INTRODUCTION
A cardiomyopathy is described as a myocardial disorder in which the heart muscle is structurally and functionally abnormal, in the absence of coronary artery disease, hypertension, valvular disease or congenital heart disease sufficient to cause the observed myocardial abnormality[1]. It is estimated that cardiomyopathy cause over 26 000 deaths each year in the United States and follows coronary heart disease as the commonest cause of sudden death[2].
Cardiac magnetic resonance imaging (CMRI) has emerged as a useful non-invasive imaging modality capable of producing high-resolution images of the heart in any desired image plane and without ionizing radiation. As a result it has become a primary imaging modality for many cardiomyopathies[3,4]. In this two-part review, we outline the utility of CMRI in the investigation of cardiomyopathies. Part I focused on the basic sequences used in characterizing the common cardiomyopathies, reviewed the commonest cardiomyopathy classification systems in use and illustrated the imaging spectrum of the common phenotypes. Part II focuses on showing the imaging spectrum of the more rare phenotypes.
RARE CARDIOMYOPATHIES CHARACTERIZED BY HYPERTROPHY
Hypertrophic cardiomyopathy (HCM) is characterized by increased ventricular wall thickness or mass in the absence of a loading condition such as valvular disease or hypertension. Loading conditions can be volume loading (aortic regurgitation) or pressure loading (aortic stenosis and chronic systemic hypertension).
Friedrich’s ataxia
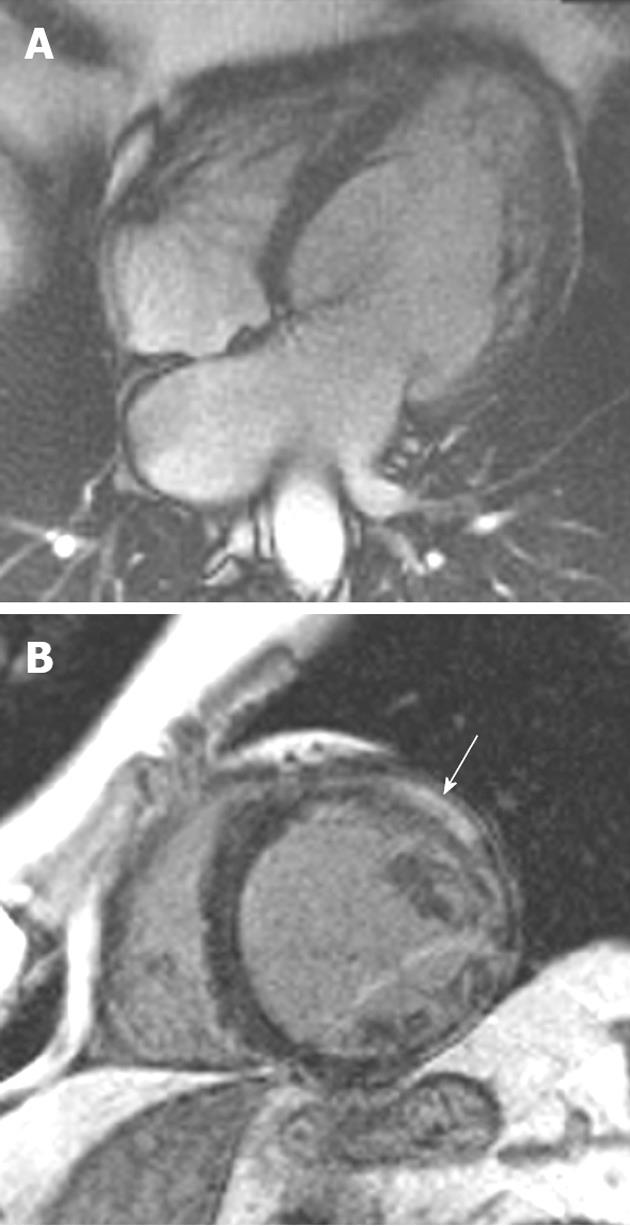
Friedrich’s ataxia (FA) is the most common inherited ataxia syndrome. Reduced fraxetin synthesis causes an unstable expansion of a GAA trinucleotide repeat. Clinical features include progressive ataxia, dysarthria, sensory neuropathy, lower limb areflexia, extensor plantar response and weakness secondary to degeneration of dorsal columns and pyramidal tracts[5,6]. Glucose intolerance and various skeletal abnormalities may also be seen. Cardiac involvement in FA is commonly encountered and defined by concentric and symmetrical increased ventricular wall thickness and a normal or small left ventricular (LV) cavity. The systolic function is normal[7].
LV mass on CMRI has been shown to positively correlate with both the genotypic and phenotypic severity of the disease[8]. Increased trinucleotide repeats correlates with increasing LV mass[9]. In particular, septal and posterior wall hypertrophy is more severe in patients with more trinucleotide repeats[8,10]. Larger LV mass is seen with severe and early onset disease. Conversely longer disease duration is often associated with a smaller LV mass. Echocardiography has been the traditional primary imaging tool in FA cardiomyopathy. CMRI offers several additional advantages. It provides a more accurate assessment of the presence, extent and location of myocardial hypertrophy than echocardiography (Figure 1)[8]. It provides these parameters with lower interobserver variation. Perfusion and late contrast enhancement abnormalities indicating myocardial fibrosis can be detected with CMRI early in the pathogenesis of FA cardiomyopathy, earlier than the onset of measurable hypertrophy[11].
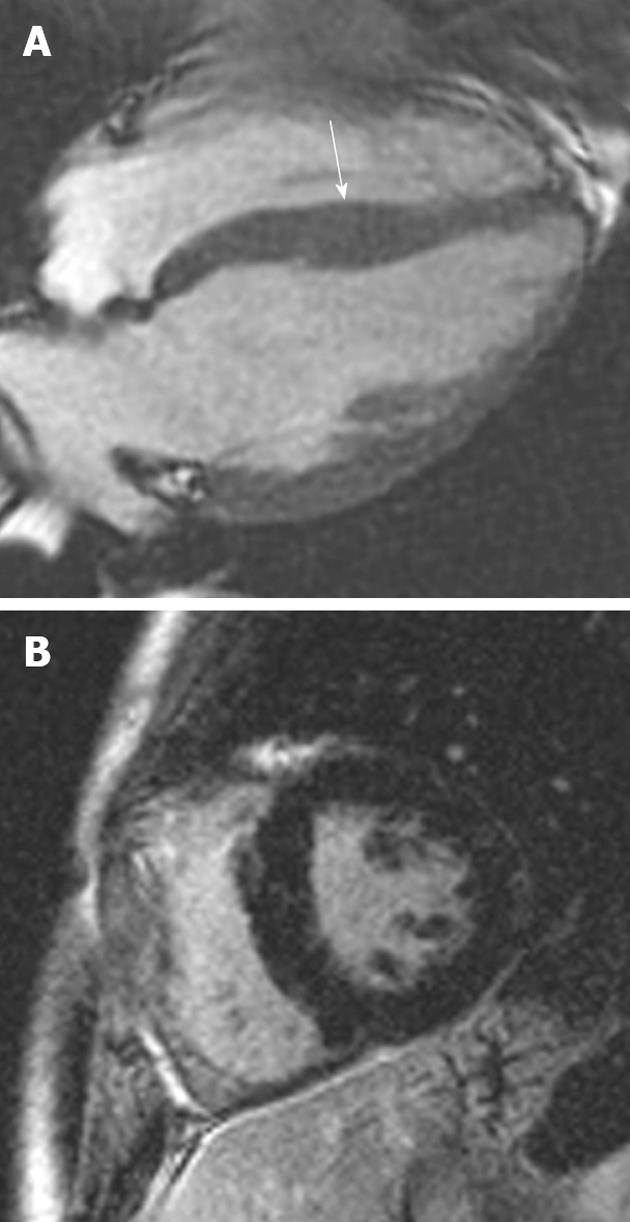
Figure 1 A 19-year-old man with Friedrich’s ataxia who presented with sudden onset chest pain.
A: Horizontal long-axis SSFP sequence showing circumferential hypertrophy (arrow, 14 mm thickness) of the anterobasal segment of the left ventricular; B: Late-enhanced sequence showing absence of high signal, characteristic of the hypertrophy seen in this disorder.
Noonan syndrome
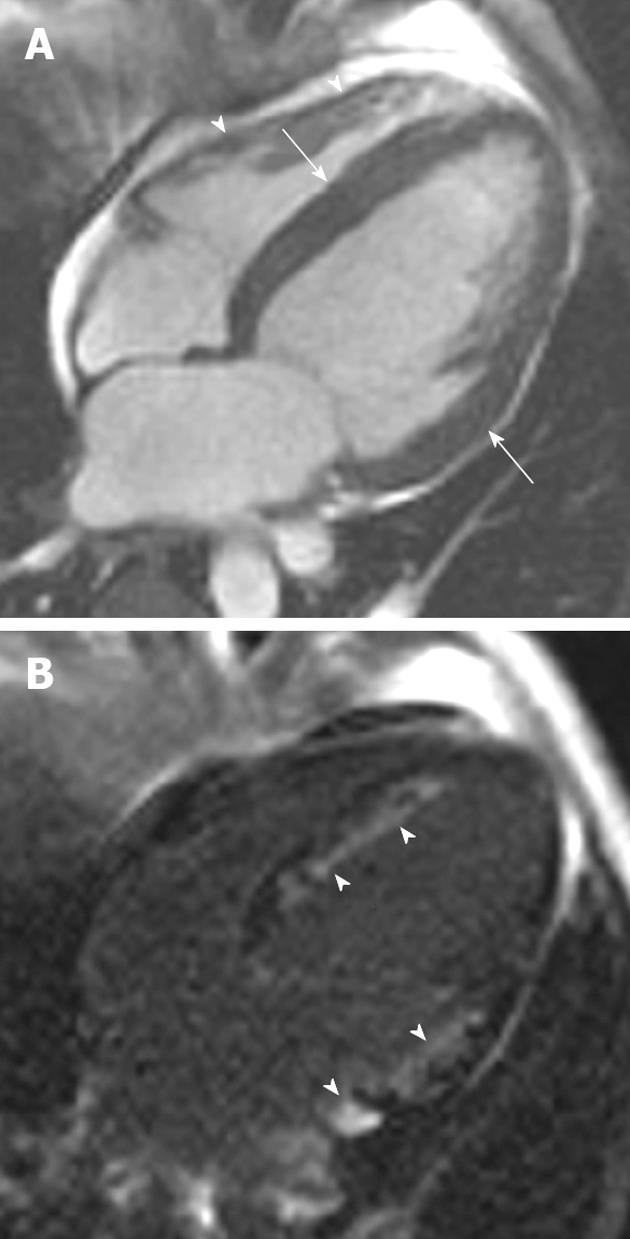
Noonan syndrome occurs as a sporadic or autosomal dominant mutation with equal sex predominance[12]. Clinical features include congenital heart defects, bleeding diathesis and mental retardation. Phenotypical features include short stature, hypertelorism, down-slanting eyes, epicanthic folds, low-set posterior ears, micrognathia, a webbed neck and chest deformities[13,14]. Valvular disease is the most common cardiac abnormality. Pulmonary valve stenosis is the most common defect, affecting approximately 50% of patients[15]. Thus, it is important to acquire steady-state free precession sequences of the right ventricular outflow tract (RVOT) and pulmonary valve when evaluating patients suspected of cardiac involvement. Asymmetric septal hypertrophy is seen in 20% of patients with Noonan syndrome (Figure 2)[16,17]. Atrial septal defects occur in approximately 10%, persistent ductus arteriosus in 3% and ventricular septal defects in 5%[12]. A previous case report has described the CMRI appearances in a patient with Noonan syndrome[17]. In that 27-year-old female the presence of septal hypertrophy was detected with CMRI and shunts were excluded. Late gadolinium enhancement (LGE) manifested as patchy increased signal in the anterior, anteroseptal and lateral walls[17]. Echocardiography remains the primary imaging modality in Noonan’s cardiomyopathy. In cases where imaging with echocardiography is equivocal or incongruent with other clinical parameters, CMRI can provide imaging confirmation of hypertrophy.
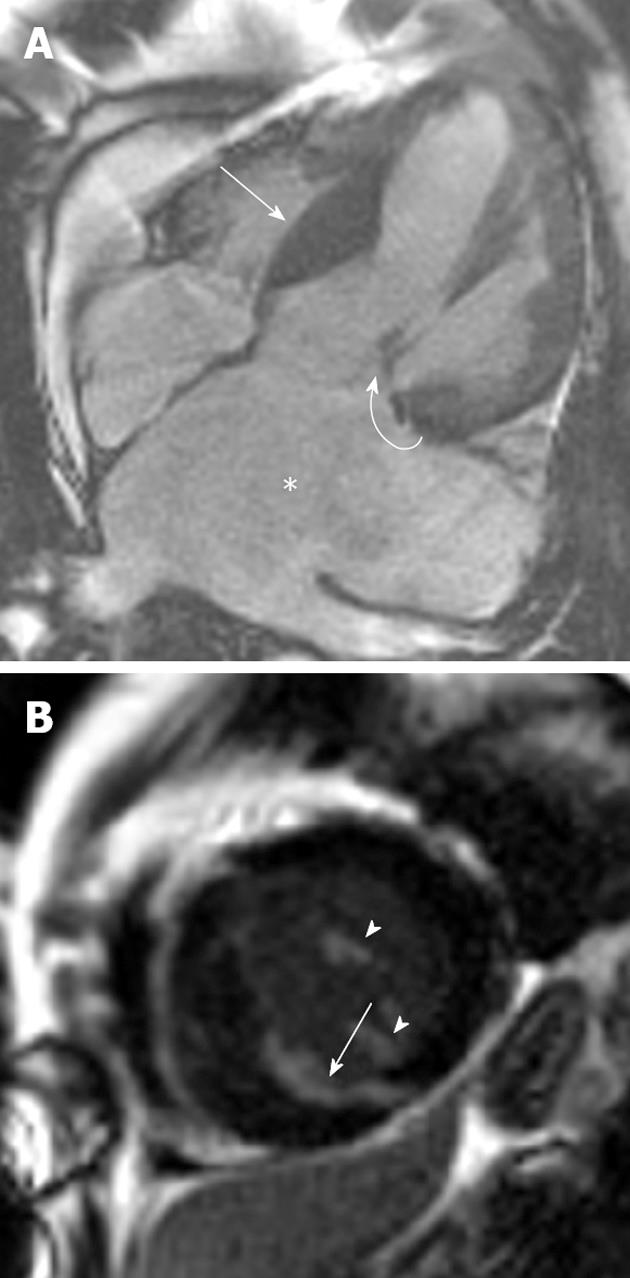
Figure 2 A 46-year-old man with Noonan’s syndrome.
A: Horizontal long-axis SSFP sequence showing septal hypertrophy (curved arrow) and a severely dilated left atrium secondary to severe mitral regurgitation (asterisk); B: Late-enhanced short axis sequence showing extensive late gadolinium enhancement (LGE) throughout the antero- and infero-septal myocardial segments (arrow). LGE was also noted in the papillary muscles (arrowheads).
RARE CARDIOMYOPATHIES CHARACTERIZED BY DILATATION
Dilatation of the cardiac chambers with impaired contraction of the ventricles is characteristic of dilated cardiomyopathies.
Arrhythmogenic right ventricular dysplasia
Arrhythmogenic right ventricular dysplasia (ARVD) is a rare genetic disorder characterized by progressive loss of myocytes with fibro-fatty replacement of right, and more recently described, LV myocardium. Patients may present with ventricular arrhythmias and left bundle branch block, syncope or sudden cardiac death. It may be responsible for up to 5% of sudden deaths in young athletes[18], although it has a higher prevalence in some countries such as Italy (25% of sudden deaths in young athletes)[19]. Because of the subtlety of the phenotype, consensus criteria were developed based on structural, functional, and electrocardiographic (ECG) manifestations[20]. In these criteria, tissue characterization depicted on CMRI such as fatty infiltration has been removed and an increased emphasis placed on right ventricular wall motion, volume and ejection fraction abnormalities.
CMRI assessment of ARVD is now primarily based on functional and volume abnormalities of the right ventricle[20] although many centers still provide tissue characterization imaging of the right ventricle. Morphological abnormalities include intra-myocardial fat deposits, focal wall thinning (< 2 mm), wall hypertrophy (> 6 mm), moderator band hypertrophy and trabeculation disarray. Functional abnormalities include focal or global RV wall hypokinesis (dilatation, focal or global RV dilatation and in severe cases, focal aneurysms). The site of involvement most commonly occurs in the “triangle of dysplasia” found in the inferior sub-tricuspid area, RV apex and RV infundibulum. The major treatment implication in suspected cases of ARVD is implantable defibrillator placement.
Important CMRI sequences in ARVD
CMRI protocols for ARVD focuses particular attention on the right ventricle and RVOT (Figure 3). A thinner slice thickness and slice gap for this protocol (5-6 mm contiguous slices are typical) is recommended. Saturation bands above and below the heart help improve image quality by reducing flow artifacts related to slow-inflowing blood. A small field of view targeted to the right ventricle helps improve the spatial resolution further, at the cost of decreasing signal-to-noise ratio. Myocardial late enhancement has been used to demonstrate scarring of the RV wall[21].
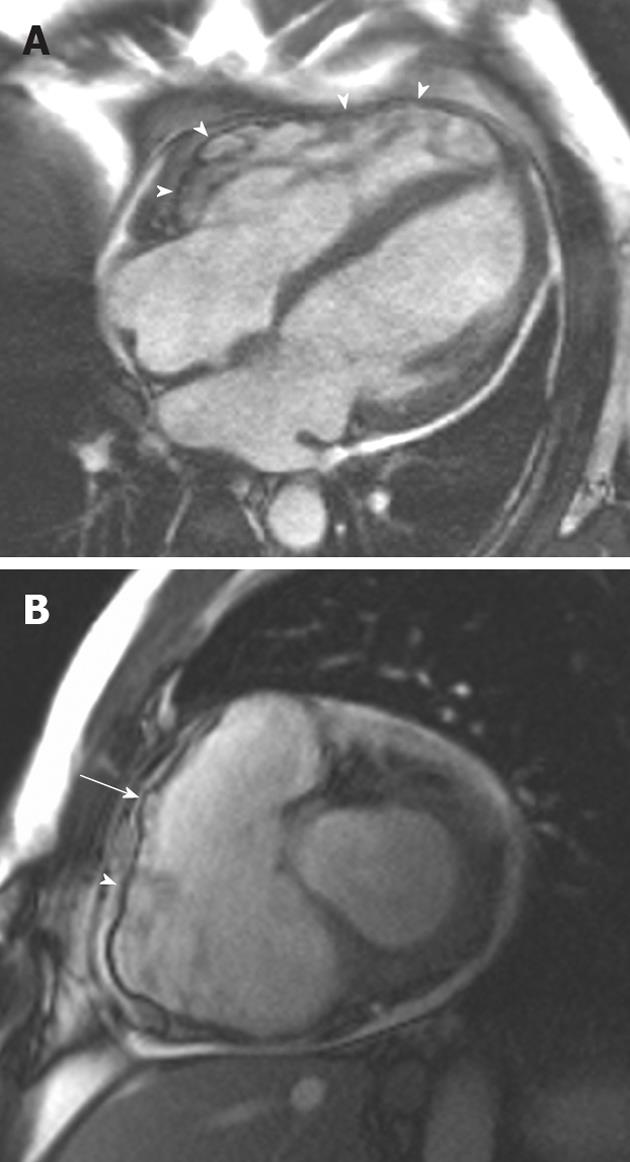
Figure 3 A 38-year-old man of Italian origin presented with palpitations and progressive shortness of breath during exercise.
A: Horizontal long-axis SSFP shows a heavily trabeculated right ventricle with thickened trabeculae and increased right ventricular (RV) volume. The RV free wall is very thin, with multiple small aneurysms (arrowheads); B: Short-axis SSFP sequence showing further small aneurysms of the RV free wall. Note again the increased RV volume.
The diagnostic accuracy of CMRI for ARVD carries a high sensitivity but low specificity when compared with traditional Task Force criteria[22]. Modified criteria have been proposed, such that the presence of any minor criterion in a first-degree relative of a proven case of ARVD is regarded as clinical disease expression[20]. When CMRI is assessed using these modified criteria it is frequently abnormal, suggesting a role in depicting initial manifestations of disease[20,23]. It is important to emphasize that the interpretation of CMRI abnormalities should be made in a multidisciplinary approach involving ECG, arrhythmic, morpho-functional, histopathological, and clinical/molecular genetic analysis before making the diagnosis of ARVD[20,24].
Peripartum cardiomyopathy
Peripartum cardiomyopathy (PPCM) is defined as heart failure occurring in the last month of pregnancy or within 5 mo of delivery. The etiology of PPCM is multifactorial. Ntusi et al[25] and Baruteau et al[26,27] divided the etiology of PPCM into inflammatory and non-inflammatory causes. Inflammatory PPCM occurs secondary to viral myocarditis, an abnormal immune response to pregnancy, an abnormal response to hemodynamic stresses of pregnancy, increased myocyte apoptosis and cytokine-mediated myocardial inflammation. Non-inflammatory causes of PPCM include malnutrition, genetic factors, increased prolactin production, abnormal hormone function and increased adrenergic tone.
CMRI appearances: It is no surprise with such a multifactorial etiology that the reported CMRI appearances of PPCM are varied. The most common finding is a dilated left ventricle (Figure 4). Reported appearances post administration of gadolinium are more varied. Marmursztejn et al[28] described the CMRI appearances of two patients with PPCM. The first patient had a normal post-gadolinium CMRI and at clinical follow-up regained normal cardiac function. The second patient had several areas of myocardial LGE and had persistent LV dysfunction on clinical follow-up. Mouquet et al[29] evaluated eight patients with PPCM with CMRI and no LGE was seen in any patient. Kawano et al[30] described a case of PPCM with diffuse epicardial and midwall LGE in the left ventricle on CMRI performed at 2 mo with reduction in the LGE on follow-up CMRI at 10 mo. One explanation for the differing reports may lie in the differing inflammatory vs non-inflammatory etiologies for PPCM. Those with a predominantly inflammatory etiology demonstrate LGE in a similar fashion to other viral myocarditis as opposed to non-inflammatory etiologies which are not associated with a T2 hypersignal or LGE[27].
Figure 4 A 34-year-old woman 1 wk post-partum who developed progressive shortness of breath.
A: Short-axis SSFP sequence showing dilation of the left ventricle (end-diastolic diameter = 62 mm); B: The systolic phase demonstrating moderate circumferential hypokinesis (left ventricular ejection fraction 45%); C: Late gadolinium enhancement sequence showing an absence of high signal in this case.
Muscular dystrophy
Duchenne and Becker muscular dystrophies are X-linked genetic mutations affecting the dystrophin gene. Dystrophin is totally absent or dysfunctional in Duchenne muscular dystrophy (DMD) and dystrophin is mildly dysfunctional or reduced in expression in Becker muscular dystrophy (BMD). DMD is associated with a more severe disorder and patients die of respiratory failure, rarely surviving past the third decade. In contrast, BMD is a milder form of dystrophy with patients surviving until the sixth decade; cardiomyopathy is the main cause of death[31]. Another important group of dystrophies that may exhibit cardiomyopathy are the limb girdle muscular dystrophies. The subtypes most commonly associated with cardiac involvement include those associated with a defect in the genes coding for the α (LGMD2D), β (LGMD2E), γ (LGMD2C), or θ (LGMD2F) subunits of the dystrophin associated sarcoglycan complex.
CMRI appearances: The subepicardium of the inferolateral wall is initially affected in BMD. This occurs in the third decade of life and increases in extent with age. There is a progressive loss of contractility and decrease in LV systolic dysfunction secondary to ongoing myocardial damage. Cardiac involvement is not commonly seen in patients under the age of 16 with BMD, but increases to 70% by the age of 40 years[32]. Yilmaz et al[33] demonstrated that patients with BMD and reduced LV ejection fraction were older, had heavier hearts and regional wall motion abnormalities. In DMD, almost all patients that survive to > 30 years of age demonstrate cardiomyopathy. LGE has been reported involving the inferolateral free wall, the basal inferior and anterolateral region of the left ventricle (Figure 5)[34,35]. The LGE likely occurs because of myocardial damage resulting from mechanical stress on top of a metabolically and structurally abnormal myocardium, although the precise mechanism remains to be elucidated. Whether the inferolateral wall is particularly vulnerable because of regional molecular changes or from exposure to higher mechanical stress is unknown. It is interesting that the LGE pattern is remarkably similar to that of myocarditis, and that enterovirus infection has been shown to produce myocardial damage via cleavage of dystrophin[36]. Perhaps this explains the similarity in the LGE pattern between myocarditis and dystrophin-associated cardiomyopathy. Performing CMRI in DMD/BMD is useful because early commencement of standard heart failure therapy can delay the onset and progression of LV systolic dysfunction and possibly even reverse the remodeling process. Furthermore, myocardial fibrosis detected by LGE imaging may be observed in the presence of normal echocardiography[34]. For the limb-girdle muscular dystrophies, several authors have shown LGE in the basal interventricular septum in these cardiomyopathies before the onset of ventricular dilatation and systolic dysfunction[37].
Figure 5 A 46-year-old man with progressive heart failure and Becker’s muscular dystrophy.
A: Short-axis SSFP sequence showing moderate dilation of the left ventricle; B: Late gadolinium enhancement (LGE) sequence showing extensive transmural LGE throughout the lateral segments (arrow).
RARE CARDIOMYOPATHIES CHARACTERIZED BY RESTRICTION
Carcinoid heart disease
Carcinoid tumors arise from neuroendocrine tumors and secrete vasoactive substances including 5-hydroxytryptamine, histamine and bradykinin. Clinical symptoms of carcinoid syndrome occur when there is metastatic disease in the liver and symptoms include episodic flushing, diarrhea and bronchospasm. Carcinoid heart disease occurs in two-thirds of patients with carcinoid syndrome[38]. Most cardiac lesions affect the right side of the heart, which is postulated to be due to paraneoplastic effects of the vasoactive substances secreted into the hepatic veins from liver metastases[39].
CMRI appearances: Right heart valvular dysfunction is the most common abnormality and the tricuspid valve is most commonly involved[40]. Right atrial and right ventricular enlargement are present in up to 90% of cases of carcinoid heart disease. One study of 252 patients with carcinoid heart disease demonstrated tricuspid valve involvement in 90%, pulmonary valve in 69%, mitral valve in 29% and aortic valve in 27%. Thirteen out of 15 (87%) patients with left-sided cardiac involvement had a patent foramen ovale. Myocardial metastases were seen in 3.8%[41]. Imaging appearances of carcinoid heart disease are of plaque-like deposits causing fibrous endocardial thickening, with retraction and fixation of the valvular cusps and the subvalvular apparatus (chordae and papillary muscles)[42]. Tricuspid valve regurgitation leads to volume overload, which in turn causes right atrial and RV dilatation. In contrast to the tricuspid valve involvement, pulmonary valve involvement typically causes pulmonary stenosis rather than regurgitation[40].
Post contrast delayed CMRI may also demonstrate myocardial fibrosis or scarring as areas of high signal intensity. Several case reports describe similar appearances of cardiac carcinoid on CMRI with right heart chamber dilatation and fixed retraction of the tricuspid valve leaflets. Post contrast images demonstrated enhancement of the tricuspid septal leaflet, most marked at the annulus[43,44].
Hypereosinophilic syndrome
Idiopathic hypereosinophilic syndrome is a syndrome of unknown cause characterized by peripheral eosinophilia and multiorgan dysfunction. Cardiac involvement is common and is characterized by endomyocardial fibrosis and thrombus formation. Three stages are recognized. Stage one is an acute necrotic stage and is clinically silent. There is thrombus formation in stage two. Stage three is characterized by endomyocardial fibrosis and a restrictive cardiomyopathy[45].
CMRI appearances: There are several case reports documenting the appearances of cardiac involvement in hypereosinophilic syndrome on CMRI. A two- or three-layered appearance post contrast is most commonly described, consisting of a normal appearing epicardium, a hyperintense subendocardium indicating eosinophilic infiltration and frequently a linear hypointense layer of thrombus in the endocardium (Figure 6)[46,47]. Histological evaluation has confirmed the presence of endomyocardial eosinophilic infiltration and areas of myocyte necrosis. Debl et al[48] have suggested the potential for CMRI to evaluate response to treatment, with a decrease in the extent of late enhancement occurring with successful steroid therapy. Syed et al[46] has also described severe hypertrophy of the LV myocardium and partial obliteration of the LV cavity in systole.
Figure 6 A 38-year-old man presented with progressive shortness of breath.
Serum measurements demonstrated hypereosinophilia. A: Horizontal long-axis SSFP sequence showing mild circumferential hypertrophy of the left ventricle (arrows) Note the normal thin right ventricular free wall (arrowheads), a useful differentiating feature from cardiac amyloid; B: Late-enhanced horizontal long-axis sequence showing extensive high signal in the typical subendocardial distribution (arrowheads) of eosinophilic myocardial infiltration. Cardiac amyloid is the principal other cardiomyopathy that mimics this late gadolinium enhancement appearance.
Metastatic disease
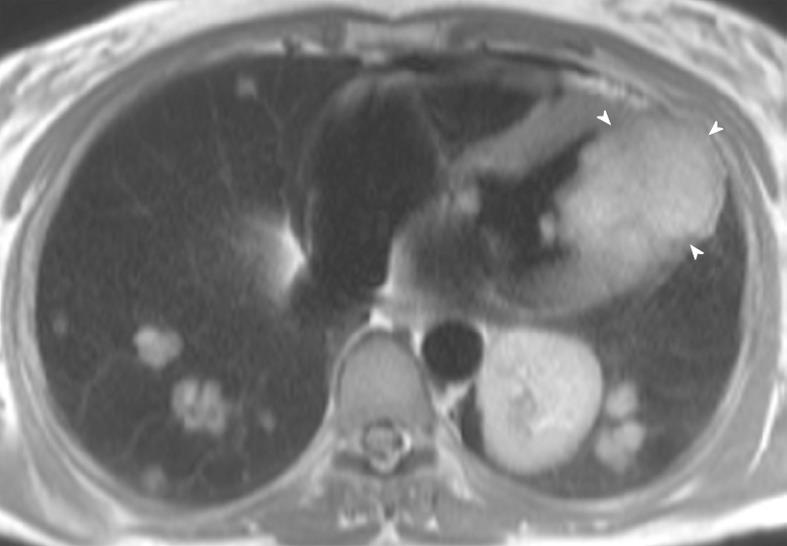
Metastatic disease to the heart and pericardium is more common than primary cardiac tumors[49]. Lung carcinoma, lymphoma, breast carcinoma, esophageal carcinoma and melanoma are the most common metastases to the heart[50,51]. Lung carcinoma typically involves the heart through direct invasion, sometimes by transvenous growth through the pulmonary veins. Cardiac metastatic involvement heralds a poor prognosis. “Charcoal heart” is the term peculiar to extensive cardiac melanoma metastases, in which the pathological appearance of the infiltrated myocardium is heavily pigmented.
CMRI appearances: Most cardiac tumors are low signal on T1-weighted sequences and higher signal on T2-weighted sequences (Figure 7)[52]. Typically, metastases enhance post contrast administration. A first pass perfusion sequence may be used to image the enhancement of the tumor with high temporal resolution. If this sequence is used, its duration should be for longer than the typical 30 s used for ischemic cases, as often the metastases take longer to demonstrate perfusion. Another useful sequence to differentiate metastases from a thrombus mass is the late-enhanced sequence in which the inversion time is set to 600 ms. Metastases enhance whilst a thrombus remains black (no signal).
Figure 7 A 56-year-old woman with metastatic sarcoma.
Note the diffuse metastatic infiltration of the left ventricle (arrowheads), resulting in restrictive pathophysiology. There are also diffuse pulmonary metastases throughout both lungs.
UNCLASSIFIED CARDIOMYOPATHIES
LV non-compaction (LVNC) and Takotsubo cardiomyopathy (TTC) are unclassified cardiomyopathies.
LV non-compaction
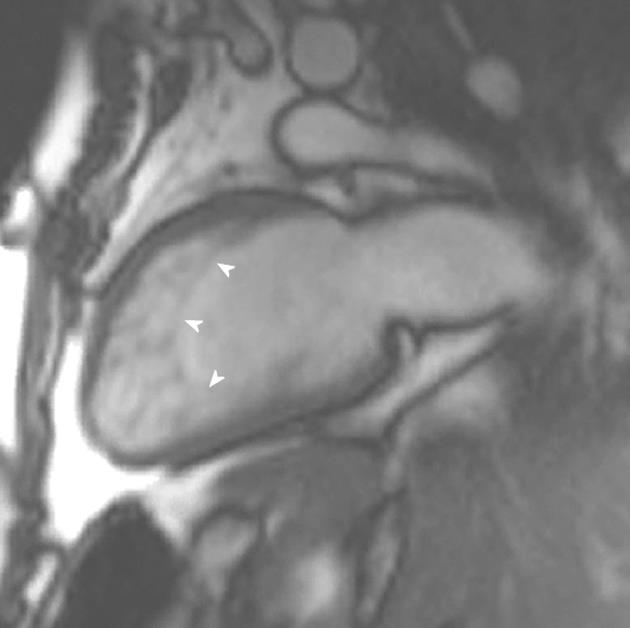
LVNC is a myocardial disorder characterized by prominent, excessive trabeculations and deep intratrabecular recesses (Figure 8). The intratrabecular recesses communicate with the ventricular cavity[53]. It is thought to be due to an arrest in the normal myocardial maturation process. The fetal myocardium has deep recesses between loosely interwoven fibers that communicate with the ventricular cavity. These trabeculations aid oxygen exchange by creating a larger surface area for diffusion. Once the coronary circulation develops, between the 5th to 8th weeks of life, these trabeculations are no longer needed and the loosely woven fibers undergo compaction[54]. Compaction moves from base to apex, epicardium to endocardium and from the septal to the lateral wall. The severity of LVNC will depend on the timing of the arrest in the myocardial maturation and compaction process. The apex and midventricular lateral segments are more commonly involved in LVNC and this is explained by the compaction process as these areas are last to undergo compaction[55].
Figure 8 A 23-year-old man with Rubenstein-Taybi syndrome who presented with increasing shortness of breath.
Vertical long-axis view demonstrating increased trabeculations at the apical ventricular level fulfilling cardiac magnetic resonance imaging criteria for left ventricular noncompaction.
The recesses in LVNC are lined by ventricular endothelium that is in continuity with the ventricular cavity. There is focal ischemic necrosis within the trabeculations and endocardial layer, compensatory hypertrophy of the myocardium, interstitial fibrosis and scarring[56,57]. LVNC was originally described in association with other severe congenital abnormalities and can present as fetal hydrops, neonatal heart failure and ventricular fibrillation[54]. In adults, arrhythmias and thromboembolic events are seen more commonly than in pediatric patients. Cardiac failure is the most common finding in LVNC[53,58]. LVNC may also be seen in association with other congenital cardiac defects. The commonest association is ventricular septal defect, though subaortic obstruction, bicuspid aortic valve, coarctation of the aorta, Ebstein’s anomaly, tetralogy of Fallot, pulmonary stenosis and pulmonary atresia are also seen in association with LVNC[59,60].
CMRI appearances: Three basic characteristics of LVNC on CMRI are described; trabeculations of the ventricular wall with deep recesses of the ventricular myocardium, extensive spongiform transformation of the LV myocardium and a dysplastic myocardium that is thinned with excessive trabeculations[61].
The end-diastolic non-compacted to compacted ratio (NC/C) is higher in patients with LVNC. A compaction ratio is calculated by measuring the thickness (in millimeters) of the non-compacted to compacted myocardium. The NC/C parameter separates out pathological non-compaction from less severe forms of non-compaction[62]. This measurement is typically obtained on SSFP sequences with high-resolution thin slices (4-5 mm) on radial slice projections with the fulcrum passing through the center of the left ventricle. The diastolic ratio of > 2.3 on CMRI is diagnostic of LVNC. Further imaging findings on CMRI in LVNC includes trabecular fibrosis, which is seen as delayed enhancement within the trabeculae. Interestingly, delayed enhancement can be seen in non-compacted segments indicating that fibrosis can also affect normal segments[61].
TTC
TTC, also called apical ballooning syndrome or stress cardiomyopathy, is characteristically accompanied by acute chest pain and when associated with ECG changes and elevated troponin, can be erroneously diagnosed as acute coronary syndrome. Clinical criteria for the diagnosis of TTC include transient akinesia or dyskinesia of the LV apical, mid or basal segments beyond the distribution of a single coronary artery, new ECG abnormalities or an elevated troponin, no angiographic evidence of acute plaque rupture or obstructive coronary artery disease, no recent head trauma/intracranial bleeding, no HCM or pheochromocytoma[63,64].
The pathogenesis of TTC is not fully understood. Proposed etiologies include coronary spasm, coronary emboli with spontaneous fibrinolysis, regional myocarditis and abnormalities in the coronary microvascular function. Several studies have demonstrated an association with catecholamine excess. Emotional and physical stressors are often seen in patients with TTC. Plasma levels of neuropeptides and catecholamines in patients with TTC have been found to be markedly elevated compared to patients with myocardial infarction[65]. One such study described TTC in nine patients, occurring after intravenous infusion of epinephrine or dobutamine[66]. Eitel et al[67] evaluated a large group of patients presenting with TTC. In 136 patients with TTC, 121 had significant stressful events 12 h preceding their TTC. Sixty-four patients had emotional stress and 57 had physical stress. Post-menopausal women were more commonly affected, 10% of patients were pre-menopausal, 10% were male and 10% did not recover LV function immediately. Endocardial biopsies in patients with TTC demonstrated changes consistent with catecholamine excess with contraction bands, increased inflammatory cells and interstitial fibrosis[68].
CMRI appearances: There are several patterns of regional wall motion abnormalities described in TTC[66]. An apical ballooning variant has apical akinesis with sparing of the base and mid-ventricle. A midventricular ballooning variant has midventricular akinesis with sparing of the apex and base (Figure 9). A basal ballooning variant has midventricular and basal akinesis but spares the apex. RV akinesia has also been described in some patients and is associated with lower LV ejection fractions[69]. LGE is seen in patients with myocardial infarctions and myocarditis but not typically in patients with TTC, making CMRI a valuable investigational tool in the work-up of patients suspected of TTC.
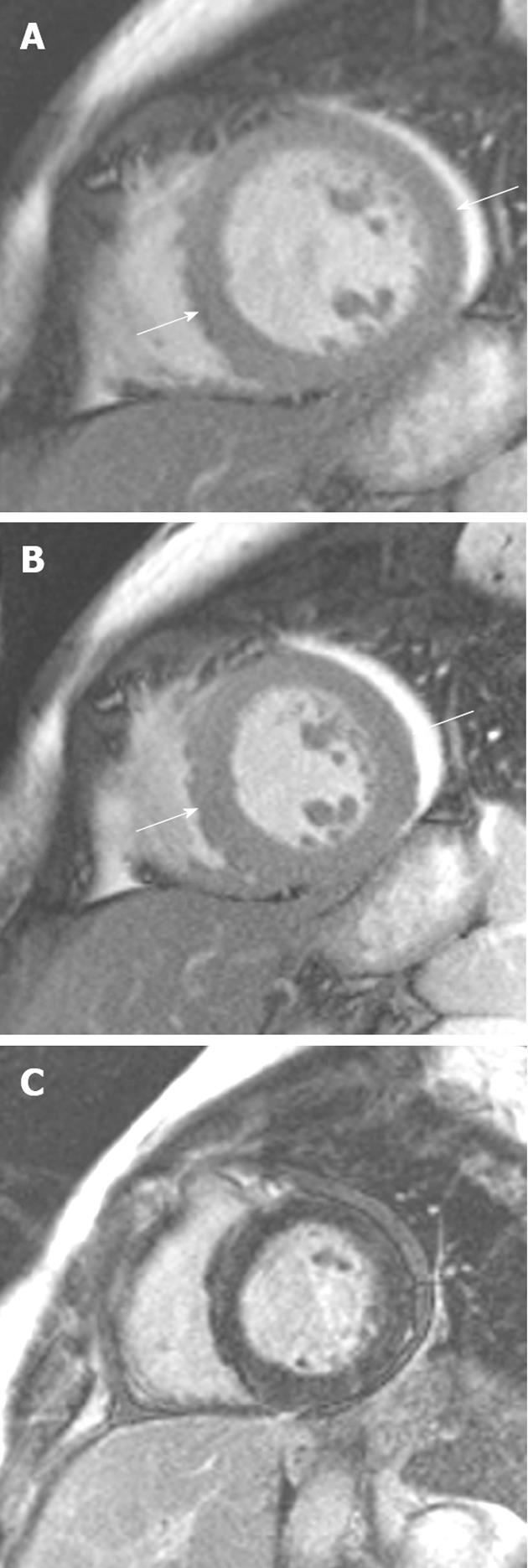
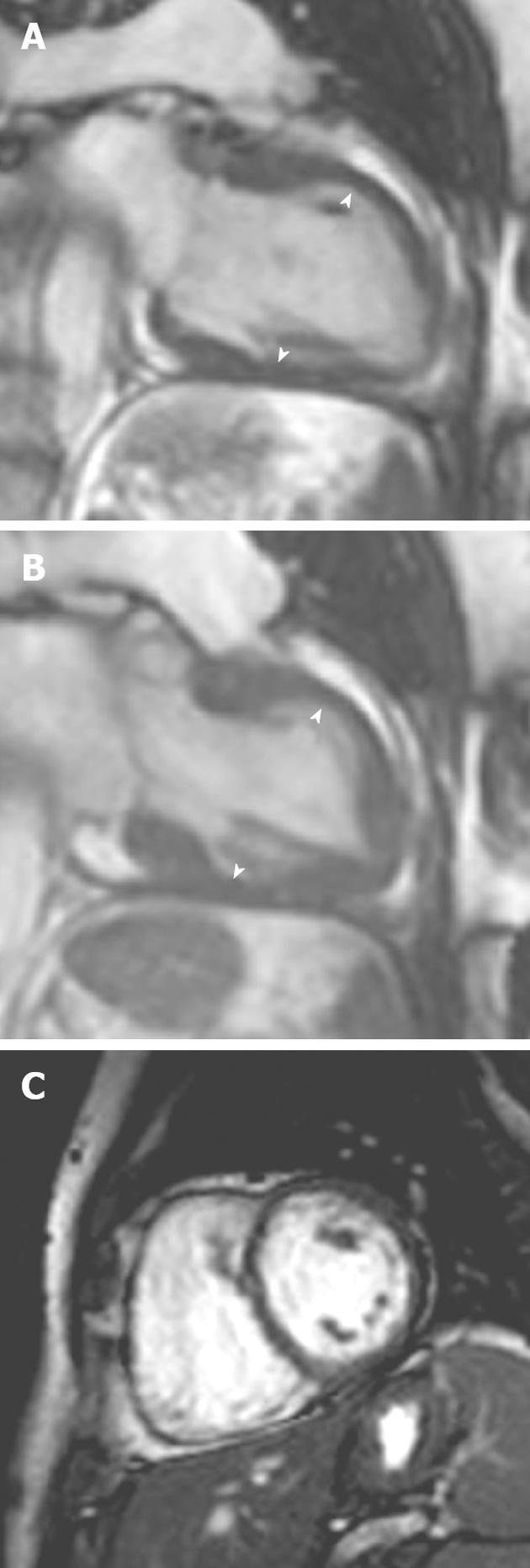
Figure 9 A 54-year-old woman with acute onset chest pain and palpitations following a road traffic accident.
A, B: Vertical long-axis SSFP sequence in (A) diastole and (B) systole showed akinesis of the left ventricular myocardial segments at the midventricular level; C: Late gadolinium enhancement showing no myocardial enhancement in the involved segments. Subsequent echocardiography 1 mo later showed normal mid-wall contraction.