Published online May 26, 2012. doi: 10.4330/wjc.v4.i5.183
Revised: May 15, 2012
Accepted: May 22, 2012
Published online: May 26, 2012
AIM: To investigate the prevalence of clinically unrecognized mitral regurgitation (MR) in lone atrial fibrillation (AF).
METHODS: We studied the prevalence and severity of MR by transesophageal echocardiography (TEE) in patients with “lone” AF as compared to a matched cohort of patients in normal sinus rhythm (NSR) undergoing TEE for other indications besides recognized valvular heart disease.
RESULTS: A total of 157 subjects (57 in the AF group and 100 in the NSR group) with structurally normal cardiac valves were included in the study. In the AF group, moderate MR or more was noted in 66% of the patients, mild MR in 18%, trace or no MR in 16%. In the control group, moderate MR was noted in 6% of patients, mild MR 31%, trace or no MR in 63 % of patients. Moderate MR or greater was significantly more prevalent in the AF group compared to the NSR group (66% vs 6%, P < 0.0001).
CONCLUSION: Clinically unrecognized moderate MR is prevalent in “lone” AF -either as an etiologic factor leading to “lone” AF or developing after onset of AF.
- Citation: Sharma S, Lardizabal J, Monterroso M, Bhambi N, Sharma R, Sandhu R, Singh S. Clinically unrecognized mitral regurgitation is prevalent in lone atrial fibrillation. World J Cardiol 2012; 4(5): 183-187
- URL: https://www.wjgnet.com/1949-8462/full/v4/i5/183.htm
- DOI: https://dx.doi.org/10.4330/wjc.v4.i5.183
There is scant data regarding the prevalence of mitral regurgitation (MR) in lone atrial fibrillation (AF). Significant MR from structural valvular abnormality is associated with subsequent development of chronic AF. However, little is known regarding the relationship between lone AF and clinically unrecognized moderate MR in patients with structurally normal valves. We noted that patients who were referred for transesophageal echocardiography (TEE) guided cardioversion for AF had an unusually higher prevalence of TEE evident moderate MR than a control group who underwent TEE for other indications. We sought to determine the exact prevalence of MR by TEE in patients with lone AF as compared to patients in normal sinus rhythm (NSR) by blinded observation in a cohort of consecutive patients who underwent TEE at our institution.
Over a 50-mo period, 57 consecutive patients with a diagnosis of lone AF underwent TEE in our institution to exclude intra-cardiac thrombus prior to external direct current cardioversion. These patients comprised the AF group. Within the same period, a cohort of 100 patients in NSR who underwent TEE for a variety of indications (evaluation for suspected endocarditis, aortic dissection, and pulmonary hypertension) were enrolled as age- and sex-matched controls. At the time of the initial diagnosis, patients in the AF group were < 60 years and were without concomitant heart disease, hypertension or diabetes mellitus. Subjects from both groups were included if they had structurally normal mitral valves. Exclusion criteria included uncontrolled hypertension, heart failure, cardiomyopathy, structural abnormality of any of the valves (including valvular stenosis and mitral valve prolapse), history of cardiac surgery (including valve repair or replacement), and congenital or pericardial heart disease.
Standard TEE with doppler color flow mapping was employed to assess the variables. All measurements were in conformity with the American Society of Echocardiography guidelines, and were verified by two independent, blinded observers. The presence or absence of MR was verified, and its severity was graded semi-quantitatively as follows: 0 (none), 1 (trace), 2 (mild), 3 (moderate), 4 (severe). Any discordance of the severity of MR was resolved by joint reading by the two observers. The following variables were also measured: left ventricle (LV) ejection fraction, LV diameter, left atrial diameter (LA) and mitral annulus diameter in the 4 chamber views.
Patient demographics, severity of MR, and cardiac dimensions between groups were compared with the use of the Student’s t test for continuous variables, and the Fisher’s exact test for categorical variables. A two-sided P value of 0.05 or less was considered to indicate statistical significance.
A total of 157 subjects (57 in the AF group and 100 in the NSR group) with structurally normal cardiac valves were included in the analysis. Both groups were similar in terms of age, sex, and co-morbidities (hypertension, chronic kidney disease and cerebrovascular disease) except for a greater prevalence of diabetes and lung disease in the control group. Baseline characteristics of the subjects are listed in Table 1.
| Demographic data | AF group(n = 57) | NSR group(n = 100) | P value(a = 0.05) |
| Age (mean ± SD) | 50.2 ± 7.3 | 51.7 ± 6.1 | 0.17 NS |
| Male sex | 39 (68) | 66 (66) | 0.86 NS |
| Comorbidities | |||
| Hypertension (controlled) | 8 (14) | 19 (19) | 0.51 NS |
| Diabetes mellitus | 0 (0) | 13 (13) | < 0.05 |
| Chronic lung disease | 0 (0) | 9 (9) | < 0.05 |
| Chronic kidney disease | 0 (0) | 6 (6) | 0.08 NS |
| Cerebrovascular disease | 0 (0) | 3 (3) | 0.55 NS |
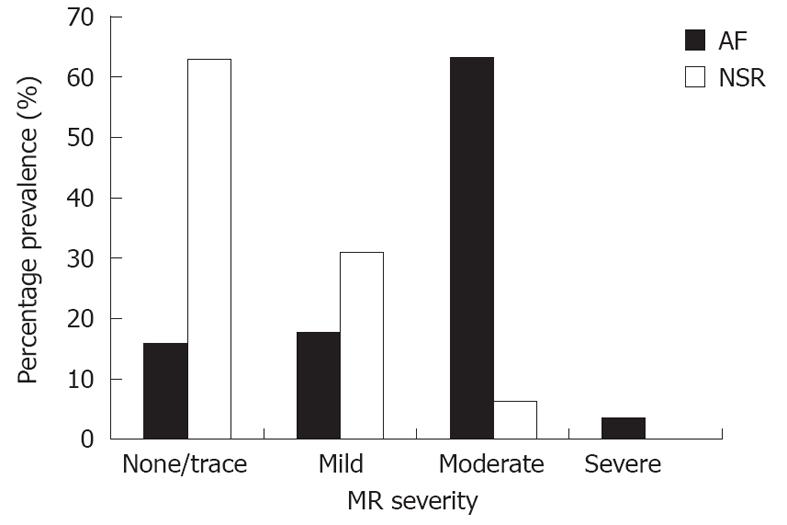
The severity of MR noted in the patients in the AF and NSR cohorts is outlined in Figure 1. In the AF group (n = 57), moderate MR or more was noted in 38 patients (66%), mild MR in 10 patients (18%), trace or no MR in 9 patients (16%). In the control group (n = 100), moderate MR was noted in 6 patients (6%), mild MR 31 patients (31%), trace or no MR in 63 patients (63%). Moderate MR or greater was significantly more prevalent in the AF group compared to the NSR group (66% vs 6%, P < 0.0001). All of the subjects had left ventricular ejection fraction > 50%, and there was no difference in LV chamber sizes between groups. LA between the two groups was not statistically significant between the two groups. Mitral annular diameter was statistically greater in the AF group than the NSR group (Table 2).
| Measurements | AF group(n = 57) | NSR group(n = 100) | P value(a = 0.05) |
| LV ejection fraction (%) | 68 ± 6 | 66 ± 9 | 0.13 NS |
| LV end diastolic diameter (cm) | 5.4 ± 0.7 | 5.5 ± 0.5 | 0.30 NS |
| LA diameter (cm) | 3.7 ± 0.5 | 3.6 ± 0.6 | 0.28 NS |
| Mitral annulus diameter (cm) | 4.0 ± 0.4 | 3.4 ± 0.5 | < 0.0001 |
AF is the most common cardiac rhythm disorder, affecting about 2% of the general adult population and is commonly associated with structural heart disease[1]. AF induces electrical, contractile and structural remodeling of the atrial myocardium that leads to AF progression and permanence[2]. However, lone AF (defined AF in the absence of demonstrable underlying cardiac disease or a history of hypertension in subjects < 65 years) is uncommon, comprising less than 3% of the total cases of AF[1-4]. Numerous mechanisms are postulated in pathogenesis of AF including acute atrial stretch, structural and electrophysiological alterations, systemic inflammation, oxidative stress, autonomic imbalance, atrial fibrosis, or localized atrial myocarditis, genetic predisposition, obesity, sleep apnea, metabolic syndrome, alcohol consumption, endurance sports suggest that apparently “lone” AF may not be necessarily idiopathic or “lone” in many patients[2-8].
In this study, we propose the possibility that clinically unrecognized moderate MR may predispose to occurrence of AF, or alternatively MR develops slowly in patients with AF related to mitral annular dilation as was noted in our subset of patients with AF. We cannot exactly pinpoint the time duration of the existence of AF in our subset of patients, since these patients were all recently or incidentally diagnosed with AF and were referred for TEE guided cardioversion to our clinic. Most of these subjects with lone AF had no transthoracic echocardiography carried out before since their TEE was scheduled essentially within the same week of their diagnosis.
Data are scant on the prevalence of MR in lone AF. In a previous report, using transthoracic 2-dimensional echocardiography, moderate or severe MR was not observed in patients with lone AF[9]. Some reports suggest that MR may arise from isolated annular dilatation secondary to lone AF and associated atrial remodeling[10-12]. Indeed, in our study, severe MR was not observed frequently. However, in our study utilizing TEE, which is more sensitive in evaluating MR, we noted that moderate MR was present in more than half of the subjects with lone AF, a prevalence that is significantly higher than that of the matched controls.
It is known that significant MR (from degenerative causes or organic valvular abnormalities) is associated with development of chronic AF, at a rate of about 5% per year[13,14]. The risk of AF is correlated with increase in LA dimension[15]. Even asymptomatic organic MR has been shown to increase the risk of AF and adverse cardiovascular outcomes. MR from organic causes enlarges the left atrium, but most patients are initially asymptomatic because atrial compliance may normalize left atrial pressure even in the presence of severe regurgitation[16]. In patients with lone AF, atrial compliance may remain abnormal even after restoration of NSR which could be related to atrial fibrosis in these patients[17-19].
In our study on subjects with structurally normal mitral valves, none of the subjects had any significant LA enlargement. However, mitral annular dilation was more frequent in the AF subgroup. It is conceivable that MR follows some mitral annular dilation and the left atrial dilation takes a longer time to develop and does not become apparent in the early stages of lone AF. Whether AF exerts some anatomical effect on the mitral annulus remains hypothetical, since the number of patients is small. It is also possible that the atrial dyssynchrony produces an anatomically variable contraction of the mitral annulus leading to varying degrees of mitral closure with an irregular R-R interval, possibly predisposing to slow development of MR. One study suggests development of “functional MR” in patients with AF that improves when sinus rhythm is restored[20].
Atrial hypertension and stretch induced by MR may also be a likely explanation for development of “lone” AF. In animal models, LA dilation of moderate severity has been shown to result in significant changes in the cellular action potential and calcium current in the atrial myocardium rendering the atria vulnerable to AF[21]. Chronic atrial dilation causes atrial conduction delays and a higher contribution of anatomically defined re-entrant circuits, creating a wider excitable gap during AF[22]. Increased left atrial size leads to greater recurrences of “lone” AF[23]. Treatment with ACE inhibitors prevents long term recurrences of “lone” AF and facilitates maintainence of sinus rhythm after cardioversion[24,25]. Cardioversion to NSR reverses the atrial enlargement in patients with AF and MR[26]. There is also an increased amount of atrial fibrosis in AF patients with mitral valve disease than in patients with lone AF[27].
Theoretically, it seems plausible that moderate MR may be a risk factor for the development of lone AF, primarily by causing mechanical stretch of the left atrium (“left atrial hypertension”). Or conversely, AF may predispose to slow development of “silent” MR that may progress with time. It is also conceivable that the MR is a transient atrial dyssynchrony predisposed phenomenon that resolves after NSR is restored; but we do not have longitudinal TEE follow-up on our patients to make that assumption. Lastly, it is conceivable that the greater degree of MR noted on TEE may be related to the greater sensitivity of TEE to evaluate MR; however the presence of a control group should have normalized for that observation bias. Our study suggests that clinically unrecognized moderate MR may be prevalent in patients with lone AF. Whether moderate unrecognized MR may be an etiologic factor related to development of “lone” AF or vice versa needs to be studied in long-term longitudinal studies.
This was a single-center, nonrandomized, retrospective, single time point observational study. Hence, it was not designed to prove causality. The basis of our study was to explore an association between “silent” unrecognized moderate MR and “lone” AF. Our observations are subject to the same limitations imposed by retrospective study designs, and it is possible for bias to exist during the review process. It is conceivable that AF, by inducing atrial wall motion dyssynchrony, may by itself induce MR. Moderate MR may be a more likely explanation for development of “lone” AF. The etiologic basis of this hypothesis would require long term longitudinal follow-up studies of patients with moderate MR who are in NSR at the time of the initial diagnosis of moderate MR. Another limitation of our data relates to the greater sensitivity of recognizing moderate MR with TEE, though this bias was possibly neutralized by inclusion of the control group of patients who were in NSR.
In our study utilizing TEE, moderate MR is prevalent in patients with lone AF. Longitudinal studies may be required to explore whether “silent” unrecognized moderate MR leads to development of “lone” AF or vice versa.
Clinically significant mitral regurgitation (MR) often leads to development of atrial fibrillation (AF). However, whether a stage of clinically unrecognized moderate MR (preceding development of clinically obvious significant MR) in patients with structurally normal valves can also lead to development of AF is not known.
MR and AF are common cardiac conditions that often co-exist. Moderate MR is often clinically unrecognized as it is asymptomatic. “Lone” AF often occurs in patients without any preceding established cardiac disease. Whether there is an etiologic relationship between moderate MR and “lone” AF has not been studied. In the present study the authors demonstrate that clinically unrecognized moderate MR often co-exists in patients with lone AF and there may be an etiologic relationship between these two entities.
The study highlights the relationship between the presence of AF without any clinically obvious cardiac disease and clinically unrecognized moderate MR. It highlights that a clinically unrecognized stage of moderate MR may be a preceding step before the clinical manifestations become obvious in the form of development of AF. It is also possible that atrial dyssynchrony induced by AF may make MR more manifest by echocardiography.
By understanding the relationship between moderate MR and lone AF, the study generates interest in whether a therapeutic strategy for preventing progression of MR by afterload reduction therapy may be of benefit in preventing onset of AF.
“Lone” AF is defined as AF in the absence of demonstrable underlying cardiac disease or a history of hypertension. MR is the presence of leakage of the mitral valve that is present in trace-to mild degree almost universally on echocardiography. However, moderate or severe MR is more uncommon and is sometimes associated with presence of other heart disease.
It is well written, concise paper and interesting.
Peer reviewers: Tomás F Cianciulli, MD, FACC, Professor, Director Echocardiography Laboratory, Division of Cardiology, Hospital of the Government of the City of Buenos Aires “Dr. Cosme Argerich”, C1155AHB Buenos Aires, Argentina; Athanassios N Manginas, MD, FACC, FESC, Interventional Cardiology, 1st Department of Cardiology, Onassis Cardiac Surgery Center, 356 Sygrou Ave, 17674 Athens, Greece
S- Editor Cheng JX L- Editor A E- Editor Zheng XM
| 1. | Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Ilstrup DM, Frye RL. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669-674. [PubMed] |
| 2. | Potpara TS, Lip GY. Lone atrial fibrillation: where are we now? Hosp Pract (Minneap). 2011;39:17-31. [PubMed] |
| 3. | Kopecky SL, Gersh BJ, McGoon MD, Chu CP, Ilstrup DM, Chesebro JH, Whisnant JP. Lone atrial fibrillation in elderly persons: a marker for cardiovascular risk. Arch Intern Med. 1999;159:1118-1122. [PubMed] |
| 4. | Falk RH. Atrial fibrillation. N Engl J Med. 2001;344:1067-1078. [PubMed] |
| 5. | Parvez B, Darbar D. Lone AF - etiologic factors and genetic insights into pathophysiolgy. J Atr Fibrillation. 2010;1:675-684. [PubMed] |
| 6. | Rosiak M, Dziuba M, Chudzik M, Cygankiewicz I, Bartczak K, Drozdz J, Wranicz JK. Risk factors for atrial fibrillation: Not always severe heart disease, not always so 'lonely'. Cardiol J. 2010;17:437-442. [PubMed] |
| 7. | Korantzopoulos P, Liu T, Milionis HJ, Li G, Goudevenos JA. 'Lone' atrial fibrillation: hunting for the underlying causes and links. Int J Cardiol. 2009;131:180-185. [PubMed] |
| 8. | Kozlowski D, Budrejko S, Lip GY, Rysz J, Mikhailidis DP, Raczak G, Banach M. Lone atrial fibrillation: what do we know? Heart. 2010;96:498-503. [PubMed] |
| 9. | Zhou X, Otsuji Y, Yoshifuku S, Yuasa T, Zhang H, Takasaki K, Matsukida K, Kisanuki A, Minagoe S, Tei C. Impact of atrial fibrillation on tricuspid and mitral annular dilatation and valvular regurgitation. Circ J. 2002;66:913-916. [PubMed] |
| 10. | Vohra HA, Whistance RN, Magan A, Sadeque SA, Livesey SA. Mitral valve repair for severe mitral regurgitation secondary to lone atrial fibrillation. Eur J Cardiothorac Surg. 2012;Epub ahead of print. [PubMed] |
| 11. | Kihara T, Gillinov AM, Takasaki K, Fukuda S, Song JM, Shiota M, Shiota T. Mitral regurgitation associated with mitral annular dilation in patients with lone atrial fibrillation: an echocardiographic study. Echocardiography. 2009;26:885-889. [PubMed] |
| 12. | Silbiger JJ. Mitral regurgitation in lone atrial fibrillation: more than a matter of annular size. Echocardiography. 2010;27:218; author reply 219. [PubMed] |
| 13. | Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, Boone J, Sheldon R, Dorian P, Newman D. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149:489-496. [PubMed] |
| 14. | Grigioni F, Avierinos JF, Ling LH, Scott CG, Bailey KR, Tajik AJ, Frye RL, Enriquez-Sarano M. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. 2002;40:84-92. [PubMed] |
| 15. | Parkash R, Green MS, Kerr CR, Connolly SJ, Klein GJ, Sheldon R, Talajic M, Dorian P, Humphries KH. The association of left atrial size and occurrence of atrial fibrillation: a prospective cohort study from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2004;148:649-654. [PubMed] |
| 16. | Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875-883. [PubMed] |
| 17. | Donal E, Ollivier R, Veillard D, Hamonic S, Pavin D, Daubert JC, Mabo P. Left atrial function assessed by trans-thoracic echocardiography in patients treated by ablation for a lone paroxysmal atrial fibrillation. Eur J Echocardiogr. 2010;11:845-852. [PubMed] |
| 18. | Tondo C. Atrial fibrosis and lone atrial fibrillation: an ominous association from the beginning? Heart Rhythm. 2010;7:1482-1483. [PubMed] |
| 19. | Kottkamp H. Atrial fibrillation substrate: the "unknown species"-- from lone atrial fibrillation to fibrotic atrial cardiomyopathy. Heart Rhythm. 2012;9:481-482. [PubMed] |
| 20. | Gertz ZM, Raina A, Saghy L, Zado ES, Callans DJ, Marchlinski FE, Keane MG, Silvestry FE. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. 2011;58:1474-1481. [PubMed] |
| 21. | Deroubaix E, Folliguet T, Rücker-Martin C, Dinanian S, Boixel C, Validire P, Daniel P, Capderou A, Hatem SN. Moderate and chronic hemodynamic overload of sheep atria induces reversible cellular electrophysiologic abnormalities and atrial vulnerability. J Am Coll Cardiol. 2004;44:1918-1926. [PubMed] |
| 22. | Neuberger HR, Schotten U, Blaauw Y, Vollmann D, Eijsbouts S, van Hunnik A, Allessie M. Chronic atrial dilation, electrical remodeling, and atrial fibrillation in the goat. J Am Coll Cardiol. 2006;47:644-653. [PubMed] |
| 23. | Zacà V, Galderisi M, Mondillo S, Focardi M, Ballo P, Guerrini F. Left atrial enlargement as a predictor of recurrences in lone paroxysmal atrial fibrillation. Can J Cardiol. 2007;23:869-872. [PubMed] |
| 24. | Grecu M, Olteanu RO, Olteanu SS, Georgescu CA. Does treatment with ACE inhibitors prevent the long term recurrences of lone atrial fibrillation after cardioversion? Rom J Intern Med. 2007;45:29-33. [PubMed] |
| 25. | Belluzzi F, Sernesi L, Preti P, Salinaro F, Fonte ML, Perlini S. Prevention of recurrent lone atrial fibrillation by the angiotensin-II converting enzyme inhibitor ramipril in normotensive patients. J Am Coll Cardiol. 2009;53:24-29. [PubMed] |
| 26. | Gosselink AT, Crijns HJ, Hamer HP, Hillege H, Lie KI. Changes in left and right atrial size after cardioversion of atrial fibrillation: role of mitral valve disease. J Am Coll Cardiol. 1993;22:1666-1672. [PubMed] |
| 27. | Geuzebroek GS, van Amersfoorth SC, Hoogendijk MG, Kelder JC, van Hemel NM, de Bakker JM, Coronel R. Increased amount of atrial fibrosis in patients with atrial fibrillation secondary to mitral valve disease. J Thorac Cardiovasc Surg. 2011;Epub ahead of print. [PubMed] |