Published online Apr 26, 2012. doi: 10.4330/wjc.v4.i4.130
Revised: March 14, 2012
Accepted: March 21, 2012
Published online: April 26, 2012
Cardiac ischemia with a normal coronary angiogram can be caused by coronary microvascular dysfunction. A favorable prognosis, with excellent long-term clinical outcome, without major acute coronary events, has been consistently reported in these patients. We report a patient with a normal coronary angiogram and 3 episodes of myocardial infarctions, where the formation of a ventricular aneurysm and progressive deterioration of left ventricular function was documented, and hypoperfusion of the myocardium was confirmed by cardiovascular magnetic resonance imaging. This case suggests that myocardial ischemia caused by coronary microvascular dysfunction could have a poor prognosis. Whether this case represents a special clinical condition which is between the cardiac syndrome X and coronary artery disease remains to be investigated.
- Citation: Feng QZ, Cheng LQ, Li YF. Progressive deterioration of left ventricular function in a patient with a normal coronary angiogram. World J Cardiol 2012; 4(4): 130-134
- URL: https://www.wjgnet.com/1949-8462/full/v4/i4/130.htm
- DOI: https://dx.doi.org/10.4330/wjc.v4.i4.130
It has been demonstrated that myocardial ischemia or infarction is the result of a decrease or interruption in the blood supply to the myocardium due to coronary artery disease. However, in some patients with myocardial ischemia or infarction, the coronary artery angiogram is completely normal, and a favorable prognosis, with an excellent clinical outcome at long-term follow-up, without major acute coronary events, has been reported[1]. Myocardial infarction with an absolutely normal coronary angiogram is also rarely complicated by ventricular aneurysm[2]. Here, we report a case of a patient with a normal coronary angiogram and 3 episodes of myocardial infarction (MI), formation of a ventricular aneurysm, and progressive deterioration of the left ventricular function.
A 67-year-old woman with 28 years of recurrent chest pain was admitted to hospital after 20 d of shortness of breath.
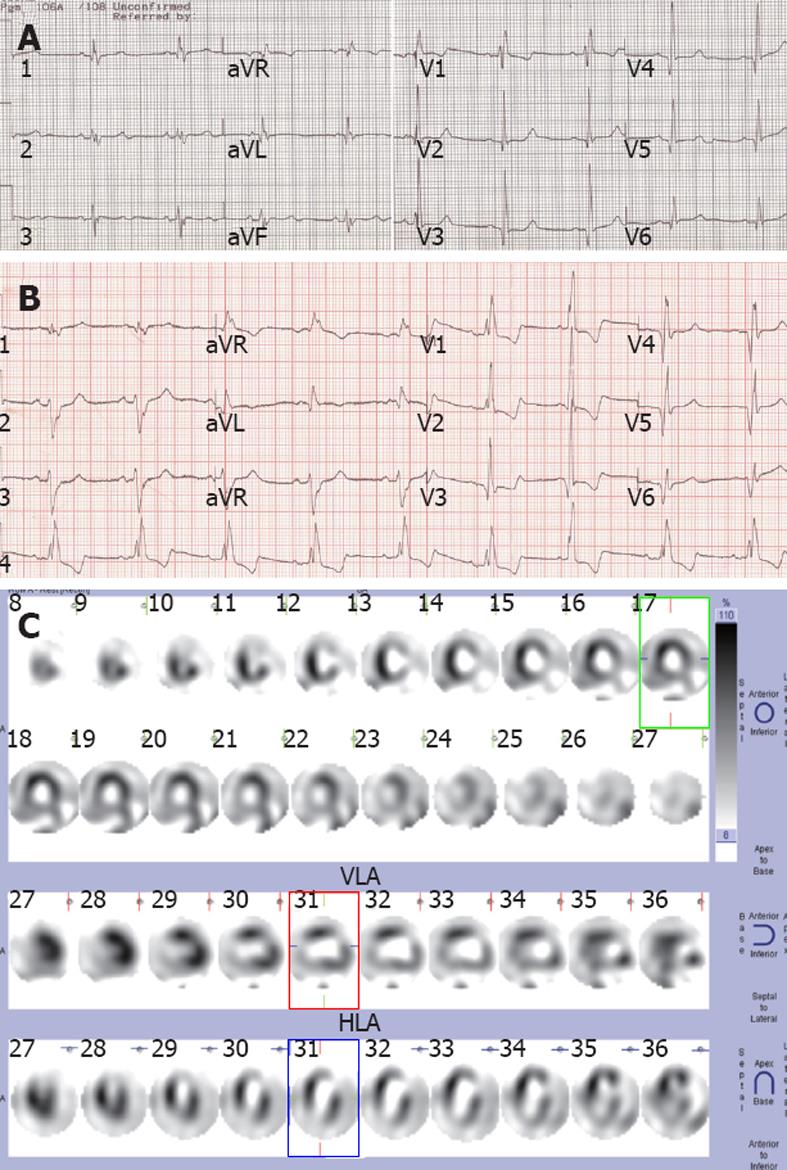
At the age of 39 years, she was diagnosed of an acute anterior wall MI based on her symptoms, typical evolution of ST-T abnormalities and elevation of cardiac enzymes. At that time, chest pain was induced by severe emotional stress, associated with radiation to the left shoulder, and heavy sweating, and was not relieved by nitroglycerin. The patient was hospitalized and treated accordingly. After discharge, she suffered from chest pain once or twice a week, relieved by rest or nitroglycerin. Again, 7 years and 11 years later, the patient had an acute anterior wall MI presenting as severe chest pain during sleep. In the third hospitalization, an echocardiogram revealed an anterior wall ventricular aneurysm. After discharge, chest pain occurred 3-5 times during the day, and occasionally at night. Each episode lasted 10-30 min, and symptoms could be relieved by rest or nitroglycerin. One year after the third MI, at the age of 53, she was hospitalized because of shortness of breath on exertion. An electrocardiogram (ECG) showed an old anterior wall infarction with right bundle branch block (Figure 1A); there was also evidence of mild enlargement of the left ventricle, and an ejection fraction of 50%. Cardiac catheterization was performed for coronary intervention, but the coronary angiogram indicated an absolutely normal coronary artery, and the left ventriculogram showed a left anterior ventricular aneurysm. The left ventricular end diastolic pressure was normal at 10 mmHg. Since then, she has been stable with intermittent chest pain until 13 years later. Last year, her condition gradually deteriorated, and 20 d after she began to have shortness of breath after light activity, with mild chest pain (relieved by rest) and ankle edema, she was hospitalized again for further treatment.
She had no history of smoking, diabetes mellitus, hyperlipidemia, hypertension, coagulation disorders, oral contraceptive use, or cocaine abuse.
On physical examination, there was no jugular venous distention. Auscultation revealed a soft first heart sound. No murmur was heard at the cardiac apex. The resting ECG showed sinus rhythm with an old anterior wall MI and right bundle branch block, and more depression of ST segment in V1-3 leads when compared with the previous ECG taken when she was 53 years of age (Figure 1B). Echocardiography revealed a ventricular aneurysm at the apex and anterolateral wall with systolic bulging, mild mitral, aortic, and pulmonary regurgitation, and generalized hypokinetic wall motion. The left ventricular ejection fraction (LVEF) was 27% by echocardiography. Resting single-photon emission computed tomography myocardial perfusion imaging showed fixed defects in the anterolateral wall and the apex, and hypoperfusion at the inferiolateral wall (Figure 1C). All laboratory examinations, including biochemical and serological tests, showed no evidence of MI, and pro-brain natriuretic peptide was 943.8 pg/mL.
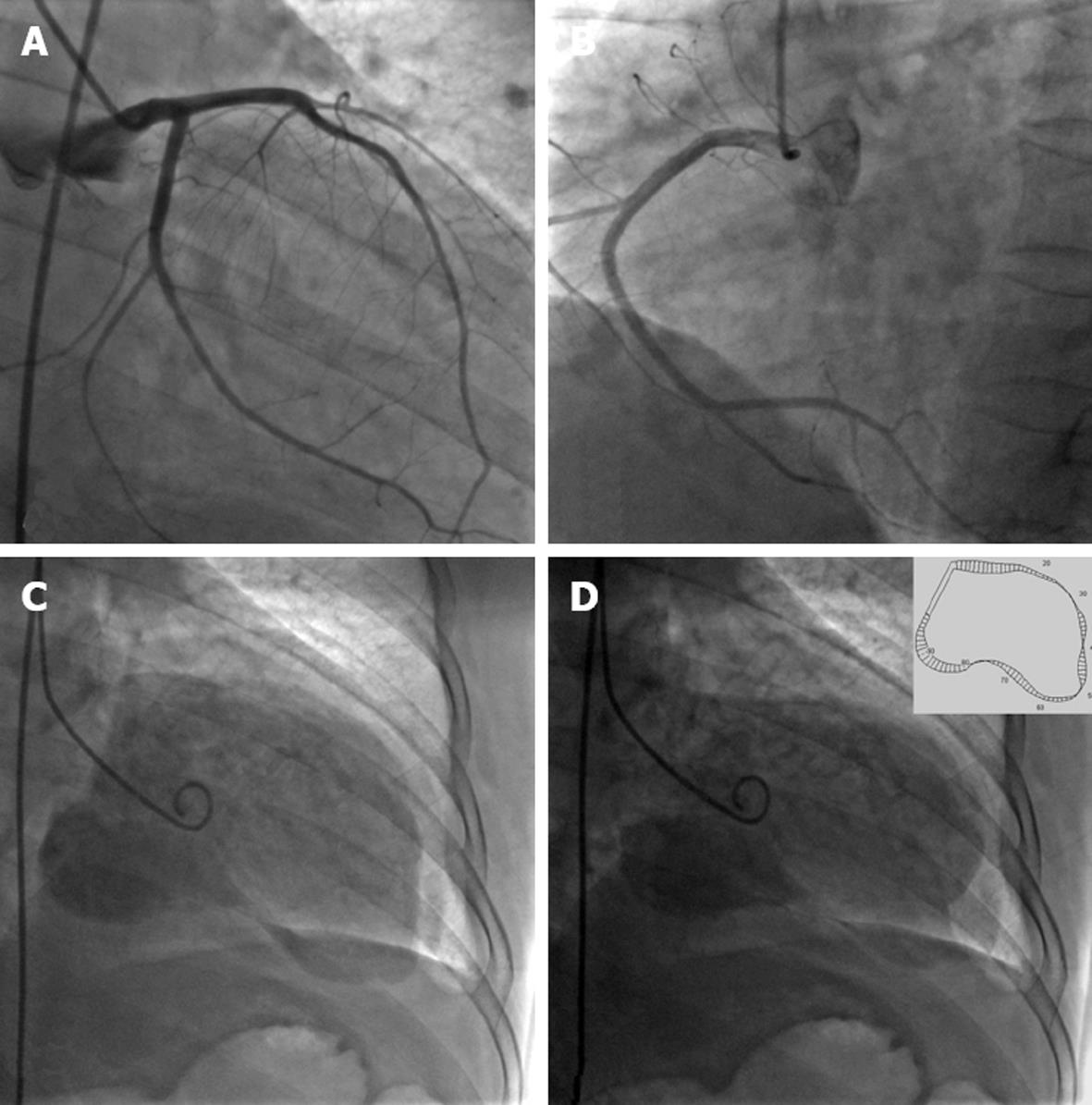
She was diagnosed with coronary heart disease, unstable angina pectoris, old myocardial infarction complicated with ventricular aneurysm, and congestive heart failure (New York Heart Association class III). For further coronary intervention, cardiac catheterization was performed and showed normal coronary arteries. A left ventriculogram showed aneurysms with systolic bulging in the apex and anterolateral wall and mild mitral regurgitation. LVEF was 22.5% (Figure 2).
For resolution of the clinical dilemma of recurrent MI in the normal coronary artery, cardiovascular magnetic resonance imaging (CMRI) was performed on a 1.5-T Signa Excite HD MR Scanner (GE Healthcare Systems). First-pass perfusion imaging was obtained using a segmented echo-planar imaging pulse sequence with a notched saturation pulse[3]. All perfusion images were acquired in the short-axis orientation only. The maximum number of slices was limited by heart rate. Gadolinium-diethylenetriamine pentaacetic acid (God-DTPA) was delivered at a rate of 4 mL/s using a power injector, with a total volume of 0.1 mmol/kg body weight. Delayed-enhancement images were obtained approximately 10 min after injection of intravenous God-DTPA using a segmented inversion recovery prepared fast gradient echo sequence[4].
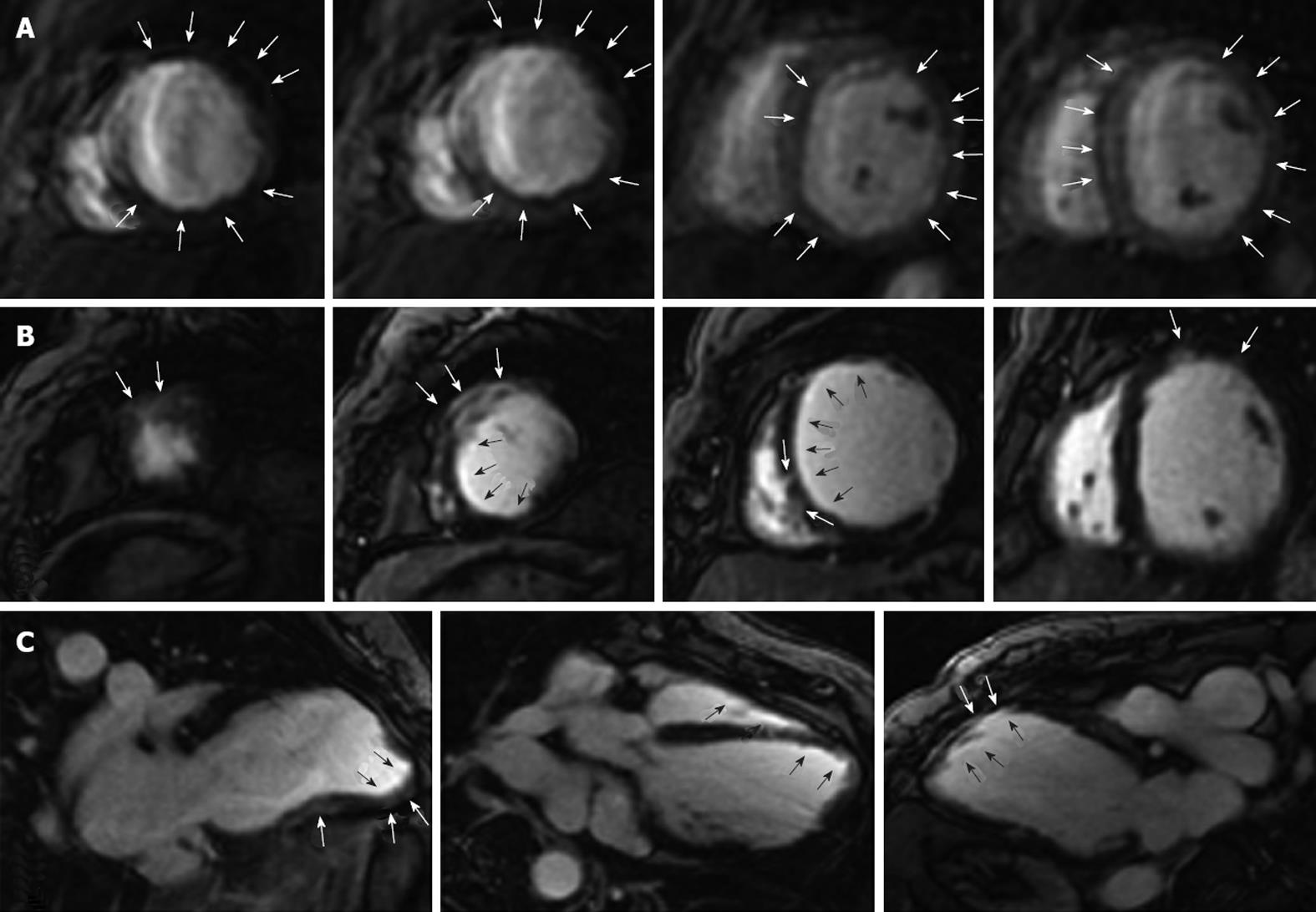
Images of the myocardium at peak myocardial enhancement during the first pass of gadolinium showed diffuse hypoperfusion of the myocardium, especially the subendocardium and anterolateral wall (Figure 3A). Delayed contrast-enhanced images showed transmural, subendocardial, and intramural enhancement in different segments of the left ventricle, especially in the anterior wall (Figure 3B and C).
Myocardial ischemia/infarction with a normal coronary angiogram is commonly characterized by coronary microvascular dysfunction, coronary spasm and coronary embolism with subsequent spontaneous clot lysis[5], retraction, or recanalization[6]. Only effort angina a with normal coronary artery angiogram and no other cardiac or systemic diseases (for example, hypertension or diabetes) known to influence vascular function was called cardiac syndrome X (CSX), which is believed to be caused by coronary microvascular dysfunction[7]. Recurrent myocardial ischemia or infarction with a normal coronary angiogram, which constitutes approximately 1% of patients with documented MI[6], is thought to be caused by coronary spasm, acquired or inherited coagulation disorders, toxic conditions, or embolization[1]. Myocardial infarction with absolutely normal coronary angiogram usually has a good prognosis[1,8], and is rarely complicated by left ventricular aneurysm. It was reported that the incidence of left ventricular aneurysm in MI with absolutely normal coronary angiogram was 0.47%[2]. The present case with 3 episodes of MI associated with left ventricular aneurysm and absolutely normal coronary angiogram has not previously been reported.
Those patients exhibiting MI with a normal coronary angiogram tend to be young and to have relatively few coronary risk factors such as hypertension, hyperlipoproteinemia, or diabetes, except that they often have a history of cigarette smoking. The onset of acute MI in these patients was usually abrupt and without prodromes, and subsequent angina and reinfarction was rare[1,8]. Coronary spasm was considered as one of the reasons. It has been shown, however, that patients with angina caused by spasm of normal coronary arteries have a distinctive clinical pattern characterized by recurrent, usually nocturnal, attacks of chest pain, with ST segment elevation on the ECG[9]. The angina in CSX, which is believed to be caused by microvascular dysfunction, is characterized by recurrent, usually exertional, chest pain and ST segment depression in the ECG.
In recent years, CMRI has been used for the detection of myocardial ischemia or infarction because of its high spatial resolution and diagnostic accuracy. First-pass perfusion CMRI has been used for the assessment of myocardial perfusion. With the first-pass perfusion CMRI technique, some patients with CSX were found to have stress-induced subendocardial perfusion defects on visual inspection[10,11]. Delayed contrast-enhanced CMRI has been shown to be a powerful technique to distinguish left ventricular systolic dysfunction related to coronary artery disease (CAD) from other heart diseases[12-14]. The patients with left ventricular dysfunction resulting from CAD were reported to have subendocardial or transmural enhancement in delayed contrast-enhanced CMRI[12].
This patient had the first episode of MI at the age of 39 years, without prodromes. Since then, she had recurrent angina and the second and the third MI in the span of 14 years. In the third episode of MI, the left ventricular aneurysm was complicated. Her left ventricular function gradually deteriorated. She had no risk factors. Two coronary angiograms, 1 and 16 years after the third episode of MI showed absolutely normal coronary arteries. However, CMRI revealed a diffuse hypoperfusion of the myocardium. This was different from the normal features of MI with a normal coronary angiogram. Three episodes of anterior wall MI disclosed that each attack of MI did not result in a large area of necrosis of the anterior wall of the myocardium. The intramural and transmural enhancement in different segments of the left ventricle, including the anterior wall, in a delayed contrast-enhanced image of CMRI demonstrated the feature of focal myocardial necrosis. Coronary spasm complicated with embolism and subsequent spontaneous clot lysis was reported to result in MI with a normal coronary angiogram[5]. This patient had both rest and nocturnal chest pain, which suggest coronary spasm. However, microvascular dysfunction was actually involved in the myocardial ischemia. Though evaluation of endothelial function and sophisticated tests such as intravascular ultasound and coronary or myocardial flow reserve were not performed in this patient, both the clinical presentation and the results of CMRI suggested that the recurrent myocardial ischemia and infarction were, at least to some extent, attributable to coronary microvascular dysfunction.
Recurrent effort angina, a normal coronary angiogram, microvascular dysfunction, and good prognosis were believed to be features of CSX[7]. All studies on patients with CSX before the Women’s Ischemia Syndrome Evaluation (WISE), reported an excellent clinical outcome at long-term follow-up, with a total absence of major acute coronary events (that is, cardiac death or acute MI)[15]. However, WISE showed that women with ischemic symptoms and signs but without obstructive CAD were at elevated risk for cardiovascular events compared with asymptomatic community-based women[16]. This patient was characterized by having a normal coronary angiogram, microvascular dysfunction, recurrent rest angina, infarction, and progressive deterioration of left ventricular function, which supports the results of WISE. Progressive deterioration of left ventricular function in patients with a normal coronary angiogram may be mainly caused by extensive microvascular dysfunction, resulting in poor long-term prognosis because of many patches of myocardial hibernation, necrosis and left ventricular remodeling; coronary vasospasm might also be involved in the process. Whether this case represents a special clinical condition which resides between CSX and CAD remains to be investigated.
We greatly thank Thach Nguyen, Director of Cardiology at Community Healthcare System, St Mary Medical Center, Hobart, IN, United States for his critical review.
Peer reviewers: Tommaso Gori, MD, PhD, II Medizinische Klinik, Universitätsmedizin der Johannes Gutenberg Universitats Mainz, 55131 Mainz, Germany; Nipon Chattipakorn, MD, PhD, Professor, Cardiac Electrophysiology Research and Training Center, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
S- Editor Cheng JX L- Editor Cant MR E- Editor Li JY
| 1. | Da Costa A, Isaaz K, Faure E, Mourot S, Cerisier A, Lamaud M. Clinical characteristics, aetiological factors and long-term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3-year follow-up study of 91 patients. Eur Heart J. 2001;22:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Toda G, Iliev II, Kawahara F, Hayano M, Yano K. Left ventricular aneurysm without coronary artery disease, incidence and clinical features: clinical analysis of 11 cases. Intern Med. 2000;39:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Eichenberger AC, Schuiki E, Köchli VD, Amann FW, McKinnon GC, von Schulthess GK. Ischemic heart disease: assessment with gadolinium-enhanced ultrafast MR imaging and dipyridamole stress. J Magn Reson Imaging. 1994;4:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215-223. [PubMed] |
| 5. | Benacerraf A, Scholl JM, Achard F, Tonnelier M, Lavergne G. Coronary spasm and thrombosis associated with myocardial infarction in a patient with nearly normal coronary arteries. Circulation. 1983;67:1147-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Arnett EN, Roberts WC. Acute myocardial infarction and angiographically normal coronary arteries. An unproven combination. Circulation. 1976;53:395-400. [PubMed] |
| 7. | Lanza GA. Cardiac syndrome X: a critical overview and future perspectives. Heart. 2007;93:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Rosenblatt A, Selzer A. The nature and clinical features of myocardial infarction with normal coronary arteriogram. Circulation. 1977;55:578-580. [PubMed] |
| 9. | Selzer A, Langston M, Ruggeroli C, Cohn K. Clinical syndrome of variant angina with normal coronary arteriogram. N Engl J Med. 1976;295:1343-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 500] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 11. | Lanza GA, Buffon A, Sestito A, Natale L, Sgueglia GA, Galiuto L, Infusino F, Mariani L, Centola A, Crea F. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 813] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 13. | Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 620] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 15. | Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 307] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 434] [Article Influence: 27.1] [Reference Citation Analysis (0)] |