CASE REPORT
A 54-year-old hypertensive, diabetic male and chronic smoker presented in February 2008 with symptoms of dyspnea on exertion, New York Heart Association class III for 1 year, orthopnea and paroxysmal nocturnal dyspnea for 1 mo, and 6-mo bilateral lower limb claudication on walking 50 m. There was no history of angina, syncope or lower limb ulceration/discoloration. A general physical examination revealed a blood pressure of 130/90 mmHg in the right upper limb, feeble bilateral femoral arterial pulse, dependent pitting edema of the feet, and raised jugular venous pressure. Systemic examination revealed a left ventricular third heart sound, bilateral crepitations in half of the basal lung fields. Ankle brachial pressure index (ABPI) was 0.54 on the right and 0.68 on the left side. Blood investigations revealed hemoglobin (Hb) 10.9 g/dL, urea 94 mg/dL, creatinine 1.9 mg/dL, and fasting blood sugar 250 mg/dL. The fasting lipid profile was total cholesterol 151 mg/dL, high density lipoprotein cholesterol (HDL) 38 mg/dL, low density lipoprotein cholesterol (LDL) 81 mg/dL, and triglycerides 137 mg/dL. Electrocardiography showed a poor R wave in V1-V4 chest leads, with the rest within normal limits. Two-dimensional echocardiography revealed global left ventricular hypokinesia, an ejection fraction of 0.30, mild tricuspid regurgitation, and an estimated pulmonary artery systolic pressure of 65 mmHg. Peripheral ultrasound Doppler revealed focal stenosis of the left superficial femoral artery (SFA) in the mid thigh.
After adequate decongestive therapy and glycemic control, the patient underwent contrast angiography of the coronary and peripheral vasculature. The coronary angiogram revealed 100% block of the proximal non-dominant right coronary artery, 90% diffuse stenosis of the proximal left anterior descending artery (LAD) (Figure 1A), 90% tubular stenosis of the distal left circumflex artery (LCx) (Figure 1A), and a left dominant circulation. The left main coronary artery was normal and obtuse marginal-3 was occluded from its origin. Left ventricular end diastolic pressure was 50 mmHg. Peripheral angiography revealed 75% osteal stenosis of the left renal artery (Figure 2), 50% osteal stenosis of the right renal artery, a normal celiac trunk, 100% osteal block of the superior mesenteric artery (SMA), 50% osteal stenosis of the inferior mesenteric artery (IMA) (Figure 3A), diffuse narrowing of the infra-renal abdominal aorta, and more than 50% bilateral stenosis of the common iliac artery (right > left) (Figure 4A).
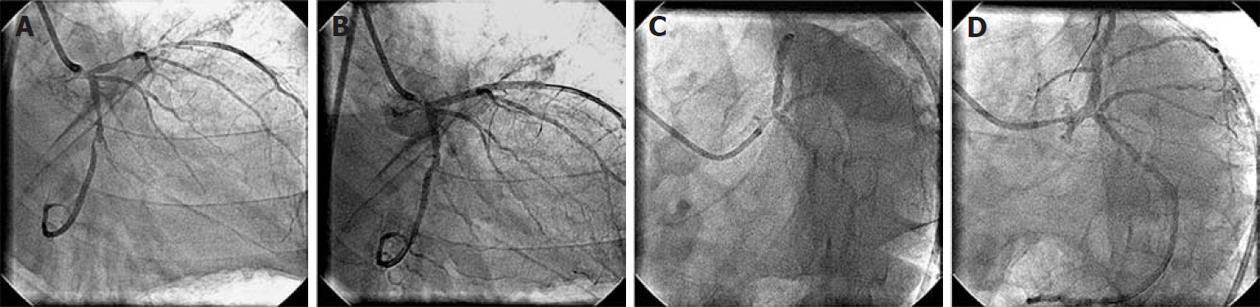
Figure 1 Coronary angiogram.
A: 90% diffuse stenosis of proximal left anterior descending artery (LAD) and 90% tubular stenosis of the distal left circumflex artery (LCx); B: No residual stenosis following proximal LAD and distal LCx stenting; C: At 15 mo of follow-up, showing patent LAD and LCx stents. There is new 70% bifurcation stenosis of the left main coronary arterywith osteal LAD stenosis. The LCx ostium is normal without any stenosis; D: No residual stenosis and normal LCx ostium following left main coronary artery crossover stenting.
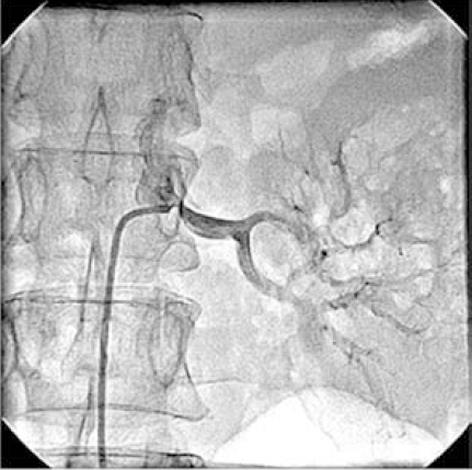
Figure 2 Left renal angiogram showing 75% osteal stenosis of renal artery.
Figure 3 Inferior mesenteric artery angiogram.
A: 50% osteal stenosis of the inferior mesenteric artery (IMA); B: Post-IMA osteal stenting with a 7 mm x 18 mm balloon-expandable stent, at 15 mo follow-up, showing no residual IMA stenosis.
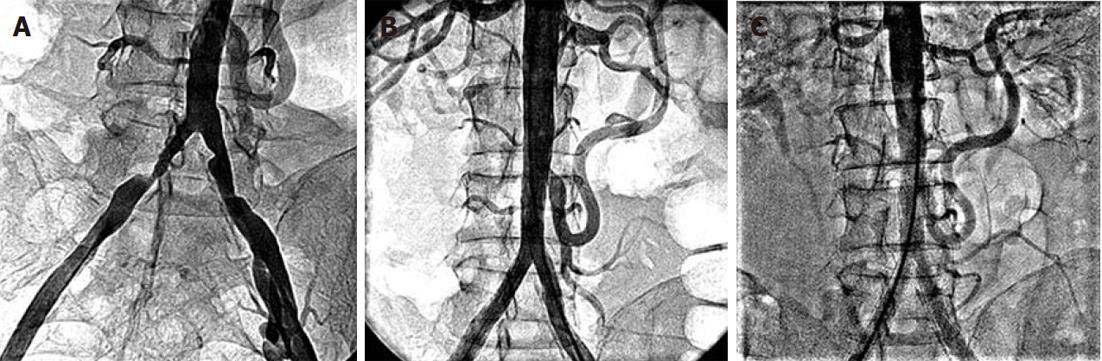
Figure 4 Abdominal aortogram.
A: Bilateral common iliac artery stenosis; B: No residual stenosis following left renal and aorto-bilateral iliac artery stenting. A big inferior mesenteric artery collateral “arch of Riolan” supplies the occluded superior mesenteric artery; C: At 30 mo follow-up, showing patent left renal and aorto-bilateral iliac stents.
Following a detailed discussion about percutaneous/surgical revascularization for his extensive CAD and PAD, he gave written informed consent for percutaneous intervention. After a gap of a week, he underwent percutaneous intervention. Bilateral femoral arterial access was achieved with a 7 F sheath. The left coronary artery was cannulated with Judkins Left 3.5, 7 F guide catheter, and a 0.014 inch All Track Wire (ATW) (Cordis Corp., Miami, Florida) was inserted into the proximal LAD lesion. After pre-dilation with a 2.5 × 15 mm Sprinter balloon (Medtronic, Inc., Minneapolis, Minnesota), a sirolimus-eluting Cypher 3 × 33 mm stent (Cordis) was deployed. Then a 0.014 inch ATW wire (Cordis) was inserted into the distal LCx lesion, which was pre-dilated with 2.5 mm × 15 mm Sprinter balloon (Medtronic), and a Cypher 2.75 mm × 28 mm stent (Cordis) was deployed (Figure 1B). After coronary intervention, the left renal artery was cannulated with a 7 F renal guide catheter (Medtronic) and a 7 mm × 15 mm GenesisTM balloon expandable stent (Cordis) was deployed across the ostium, resulting in brisk flow (Figure 4B). Then, for aorto-iliac intervention, a 0.018 inch Roadrunner floppy tip guide wire (Cook, Bloomington, Indiana, United States) was positioned across both iliac arteries, extending into the aorta. An 8 mm × 80 mm SMARTTM control Nitinol self-expanding stent (Cordis) was directly deployed from the infra-renal abdominal aorta to the right iliac artery. It was post-dilated with an 8 mm × 60 mm OPTA®Pro Peripheral balloon (Cordis). Then, another 8 mm × 80 mm SMARTTM control stent (Cordis) was deployed from the infra-renal abdominal aorta to the left iliac artery. It was post-dilated with a 5 × 20 mm and then an 8 × 60 mm OPTA®Pro Peripheral balloon (Cordis). An abdominal aortogarm showed brisk flow through the left renal artery and aorto-iliac arteries with no residual stenosis (Figure 4B). In total, 150 mL of iodixanol contrast agent and 20.7 min of fluoroscopy time was used for all these interventions. The patient had an uneventful recovery and was discharged on day 4 after the intervention. The pre-discharge serum creatinine was 1.9 mg/dL.
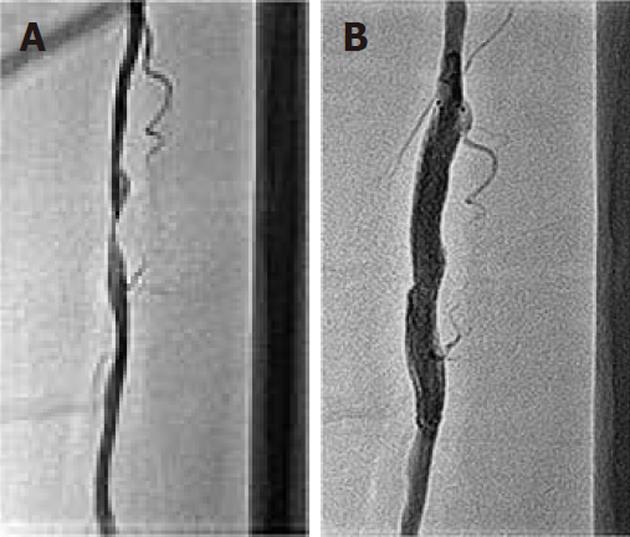
At 3-mo follow-up, the patient was admitted for left SFA intervention. This time the claudication distance of the left lower limb was 500 mm while there was no claudication in the right lower limb. A 6 F sheath was placed in the left femoral artery after a retrograde puncture. A 0.014 inch ATW wire (Cordis) was inserted into 90% of the stenosed SFA lesion (Figure 5A), pre-dilated with a 3 × 30 mm coronary angioplasty balloon, and a 7 × 60 mm Zilver self-expanding stent (Cook) was deployed. It was post-dilated with a 6 × 20 mm OPTA®Pro peripheral balloon (Cordis); a brisk flow was achieved in the SFA (Figure 5B).
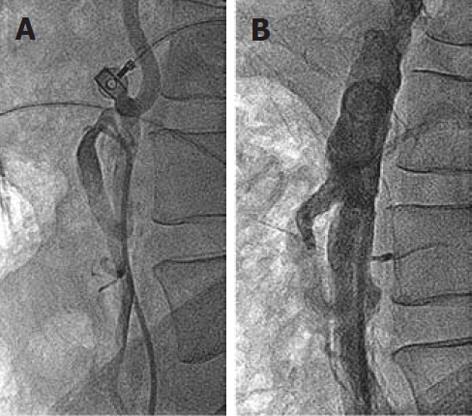
Figure 5 Left superficial femoral artery angiogram.
A: At 3 mo follow-up, showing 90% discrete stenosis in the mid part; B: Following 7 mm x 60 mm self-expanding stent deployment, there is no residual stenosis.
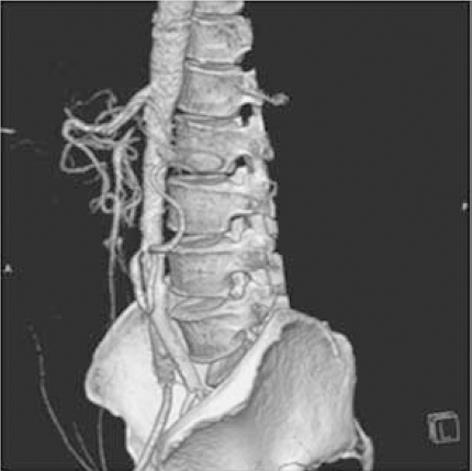
At 9-mo follow-up, the patient was relatively asymptomatic. Congestive heart failure had improved; echocardiography showed a left ventricular ejection fraction of 0.40. There was no claudication in the lower limbs, and bilateral ABPI was 0.98. Glycemia was controlled with insulin and oral hypoglycaemic agents. Serum urea and creatinine were 52 and 1.6 mg/dL, respectively. Computed tomography showed patent left renal, bilateral aorto-iliac and left SFA stents. There was 90% osteal stenosis of the IMA; a 100% occluded SMA at the ostium showed retrograde filling through an arch of Riolan, which received a collateral from the IMA (Figure 6). There were no symptoms of chronic mesenteric ischemia despite underlying SMA and IMA stenosis.
Figure 6 Computed tomography.
Image of the abdominal aorta at 9 mo follow-up, showing 90% stenosis of the inferior mesenteric artery (IMA) ostium. The totally occluded superior mesenteric artery at the ostium is filled retrogradely via the arch of Riolan from the IMA.
At 15-mo follow-up, the patient started having pain in the upper abdomen 1.5-2 h following a meal and lasting for about 1 h. This complaint was of 3 wk duration. There was no history of hematemesis or blood in the stool. Abdominal pain did not improve despite treatment with proton pump inhibitors and antacids. Abdominal ultrasound did not show any free fluid or dilated bowel loops suggestive of mesenteric infarction. Upper gastrointestinal endoscopy was within normal limits. In view of the typical postprandial abdominal pain of underlying mesenteric artery disease, a diagnosis of mesenteric ischemia was made. There was no cardiac angina, worsening heart failure, claudication, significant weight loss, hematemesis or occult blood in stool. His urea, creatinine and fasting blood sugar were 50 mg/dL, 1.6 mg/dL and 144 mg/dL, respectively. He was scheduled for percutaneous mesenteric revascularization. A 6 F sheath was placed in the right femoral artery for angiographic check of the stented coronary and peripheral arteries; a 7 F sheath was placed in the right brachial artery for IMA intervention. To our surprise, coronary angiography revealed 70% stenosis of the left main artery extending from the ostium to the bifurcation and to the osteal LAD; the Lcx ostium was normal (Figure 1C). Previously deployed stents in the proximal LAD and distal LCx were patent. Left renal and bilateral aorto-iliac stents were also patent. The IMA had 90% osteal stenosis. The right renal artery showed non-progressed 50% osteal stenosis. In view of significant left main coronary artery disease, informed written consent was obtained for both left main coronary artery and IMA stenting. In the same operation, the left coronary artery was cannulated with a Judgkins Left 3.5, 6F coronary guide catheter via the trans-femoral route. Both the LAD and LCx had a 0.014 inch ATW wire (Cordis) inserted. The left main coronary artery and osteal LAD were pre-dilated with a 2.5 × 15 mm Sprinter balloon (Medtronic). A 3.5 cm × 18 mm Cypher stent (Cordis) was deployed from the ostium of the left main coronary artery to the proximal LAD, crossing the LCx ostium. After stenting, there was TIMI-3 flow in the left main coronary artery, LAD and LCx; the LCx ostium was normal with no stenosis (Figure 1D). Then, the IMA was cannulated with a Judgkins Right 3.5, 7 F coronary guide catheter via the right brachial route. A 0.014 inch ATW wire (Cordis) was inserted into the IMA lesion and a 7 mm × 18 mm Genesis balloon-expandable stent (Cordis) was deployed across the ostium of the IMA. Brisk flow was achieved in the IMA (Figure 3B). During this intervention, the fluoroscopy time was 24.5 min and 200 mL of iodixanol contrast agent was used. After the procedure, the creatinine at 72 h was 1.58 mg/dL. Repeat biochemistry revealed total cholesterol of 142 mg/dL, HDL 35 mg/dL, LDL 72 mg/dL and triglycerides 150 mg/dL; other parameters included lipoprotein(a) 37.3 mg/dL, high-sensitive c-reactive protein (hsCRP) 6.73 mg/L, homocysteine 15.86 μmol/L and HbA1c 10.10% The patient had an uneventful recovery and was discharged on day 4 following the intervention. There were no further symptoms of post-prandial abdominal pain at follow-up.
At 30-mo follow-up, the patient was asymptomatic and underwent an angiogram for academic reasons. It revealed a patent left main coronary artery, LAD and LCx stents; and left renal artery (Figure 4C), bilateral aorto-iliac (Figure 4C), IMA (Figure 4C) and left SFA stents.
During each session of angiography and/or intervention, adequate hydration was maintained, N-acetyl cysteine was given and the procedure was performed with the minimum permitted amount of iodixanol contrast agent to avoid contrast-induced nephropathy. At 40-mo follow-up in July 2011, he was asymptomatic and was on dual anti-platelet therapy, atorvastatin 40 mg, ramipril 10 mg, β-blockers and insulin and diuretics. His fasting blood sugar and creatinine were 110 mg/dL and 1.6 mg/dL, respectively.
DISCUSSION
Physicians frequently see patients with both CAD and PAD in routine clinical practice. In a study of 28 649 patients of angiography-proven CAD, 9% of patients were found to have associated PAD[1]. On the other hand, in an another study of 110 patients with abdominal aneurysm, 71% of patients had associated CAD[2]. Although there has been a significant improvement in CAD-associated morbidity and mortality following advanced percutaneous therapeutic interventions in recent years, concomitant PAD poses a therapeutic challenge. Coronary intervention is associated with improved myocardial function and survival, while peripheral interventions are associated with different clinical outcomes, for example in the carotid artery it is associated with reduced stroke rate, in the renal artery with reduced need for renal replacement and blood pressure therapy, in the mesenteric artery with decrease mesenteric ischemia, and in the ilio-femoral and femoro-popliteal arteries with improved functional status and reduced amputation rate[3]. Hence, there has to be an integrated and comprehensive multi-disciplinary approach for therapeutic intervention in patients with CAD and PAD. The index diabetic case, who had renal, aorto-iliac, mesenteric and SFA stenosis, in addition to triple vessel CAD, had undergone multi-vascular interventions for improvement in cardiac function, claudication, renal function, hypertension and symptomatic mesenteric ischemia. He was a high-risk case for percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG), because of associated risk factors such as triple vessel disease, diabetes, uremia, congestive heart failure, left ventricular systolic dysfunction, and associated PAD[4,5]. The option of PCI instead of CABG in this patient was more appropriate because of low ejection fraction, impaired renal function and associated PAD. Multi-vessel coronary revascularization with sirolimus drug-eluting stent as in the index case, has shown comparable long-term mortality and major adverse cardiac event rate with CABG in both normal and severe left ventricular dysfunction patients[6,7]. Accelerated atherosclerosis and need for repeat revascularization of left main coronary artery stenosis at 15 mo of follow-up can be explained by diabetes mellitus, uncontrolled glycemia (HbA1c 10.10%)[8], and raised hsCRP[9]. hs-CRP is a marker of inflammation and is a strong and independent predictor for future risk of myocardial infarction, stroke, PAD and sudden cardiac death in healthy population[10]. Amano et al[11] observed that post-PCI patients with elevated CRP frequently undergo non-culprit lesion revascularization at follow-up. Also, PAD patients undergoing PCI have higher in-hospital major cardiovascular complications and non-favorable long-term outcomes[12,13]. Wildman et al[14] also demonstrated that the CRP level is independently associated with PAD. A high level of hs-CRP, i.e., 6.73 mg/L in the index case, was associated with extensive atherosclerotic CAD and PAD. More aggressive statin therapy in CAD patients with high CRP levels is associated with a better clinical outcome[15,16], and therefore the index case was put on atorvastatin 40 mg/d throughout his follow-up course. The single stent technique as performed in the index case for the left main coronary artery bifurcation lesion has shown more favorable long-term clinical outcomes in comparison with the two-stent technique[17].
Risk factors such as diabetes, raised creatinine of >1.5 mg/dL, anemia, congestive heart failure and left ventricular dysfunction, present in the index case, are associated with contrast-induced nephropathy and adverse clinical outcomes following contrast load[18]. As per the recommendation for contrast-induced nephropathy prevention[19,20], we maintained adequate hydration by adjusting diuretic therapy, used N-acetyl cysteine and iodixanol contrast agent. According to the American College of Cardiology/American Heart Association (ACC/AHA) recommendations, asymptomatic bilateral renal artery stenosis (≥ 50% stenosis) as in the index case is a class IIb indication for percutaneous revascularization[21]. Renal dysfunction with creatinine of 1.9 mg/dL can be explained by diabetic nephropathy and bilateral renal artery stenosis. Progression to total occlusion is more common in renal arteries with more severe stenosis, which may result into worsening renal function and the need for renal replacement therapy[22,23]. In the index case, we performed renal stenting in only the left kidney which had 75% stenosis, but did not intervene in the 50% stenosed right renal artery. A stabilized/decreased serum creatinine of 1.6 mg/dL at 40-mo follow-up can be explained by percutaneous left renal intervention and non-progression of right renal artery stenosis, though these points can be debated.
Symptomatic chronic mesenteric ischemia usually manifests when at least 2 of 3 mesenteric arteries, i.e., the celiac trunk, SMA and IMA, have proximal atherosclerotic stenosis[21]. A lack of clinical manifestation involving only one vessel is because of extensive inter-connected collateral formation, though there can be an exception with the SMA[21,24]. The index case was initially asymptomatic despite 100% occlusion of the SMA, because of good retrograde filling via the arch of Riolan, which received collaterals from the IMA. There is no randomized trial or clinical guideline for revascularization in such a situation, when there is asymptomatic two-vessel involvement[21,25]. With progression of osteal stenosis of the IMA from 50% at baseline to 90% at 15-mo follow-up, the patient became symptomatic with classical postprandial abdominal pain of mesenteric ischemia, for which he underwent successful endovascular mesenteric revascularization. Endovascular treatment for chronic mesenteric ischemia is considered to be a primary therapeutic strategy in most instances[26]. Silva et al[24], in his series of 59 patients with chronic mesenteric ischemia treated with percutaneous intervention, had shown a procedural success rate of 96% with no procedural mortality and 80% 5-year symptom-free survival. Isolated IMA revascularization is useful in relieving symptoms and may improve mortality, when revascularization of other visceral arteries is technically not feasible and there is an extensive and intact collateral supply from the IMA[27]. The index patient also improved following partial mesenteric revascularization by performing isolated IMA intervention, as there was a well formed arch of Riolan supplying the SMA. No intervention in the chronically occluded SMA was performed because of the technical difficulty[28], expected symptomatic improvement following isolated IMA intervention[27], and the risk of contrast-induced nephropathy in this high-risk patient. Although surgical revascularization of the isolated IMA has been reported[27], there is no published report of isolated IMA stenting for chronic mesenteric ischemia management, and the index case is first one in the published literature. An interesting observation in this case, which is again not reported, is an increase in IMA diameter following SMA occlusion, for which we could deploy a 7 mm diameter balloon-expandable stent.
According to ACC/AHA recommendations, symptomatic focal aorto-iliac and femoro-popliteal disease is a class 1 indication for endovascular interventions[21]. In this case, we performed bifurcation kissing stenting of the aorto-iliac lesion and focal stenting of the left SFA lesion, and demonstrated their long-term patency. Various authors have demonstrated about an 80% patency rate at 60 mo of follow-up for aorto-iliac stenting[29].
Patients with extensive atherosclerotic CAD and PAD require a comprehensive multi-specialty approach for management[30,31]. The index case was treated by a team of cardiologists, and an expert opinion was sought in between from various specialties such as nephrology, gastroenterology, diabetes care and radiology.
In conclusion, we hereby report an unusual case of extensive, atherosclerotic CAD and PAD with comorbid illness, who received successful percutaneous panvascular revascularization and had a favorable long-term outcome.