± 0.8 °C and the maximal registered temperature was 45.6 °C.
There was a transient drop in the LET (TDLET) just before the delivery of energy. We defined TDLET as a decrease of > 0.5 °C for 2 s just before energy delivery. TDLET was observed in 34 of 110 (30.9%) patients (Figure 4B). The LET decreased (0.82 ± 0.35 °C) before delivery of energy with a TDLET. The maximum temperature decrease was 1.89 °C. The site that caused a TDLET before energy delivery always reached the cut-off temperature within 9.2 ± 3.2 s. Most of these sites were located near the LIPV. TDLET was not observed at sites where the LET did not increase.
Twenty-nine patients underwent a second ablation procedure and eight underwent a third involving treatment for reconduction of the PVs, the LA roof line, the mitral isthmus line, ablation of new atrial premature contraction foci, or additional CFAE ablation. The final success rate, evaluated at 6 mo after the first session, was 89% (73 of 82, including eight patients with AADs) for the paroxysmal AF patients and 79% (22 of 28, including 10 with AADs) for the persistent AF patients.
The LET rose by 70.9% in all cases. There was no significant difference in the various parameters between the LET increase and non-LET increase groups. Thus, there was no parameter that could predict an LET rise before ablation.
The shortest time for LET to reach the cut-off temperature was 6 s, and the maximum time for the temperature to decrease to the pre-energy application value after energy delivery was stopped was 216.5 s. Most of the sites were located along the posterior side of the LPV, especially around the LIPV.
It was determined that 30.9% of all patients in this study had a TDLET just before energy delivery. It was also determined that the TDLET was affected by the cooling of the irrigation catheter and the close proximity of the esophagus prior to energy delivery. In addition to causing the cooling effect, it was believed that the TDLET could easily be affected by the energy delivery and the location could more easily cause esophageal injury. In fact, the site that caused a TDLET before the energy delivery reached the cut-off temperature in all cases. TDLET was not observed at sites where the LET did not increase. Thus, the TDLET before energy delivery was useful to distinguish a high risk of esophageal injury before delivery of energy. We should avoid performing CA at sites with a TDLET. The upper limit of LET in PVI is important to prevent esophageal injury[16]. Similarly, the lower limit of LET in PVI is important to prevent esophageal injury by discovering TDLET.
Reports in the literature have attested to the utility of methods for visualizing the esophagus during AF ablation[17]. In this study, we were able to establish the position of the esophagus to some extent because a probe was inserted. However, even if an ablation catheter was near to the temperature probe in fluoroscopy, we delivered energy. We might affect the LET if we avoided using an esophagus probe and delivered energy.
We set the safety limit of the LET to 42 °C because it was shown that cells exposed to that temperature underwent morphological changes, including disruption and fragmentation of the Golgi complexes and swelling of the mitochondria[18]. In addition, metabolic studies have revealed the inhibition of both respiration and glycolysis in tumor tissue and cultured tumor cells exposed to temperatures of 42-43 °C[19]. There was no atrio-esophageal fistula formation. There is a report that esophageal damage is not caused at 42 °C[20]. However there is also a report that asymptomatic esophageal damage is observed in 14.6% of cases with endoscopy[16]. We did not perform upper gastrointestinal endoscopy, and asymptomatic esophageal damage may have been overlooked. Actually, the LET often overshot 42 °C after the cessation of RF energy delivery. LET > 42 °C was within 5 °C and persisted for a relatively short duration. Such slight and short overshooting of the LET did not seem to cause any serious esophageal damage. It is thought that the maximum temperature is the most important factor influencing esophageal injury and we should use lower temperature settings so that the T max remains within 42-43 °C.
This study had several limitations. In a large survey of the incidence and causes of death during or as a consequence of CA of AF, atrio-esophageal fistula occurred in 0.01%-0.02% of patients[20,21]. Atrio-esophageal fistula due to thermal injury of the esophagus is rare. This was possibly because atrio-esophageal fistula did not occur at a high frequency and the population of this study was not large enough. All patients took PPIs after CA, which may have resulted in an absence of gastrointestinal symptoms. Therefore, we might have underestimated gastrointestinal dysfunction. We used a non-deflectable temperature probe. This probe may not have been able to be accurately placed just posterior to the ablation catheter. Lesion formation was also related to the contact force[22]. It is also likely the LET was related to the contact force as well. However, the contact force was not evaluated in this study. Furthermore, this study was performed in a clinical setting, therefore, no estimation of the intramural tissue temperature or thrombus formation on the electrode-tissue interface was carried out, which would have been of great value.
We discovered TDLET by investigating the LET at the time of delivery of energy for PVI. The lower limit of LET in PVI is important to prevent esophageal injury by discovering TDLET. Sites with a TDLET before energy delivery should be ablated with great caution or, perhaps, not at all.
A left atrial esophageal fistula due to thermal injury to the esophagus is a devastating complication after pulmonary vein isolation (PVI). A report exists that illustrates the potential for esophageal damage during the application of radiofrequency (RF) energy, and the ability to monitor luminal esophageal temperature (LET) by inserting a temperature probe in the esophagus. However, there have been no reports on the detailed examination of the progress of the LET in PVI for atrial fibrillation (AF).
This study reports on the detailed examination of the progress of the LET in PVI for AF. Specifically, this is the first study to report on transient drop in the LET (TDLET) just before the delivery of energy.
A report exists that illustrates the potential for esophageal damage during the application of RF energy, and the ability to monitor LET by inserting a temperature probe in the esophagus. However, nobody was able to anticipate whether LET increased before delivering energy. The TDLET before energy delivery may be useful to distinguish a high risk of esophageal injury before delivery of energy.
Detecting TDLET before energy delivery is useful for identifying a higher risk of esophageal injury. Therefore, the lower limit of LET in PVI is important to prevent esophageal injury by discovering TDLET.
The study shows a new aspect of the LET monitoring during AF ablation. It shows, that the esophageal sites with the highest increase of temperature during ablation can be predicted by measuring the degree of lowering the intraluminal temperature shortly prior starting ablation. The decrease of LET is caused by proximity of the irrigated catheter tip in the posterior left atrium. It seems to be evident, that the cooling effect of the catheter is more pronounced on sites that are anatomically closer, than on sites that are more distant. This finding has not been described yet in a peer reviewed journal. This is an interesting manuscript with considerable merit.
Peer reviewer: Dr. Richard G Trohman, Professor of Medicine, Rush University Medical Center, 1653 West Congress Parkway, Room 983 Jelke, Chicago, IL 60612, United States
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
| 1. | Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666. [PubMed] |
| 2. | Haïssaguerre M, Shah DC, Jaïs P, Hocini M, Yamane T, Deisenhofer I, Chauvin M, Garrigue S, Clémenty J. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000;102:2463-2465. [PubMed] |
| 3. | Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619-2628. [PubMed] |
| 4. | Oral H, Scharf C, Chugh A, Hall B, Cheung P, Good E, Veerareddy S, Pelosi F, Morady F. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355-2360. [PubMed] |
| 5. | Cummings JE, Schweikert RA, Saliba WI, Burkhardt JD, Brachmann J, Gunther J, Schibgilla V, Verma A, Dery M, Drago JL. Assessment of temperature, proximity, and course of the esophagus during radiofrequency ablation within the left atrium. Circulation. 2005;112:459-464. [PubMed] |
| 6. | Natale A, Raviele A, Arentz T, Calkins H, Chen SA, Haïssaguerre M, Hindricks G, Ho Y, Kuck KH, Marchlinski F. Venice Chart international consensus document on atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2007;18:560-580. [PubMed] |
| 7. | Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2009;53:1798-1803. [PubMed] |
| 8. | Dagres N, Hindricks G, Kottkamp H, Sommer P, Gaspar T, Bode K, Arya A, Husser D, Rallidis LS, Kremastinos DT. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J Cardiovasc Electrophysiol. 2009;20:1014-1019. [PubMed] |
| 9. | Pappone C, Oral H, Santinelli V, Vicedomini G, Lang CC, Manguso F, Torracca L, Benussi S, Alfieri O, Hong R. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724-2726. [PubMed] |
| 10. | Sosa E, Scanavacca M. Left atrial-esophageal fistula complicating radiofrequency catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:249-250. [PubMed] |
| 11. | Nakagawa H, Yamanashi WS, Pitha JV, Arruda M, Wang X, Ohtomo K, Beckman KJ, McClelland JH, Lazzara R, Jackman WM. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation. 1995;91:2264-2273. [PubMed] |
| 12. | Weiss C, Antz M, Eick O, Eshagzaiy K, Meinertz T, Willems S. Radiofrequency catheter ablation using cooled electrodes: impact of irrigation flow rate and catheter contact pressure on lesion dimensions. Pacing Clin Electrophysiol. 2002;25:463-469. [PubMed] |
| 13. | Dorwarth U, Fiek M, Remp T, Reithmann C, Dugas M, Steinbeck G, Hoffmann E. Radiofrequency catheter ablation: different cooled and noncooled electrode systems induce specific lesion geometries and adverse effects profiles. Pacing Clin Electrophysiol. 2003;26:1438-1445. [PubMed] |
| 14. | Piorkowski C, Kottkamp H, Gerds-Li JH, Arya A, Sommer P, Dagres N, Esato M, Riahi S, Weiss S, Kircher S. Steerable sheath catheter navigation for ablation of atrial fibrillation: a case-control study. Pacing Clin Electrophysiol. 2008;31:863-873. [PubMed] |
| 15. | Miyazaki S, Takahashi A, Kuwahara T, Kobori A, Yokoyama Y, Nozato T, Sato A, Aonuma K, Hirao K, Isobe M. Randomized comparison of the continuous vs point-by-point radiofrequency ablation of the cavotricuspid isthmus for atrial flutter. Circ J. 2007;71:1922-1926. [PubMed] |
| 16. | Halm U, Gaspar T, Zachäus M, Sack S, Arya A, Piorkowski C, Knigge I, Hindricks G, Husser D. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol. 2010;105:551-556. [PubMed] |
| 17. | Dixit S, Marchlinski FE. How to recognize, manage, and prevent complications during atrial fibrillation ablation. Heart Rhythm. 2007;4:108-115. [PubMed] |
| 18. | Welch WJ, Suhan JP. Morphological study of the mammalian stress response: characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblasts after heat-shock treatment. J Cell Biol. 1985;101:1198-1211. [PubMed] |
| 19. | Dickson JA, Calderwood SK. Effects of hyperglycemia and hyperthermia on the pH, glycolysis, and respiration of the Yoshida sarcoma in vivo. J Natl Cancer Inst. 1979;63:1371-1381. [PubMed] |
| 20. | Kuwahara T, Takahashi A, Kobori A, Miyazaki S, Takahashi Y, Takei A, Nozato T, Hikita H, Sato A, Aonuma K. Safe and effective ablation of atrial fibrillation: importance of esophageal temperature monitoring to avoid periesophageal nerve injury as a complication of pulmonary vein isolation. J Cardiovasc Electrophysiol. 2009;20:1-6. [PubMed] |
| 21. | Doll N, Borger MA, Fabricius A, Stephan S, Gummert J, Mohr FW, Hauss J, Kottkamp H, Hindricks G. Esophageal perforation during left atrial radiofrequency ablation: Is the risk too high? J Thorac Cardiovasc Surg. 2003;125:836-842. [PubMed] |
| 22. | Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, Ikeda A, Pitha JV, Sharma T, Lazzara R. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol. 2008;1:354-362. [PubMed] |