Published online Jun 26, 2011. doi: 10.4330/wjc.v3.i6.186
Revised: May 11, 2011
Accepted: May 18, 2011
Published online: June 26, 2011
Reperfusion therapy must be applied as soon as possible to attenuate the ischemic insult of acute myocardial infarction (AMI). However reperfusion is responsible for additional myocardial damage, which likely involves opening of the mitochondrial permeability transition pore (mPTP). In reperfusion injury, mitochondrial damage is a determining factor in causing loss of cardiomyocyte function and viability. Major mechanisms of mitochondrial dysfunction include the long lasting opening of mPTPs and the oxidative stress resulting from formation of reactive oxygen species (ROS). Several signaling cardioprotective pathways are activated by stimuli such as preconditioning and postconditioning, obtained with brief intermittent ischemia or with pharmacological agents. These pathways converge on a common target, the mitochondria, to preserve their function after ischemia/reperfusion. The present review discusses the role of mitochondria in cardioprotection, especially the involvement of adenosine triphosphate-dependent potassium channels, ROS signaling, and the mPTP. Ischemic postconditioning has emerged as a new way to target the mitochondria, and to drastically reduce lethal reperfusion injury. Several clinical studies using ischemic postconditioning during angioplasty now support its protective effects, and an interesting alternative is pharmacological postconditioning. In fact ischemic postconditioning and the mPTP desensitizer, cyclosporine A, have been shown to induce comparable protection in AMI patients.
- Citation: Perrelli MG, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J Cardiol 2011; 3(6): 186-200
- URL: https://www.wjgnet.com/1949-8462/full/v3/i6/186.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i6.186
Acute myocardial infarction (AMI) is responsible for the death of millions of persons worldwide each year, with a mortality rate of about 10%, and is the leading cause of chronic heart failure[1]. Notwithstanding, marked improvements in the strategy to reduce infarct size and to reduce all manifestations of postischemic injury, with subsequent improvement in prognosis, have been developed in recent years. Although early reperfusion is the only way to salvage an ischemic organ, during the crucial early moments of reperfusion, significant reversible and irreversible organ damage is initiated, and is referred to as reperfusion injury. Reperfusion injury include arrhythmias, transient mechanical dysfunction of the heart or “myocardial stunning”, microvascular injury and ‘‘no-reflow’’, as well as inflammatory responses. In reperfusion, cell death can occur due to apoptosis, necrosis, and autophagy[2-8]. Given that recent data indicate that the different forms of cell death are probably interrelated[6,7], a better strategy to develop cardioprotective agents is not to define the mode of cell death and its proportion occurring during ischemia/reperfusion, but to identify mediators active in all forms of cell death. In this context, the variation in mitochondrial membrane permeability appears to be one of the major regulators of all forms of cell death.
During normal perfusion mitochondria generate adenosine triphosphate (ATP), consume large amounts of O2 and contribute to a balanced generation and scavenging of reactive oxygen species (ROS). Mitochondria are also involved in cellular ion homeostasis, including calcium homeostasis.
During ischemia, the lack of of O2 inhibits electron flow, and myocardial ATP utilization becomes inefficient. The proton-translocating F0F1ATP synthase, which normally produces ATP, switches into reverse mode, i.e. becomes an F0F1ATPase, and consumes ATP to pump protons from the matrix into the intermembrane space[9,10]. In prolonged ischemia, Na+/K+ ATPase is inhibited (because of the drop in ATP levels) and the intracellular acidification (induced by lactate production and the hydrolysis of ATP) activates the Na+/H+ exchanger (NHE), i.e. the cell tries to restore the intracellular pH; the resulting increase in intracellular Na+ concentration activates the Na+/Ca2+ exchanger (NCE), which lead to Ca2+ overload. Elevated cytosolic Ca2+ concentrations may contribute to cellular damage by activation of degrading enzymes such as nucleases, phospholipases and proteases culminating in the destruction of the membrane integrity and leading to cell death if the ischemic period is of sufficient duration[11,12].
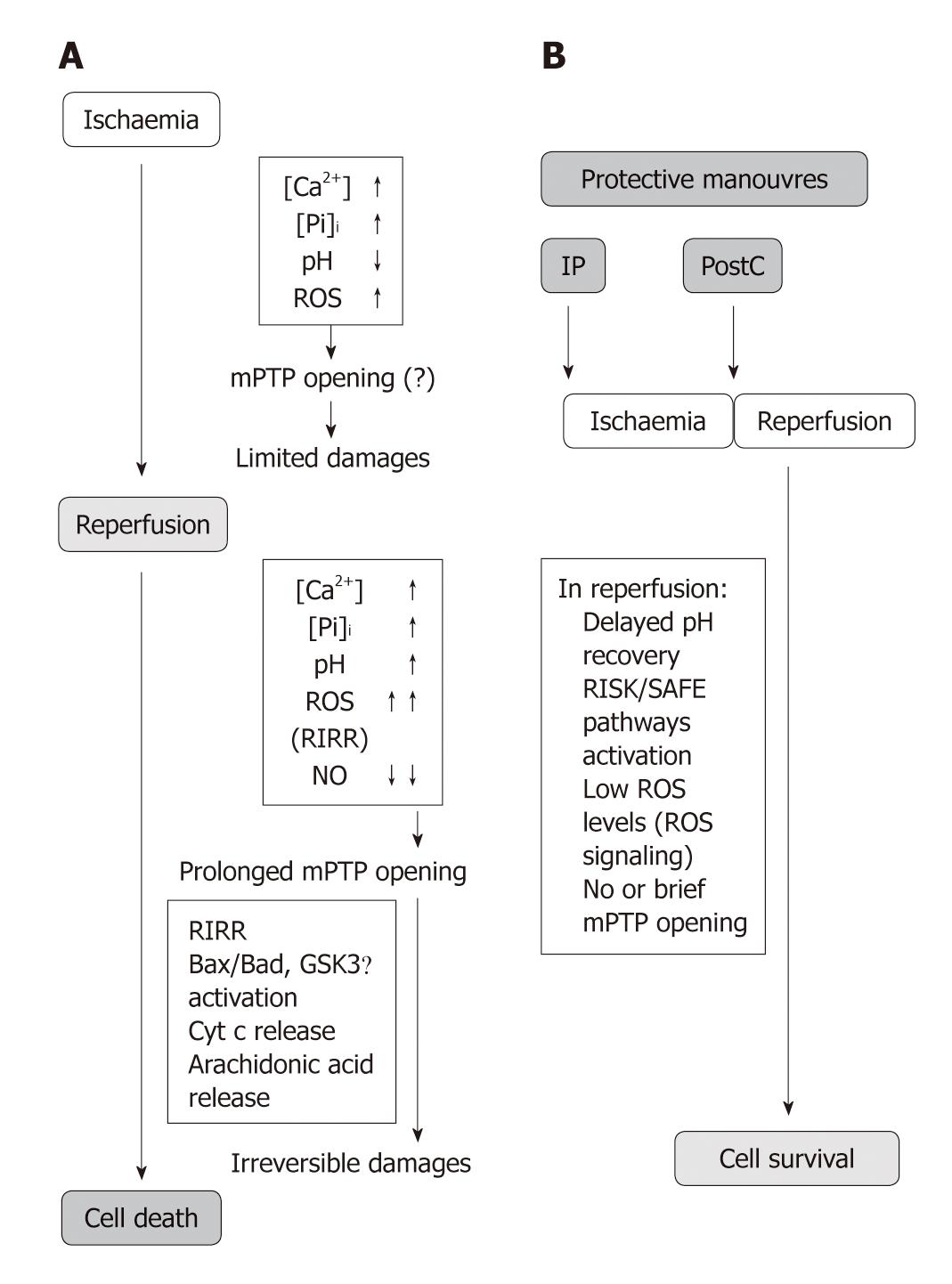
At reperfusion, intracellular and mitochondrial events such as Ca2+ overload, inadequate resynthesis of ATP, loss of membrane phospholipids, low production of nitric oxide (NO•) and oxidative stress by ROS contribute to reperfusion injury[13-16]. Yet, when an increase in ATP concentration occurs, it paradoxically contributes to reperfusion injury, leading to hyper-contracture of cardiomyocytes, membrane disruption and subsequent band necrosis[16,17]. Clearly the recovery of pH, oxidative stress and Ca2+ overload can induce the abrupt opening of the mitochondrial permeability transition pores (mPTP), a large conductance pore in the inner mitochondrial membrane (IMM), which strongly contributes to cardiomyocyte hyper-contracture, apoptosis and necrosis[18-22].
This pore is a high conductance megachannel, which builds up at the contact sites between the mitochondrial outer and inner membranes. When the mPTP is formed, it permits communication between the cytoplasm and mitochondrial matrix[23]. The molecular identity of the protein(s) forming this pore is still unknown. It has been suggested that the mPTP is formed by the voltage-dependent anion channel in the outer mitochondrial membrane (OMM), the adenine nucleotide transporter in the IMM, and cyclophilin D (Cyp D) in the matrix of mitochondria. mPTP opening seems to be facilitated by binding of the matrix protein Cyp D to the IMM in a process regulated by both Ca2+ and inorganic phosphate (Pi)[19] (Figure 1). However, even experiments with transgenic mice in each of the putative components of mPTP reached controversial results[24-31].
mPTP opening: The oxidative opening of mPTP is central in reperfusion injury (Figure 2A). Many studies have indeed revealed an important contribution of mPTP opening and have correlated cell death with the release of cytochrome c (Cyt c) after Bax and enhanced ROS levels[22,32-35].
Importantly, the opening probability of mPTP of the de-energized mitochondria is drastically reduced below pH 7.4, a condition occurring during sufficiently prolonged ischemia[36]. Low pH also reduces mitochondrial calcium uptake and favors calcium extrusion from the mitochondrial matrix, due to the activation of mitochondrial NHE and subsequent NCE[37]. However, low pH may activate uncoupling proteins (UCPs), IMM carriers of H+ that uncouple ATP synthesis from oxygen consumption[38]. For the role of UCPs in cardioprotection see below[39-41]. Low pH may also inhibit glycolysis and pyruvate production, resulting in a slower feeding of the respiratory complex chain. Therefore low pH mainly prevents mPTP opening in ischemia (Figure 1A).
On reperfusion, quite different conditions are created depending on whether or not the mitochondrial membrane potential rapidly recovers. In the case of energized respiring mitochondria, a low pH can stimulate Pi uptake increasing its intra-mitochondrial content, thus acting as an mPTP opener[42]. In contrast, in the presence of mitochondrial membrane depolarization, long-lasting opening of the mPTP take places when rapid normalization of tissue pH occurs in the presence of Ca2+ overload, Pi, ROS formation, and/or lower levels of NO•[32,43-46]. The latter condition (i.e. membrane depolarization and rapid pH normalization) is the more common scenario upon abrupt reperfusion. In fact, the probability of this pore being open is facilitated by several factors including high pH, Ca2+ overload, and burst of ROS at the onset of reperfusion. Apart from its direct action on mitochondria, the opening effect of Ca2+ is also due to indirect effects, such as phospholipase A2 and calpain activation[47-50] (Figure 1A).
Consequences of mPTP opening: Depending on the complex balance between cellular inducers and antagonists, mPTP can undergo transient or intermediate/long-lasting opening[51]. mPTP opening of short duration is likely to generate reversible cellular changes, so that this transient opening has been suggested to be involved in physiological processes and cardioprotection, such as intracellular NAD+ traffic, and transient formation of ROS (see also below)[52-55]. Actually, the transient increase in the opening probability of mPTP is involved in ROS-dependent triggering of cardioprotection by preconditioning[56,57] (see below).
While a transient/intermediate pore opening may or may not lead to apoptosis, long-lasting pore formation is followed by profound alterations of cellular bioenergetics that are considered irreversible; it results in increased mitochondrial permeability to ions and solutes with molecular weights of up to 1.5 kDa, matrix swelling and loss of critical electrochemical gradients. In this condition the F0F1ATPase actively hydrolyzes rather than synthesizes ATP, leading to inevitable cell death[11,12,33,58,59]. Actually, the mandatory consequence of long-lasting mPTP opening is the collapse of mitochondrial membrane potential. This is rapidly followed by ATP and NAD+ depletion, mitochondrial release of accumulated Ca2+, matrix swelling and rupture of the OMM leading to loss of pyridine nucleotides and release of pro-apoptotic factors such as Cyt c, which triggers apoptosis and thus also inhibits electron flow through the electron transport chain[11,19,20,32,60-62]. Many have postulated that long-lasting mPTP formation is the event that leads to irreversible changes in cellular function and cell death[13,59,62,63]. Di Lisa et al[62] were among the first to observe that addition of Ca2+ to mitochondria causes organelle swelling and profound decreases in NAD+ content.
At least two mechanisms which are not mutually exclusive have been proposed to explain mitochondrial membrane permeabilization and apoptosis. Apart the mPTP, which involves the participation of both the IMM and the OMM, a mechanism of mitochondrial death which involves only the OMM and the formation of channels across the membrane has been described. Although there is controversy concerning the structure, the regulation and the definite role of these two putative different channels, strong evidence indicates that proteins of the Bcl-2 family may contribute to both mechanisms[64,65].
Prevention of prolonged mPTP opening: All these opening factors are counteracted by “physiological” mPTP antagonists, such as adenine nucleotides (mainly ADP), elevated concentrations of protons (i.e. pH below 7.4), increased mitochondrial membrane potential, and magnesium ions, as well as by physiological levels of nitric oxide[19,66]. The pore is rapidly closed if Ca2+ is chelated[19,60]. Promotion of mPTP opening is also prevented by some drugs, including cyclosporine A (CsA), which at nM concentrations is a mPTP desensitizer[36,58,67]. Notably, in the absence of Pi the desensitizing effects of CsA are no longer present[58,67].
Since mPTP formation is likely to be a causative event in reperfusion injury and a major proportion of cell death results from mPTP formation, it is not a surprise that cardioprotective strategies demonstrated that inhibition of mPTP is the end-effector of cardioprotection (Figures 1B and 2). In fact the importance of mPTP closure as a target for myocardial protection has been described in several studies[58,68-70]. The mechanisms of cardioprotection and mPTP closure in reperfusion are described in the following section.
Lethal reperfusion injury appears to represent from 20 to 70% of the total amount of irreversible myocardial damage according to the studied species and therefore constitutes a major therapeutic target[2,71-75].
Over the last decades ischemic preconditioning and postconditioning have been recognized as protective phenomena and have been confirmed in humans; they share certain signaling elements in experimental analyses[2,76-78] (Figure 2). In 1986, Murry et al[79] reported that four 5 min circumflex occlusions, each separated by 5 min of reperfusion, followed by a sustained 40 min occlusion (index ischemia = infarcting ischemia) in the dog heart dramatically attenuated ischemia/reperfusion injury. This phenomenon was named ischemic Preconditioning (PreC). In 2003, Zhao et al[73] reported that three episodes of 30 s of reperfusion/30 s of ischemia performed immediately after index ischemia (60 min coronary occlusion) in the dog heart drastically attenuated reperfusion injury. This phenomenon was named ischemic Postconditioning (PostC). It was soon clear that the later the application of the first postconditioning ischemia, the lower the protection.
The recognition of the ischemic PostC phenomenon put an end to any discussion on the existence of reperfusion injury[80]. The term “PostC” has also highlighted the importance of intervening at the beginning of myocardial reperfusion to protect the post-ischemic heart; a clinically more relevant time-point for intervention in patients presenting with an AMI. As such, its clinical application has been rapid for both ST-elevation AMI patients undergoing primary percutaneous coronary intervention (PCI)[81,82] and for patients undergoing on-pump cardiac surgery[83] (see also below).
The protective effects observed with PostC are comparable to those observed with the powerful PreC[2,73,84,85]. In fact, PostC may reduce apoptosis, necrosis, and endothelial dysfunction/activation, thus leading to a reduced endothelium/leukocyte interaction and to reduced ROS inflammatory formation[5,73]. PostC also reduces the incidence of reperfusion arrhythmias[86-90].
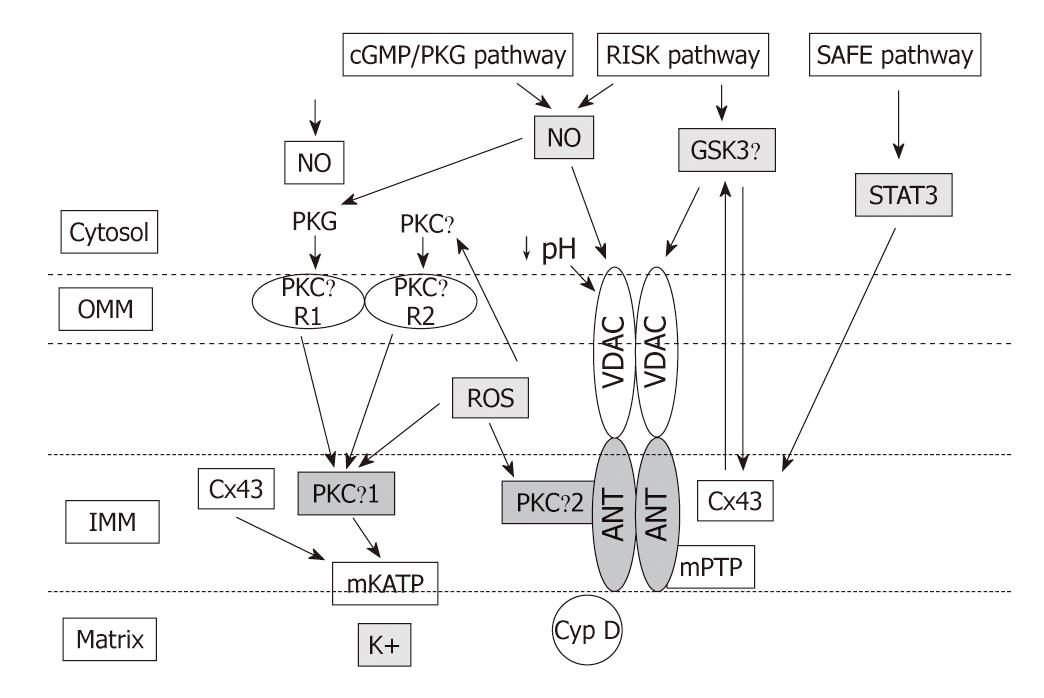
Intensive investigation of the signaling pathways underlying PreC and PostC have identified a number of signal transduction pathways conveying the cardioprotective signal from the sarcolemma to the mitochondria, some of which overlap for PreC and PostC. In fact, both PreC and PostC induce activation of signaling elements during the early reperfusion following the index ischemia[91] (Figure 2). Great attention has focused on the cyclic guanosine monophosphate (cGMP)/protein kinase G (PKG)-pathway[92-94], on the Reperfusion Injury Salvage Kinases (RISK) pathway[95,96], which involves the kinases Akt and ERK1/2, and more recently on the Survivor Activating Factor Enhancement (SAFE) pathway that has been suggested to contribute to PostC protection through the activation of tumor necrosis factor (TNF)-α, its receptor type 2, Janus kinase (JAK) and signal transducer and activator of transcription (STAT)-3[78,97]. All these pathways in preconditioning and postconditioning converge on the mitochondria via the modulation of several kinases including glycogen synthase kinase-3β, Bax/Bad and the ε isoform of protein kinase C (PKC) (for reviews see[10,78]). The modalities of mitochondrial control by cytosolic kinases depicted in Figures 2 and 3 are still controversial and are beyond the aims of this editorial.
Nevertheless, ROS signaling and acidosis in early reperfusion are two cardioprotective processes operating in early reperfusion in both preconditioning and postconditioning (Figures 2 and 3). They may act, first, directly on mPTP components to limit their opening and, then, may activate signaling pathways that have been suggested to converge again on mitochondria to decrease the susceptibility to mPTP opening and mediate protection (Figures 2 and 3). These two processes are discussed in the next sections.
Before considering the beneficial role or ROS signaling, let us consider the forms and the detrimental effects of ROS within the heart.
Forms of ROS: ROS are generated in different cellular compartments and by several enzymes, including NADPH oxidases at the plasma membrane[98,99] and cytosolic xanthine oxidases[100]. Although ROS are produced by several extracellular and intracellular processes, in cardiomyocytes the mitochondria represent the most relevant site for ROS formation[101-105]. Within the mitochondria, most of the oxygen is reduced to water at respiratory complex IV. The mitochondrial formation of ROS might be modulated by NO•[106-108] which reversibly inhibits cytochrome oxidase[105,109-112]. This inhibition can be transformed into irreversible alterations of the respiratory chain when NO• reacts with O2-• to generate a large amount of peroxynitrite, which can produce the irreversible nitration of proteins[113]. Even nitric oxide synthases can become “uncoupled” resulting in the generation of O2-• and OH•, instead of NO• under certain conditions, such as scarcity of substrate and/or cofactors[112,113]. Apart from the electron transport chain, ROS can also be produced by monoamine oxidases (MAOs) in the OMM. MAOs transfer electrons from amine compounds to oxygen to generate hydrogen peroxide[102,114,115]. Within the mitochondria, p66Shc oxidizes reduced Cyt c, which induces the partial reduction of oxygen to peroxide[116-118].
Besides being a relevant site for ROS formation, mitochondrial function and structure are profoundly altered by oxidative stress[34], especially when mPTPs have long-lasting opening. In fact, mPTPs play a central role in the so-called ROS-induced ROS release (RIRR)[61,119,120]; excessive ROS facilitate mPTP opening which in turn favors ROS formation by inhibiting the respiratory chain because of mPTP-induced loss of Cyt c and pyridine nucleotides[12,34]. This vicious cycle is likely to be established at the onset of a rapid reperfusion when a large increase in ROS formation occurs along with pH recovery, and Ca2+ overload, thus inducing injury amplification, as discussed above and below (Figure 1).
Detrimental effects of excessive ROS: Various detrimental processes can result from an imbalance between the excess formation of ROS and limited antioxidant defenses (referred to as ‘oxidative stress’). For instance, excessive ROS indiscriminately react with DNA, lipids and proteins[113,120-123]. The lack of protection of mitochondrial DNA by histones, the limited capacity of repair mechanisms and the proximity of mitochondrial DNA to the production site of ROS by RIRR render the mitochondrial DNA highly susceptible to increased oxidative stress[124]. Excessive oxidative stress, besides contributing to irreversible myocardial injury, induces long-lasting mPTP opening leading to cellular dysfunction and cell death, and may also induce reversible injury during ischemia and reperfusion[105,125-128]. The reversible contractile dysfunction following myocardial ischemia/reperfusion (“stunning”) is clearly a manifestation of excessive oxidative stress[129,130]. Whether stunning is due to RIRR has not yet been investigated.
On the other hand, as mentioned above, low levels of reactive species may act as secondary messengers, modulating cardioprotective signaling pathways by covalent modification of target molecules, referred to as ‘redox signaling’ or “ROS signaling”, which is the main topic of the next section.
It has been proposed that reintroduction of oxygen after transient ischemia induces ROS production, but it does not protect against reperfusion injury because mPTPs open and trigger RIRR before the activation of endogenous survival pathways.
The key event for cardioprotection may be prolongation of cellular acidosis by cardioprotective phenomena during early reperfusion (Figure 2B). In fact acidic perfusion in early reperfusion or PostC, delaying pH normalization, could inhibit mPTP during the first minutes of reflow and allow for endogenous protective signaling pathways to be activated by ROS signaling. The delivery of oxygen during acidic perfusion or the brief intermittent reperfusions of PostC would promote mitochondrial ROS formation which has been proposed to activate isoforms of PKC through redox signaling[131]. Different isoforms of PKC appear as critical kinases in the signaling cascade leading to a reduced probability of mPTP opening after pH normalization[131-134] (see Figure 3 and below).
Not only mPTPs may be inhibited by a limited ROS production and acidosis, but also, as mentioned above, a transient or short duration opening of the mPTP has been suggested to induce a slight, transient formation of ROS that might be relevant for cardioprotection[51-54,56,57,135]. Supporting this concept, pharmacological and genetic inhibition of Cyp D was reported to attenuate both preconditioning-induced ROS formation and protection[136,137]. In brief, in protected reperfusion, low levels of ROS may act directly on mPTP components or activate signaling pathways that have been suggested to act on mitochondria decreasing their susceptibility to prolonged mPTP opening.
Redox signaling by transient/reduced formation of ROS is also included among the triggers of PostC[138]. In fact, we were the first to show that the ROS scavengers N-acetylcysteine (NAC)[132] and N-2-mercaptopropionyl glycine (MPG) prevent the protective effects of ischemic or pharmacological PostC[94,132,139-140], and the same ROS scavenger has been shown to block the protection afforded by acidic reperfusion[141]. In our laboratory isolated rat hearts were subjected to ischemia and reperfusion. While PostC significantly reduced infarct size, protection by PostC was lost in hearts perfused with NAC for the entire reperfusion period. Infarct size was still reduced when, in postconditioned hearts, perfusion with NAC was initiated after the first 3 min of reperfusion, demonstrating an essential role of ROS formation during early reperfusion in PostC protection[132]. Cardioprotection, induced with acidic buffer for the first 2 min of reperfusion, was blocked by MPG applied for 20 min in reperfusion[141], suggesting the involvement of ROS signaling in acidosis-induced protection. Infarct size reduction by either ischemic PostC, 1.4% isoflurane or 10 mg/kg of delta-opioid receptor agonist SNC-121 in mouse hearts in vivo were attenuated by the ROS scavenger MPG when administered a few minutes before but not 10 min after the postconditioning stimulus[140]. NAC in the first minutes of reperfusion also abolished postconditioning by bradykinin or sevoflurane in isolated rat hearts[94,139]. Importantly, in the human myocardium, desflurane-induced postconditioning was mediated by adenosine and bradykinin receptors via ROS signaling[115]. These studies support a central role for ROS signaling during early reperfusion in the protection by ischemic postconditioning and in the protection by acidosis in early reperfusion.
Mitochondrial ATP-sensitive potassium (mKATP) channels in the mitochondrial inner membrane are considered targets of protective cascades, and play a pivotal role in ROS production, mainly superoxide anion derived from complex I of the electron transport chain[21,76,138,142,143]. Opening of the mKATP channels and subsequent generation of ROS is considered to be a pivotal step in the mechanisms of pre- and postconditioning[3,21,76]. We found evidence that ROS signaling is downstream of mKATP channel opening in isolated rat hearts subjected to ischemia/reperfusion with an intermittent infusion of diazoxide or diazoxide plus MPG at the onset of reperfusion, since MPG attenuated diazoxide-induced protection. However, while ROS scavenging attenuates infarct size reduction by postconditioning or diazoxide, increasing exogenous ROS formation with purine/xanthine oxidase at the onset of reperfusion does not confer protection[94]. It is likely that the type, the concentration, and/or the compartmentalization of reactive species may play a pivotal role in triggering protection at reperfusion. Nevertheless, we cannot rule out that a different ROS generator could trigger PostC protection.
In the context of cardioprotection, it has been reported that PKG- and/or Akt-dependent phosphorylation induces the opening of mKATP promoting K+ entry into mitochondria with consequent alkalinization of the mitochondrial matrix and generation of ROS with a protective signaling role. Indeed, PKG phosphorylates a protein on the OMM, which then causes the mKATP on the IMM to open. This implies that the protective signal is transmitted from the OMM to the IMM. This is accomplished by a series of intermembrane signaling steps that includes PKCε activation. The resulting ROS then activate a second PKC pool which, through another signal transduction pathway causes inhibition of the mPTP (the end effector) and reduction in cell death[3,76,144-146] (Figure 3). Pharmacological opening of mKATP channels by diazoxide contributes to the formation of small amounts of ROS[147]. Also, NO• donors can activate mKATP channels in rabbit ventricular myocytes and can potentiate the protective effect of the mKATP opener diazoxide[143]. Besides cGMP/PKG-dependent phosphorylation, mKATP could be opened by direct reaction of NO• and derivatives (S-nitrosylation), as well as by the action of H2S via S-sulfhydration[4]. However, controversy exists on the nature, existence and opening of mKATP channels, which may also be a toxic process[145,148]. PKC activation leading to the opening of mKATP channels has been challenged by the Halestrap group: they demonstrated that preconditioning inhibits opening of the mPTP in situ, by an indirect mechanism probably involving decreased ROS production and Ca2+ overload at reperfusion[11,148].
Mitochondrial uncoupling, i.e. proton influx into the mitochondrial matrix without phosphorylation of ADP, contributes to ROS formation[39,40]. Although controversy exists on the cardioprotective role of uncoupling, mild uncoupling secondary to activation of UCPs has been described to confer cardioprotection under several conditions, including myocardial reperfusion, likely by decreasing ROS production[41]. Intriguingly, it has been suggested that transient complex I inhibition during reperfusion is cardioprotective via attenuated ROS production[149,150]. Nevertheless, experimental evidence supporting the involvement of these changes in PostC protection has yet to be provided. Whether UCPs play a deleterious or protective role in ischemia tolerance is controversial[39-41].
Mitochondrial connexin-43 (Cx43) has also been implicated in ROS-signaling, though its role is not completely defined[21,151,152]. Actually, within cardiomyocytes Cx43 is mainly localized at gap junctions, but it is also present in other organelle membranes, including the IMM of sarcolemmal mitochondria[152-154] where Cx43 regulates mitochondrial potassium flux[155,156]. It seems that Cx43 transolocates to the IMM, with cardioprotection via the intervention of heat shock protein 90-TOM (translocation of the outer membrane) import pathway[154].
Mitochondrial Cx43 has been described to be essential for preconditioning protection[154,157,158], but a recent study in mice heterozygous for Cx43 (Cx43+/-) indicates that it does not play a significant role in PostC protection. Actually, Cx43 is a target of several protein kinases, and mitochondrial Cx43 is highly phosphorylated under physiological conditions[159]; it seems that in the IMM of a subset of cardiomyocyte mitochondria, subsarcolemmal mitochondria, the phosphorylated portion of Cx43 increases with ischemia[152] and decreases with PostC[138]. Since a decrease in the mitochondrial Cx43 content is sufficient to abolish the cardioprotection by diazoxide preconditioning, i.e. reduces ROS formation[147], one can speculate that Cx43 reduction in PostC may be one of the mechanisms to reduce excessive ROS production in the reperfusion phase. Recently it has been suggested that genetic ablation or pharmacological inhibition of mitochondrial Cx43 confers resistance to mKATP channel opening in response to diazoxide in patch-clamped mitoplasts (mitochondria devoid of the OMM). However, the open-probability of the mKATP channel was not affected under baseline conditions; thus it is likely that Cx43 regulates this channel activity rather than constituting the pore forming unit of the mKATP channel[156].
In the context of cardioprotection, reactive species with a signaling role are suggested to be formed during three time points: (1) during preconditioning-ischemia and/or (2) during reperfusion that follows the brief preconditioning-ischemia; and (3) in the initial part of reperfusion that follows the index ischemia; both in the postconditioning and in the preconditioning context.
During preconditioning-ischemia: A wide body of evidence exists demonstrating that appreciable formation of ROS occurs during ischemia[126,128,160-162]. In fact, mitochondrial ROS formation is favored by a decrease in electron flow resulting from respiratory chain inhibition, and is counteracted by uncoupling that is generally produced by an increased IMM permeability to protons. Therefore, the inhibition of the electron transport chain caused by insufficient oxygenation facilitates the escape of electrons that can react directly with the scarce available oxygen resulting in ROS formation.
During reperfusion that follows brief preconditioning-ischemia: Small amounts of ROS may be formed during reperfusion following a short period of preconditioning ischemia. To support this viewpoint, the group of Downey[163] used MPG (a cell-permeant ROS scavenger[164]) to test whether the ROS that triggers protection are produced during the ischemic or the reperfusion phases of the preconditioning maneuvers. These authors concluded that protective redox signaling occurs when molecular O2 is reintroduced following the brief preconditioning coronary occlusion.
In the initial part of reperfusion that follows the index ischemia in postconditioning: The above observations were done in the preconditioning phase, i.e. before the index ischemia, and extended to PostC itself. In fact, as reported above, the protective effect of PostC was attenuated by infusing, during early reperfusion, large spectrum ROS scavengers, making the oxygenated perfusate alkaline during the early reperfusion phases or making the early reperfusion buffer hypoxic[50,94,131,132,141,165].
Early reperfusion events in preconditioned heart: Clearly, both PreC and PostC, besides sharing a number of signaling elements, induce activation of signaling elements (RISK and SAFE) during early reperfusion following the prolonged index ischemia[78,91,166] (Figure 2). It is now thought that, after a triggering phase in the pre-ischemic period, the actual protection by PreC occurs in the reperfusion rather than in the ischemic phase, with the repopulation of sensitized G-protein-coupled receptors at the beginning of myocardial reperfusion following the index ischemia[167]. Hence, reintroduction of O2 at the beginning of reperfusion permits generation of signaling ROS, which will activate the PKC-dependent signaling cascade. In fact the PreC protective effect was also attenuated by infusing. during early reperfusion. large spectrum ROS scavengers, making the oxygenated perfusate alkaline during the early reperfusion phase or making the early reperfusion buffer hypoxic[56,166,167].
Targets of ROS signaling: Reactive species function as trigger molecules of protection by activating protein kinases such as PKC within and outside the mitochondria[3,145,146,168,169], as well as the mitogen-activated protein kinase p38 and/or JAK/STAT[170,171]. Several mitochondrial components are targeted by reactive species via oxidative/nitrosative processes[4,10]. Accordingly, many large spectrum scavengers of ROS, such as ascorbic acid, MPG or NAC, attenuate infarct size reduction by ischemic or pharmacological PreC or PostC, in several animal species[2-4,132,144,169,172]. Since a target of ROS in redox signaling is the PKC, hearts can be preconditioned by simply infusing free radicals into the coronary arteries, and that protection can be blocked by a PKC antagonist[76,167]. Evidence exists that ROS-activated PKC will also protect the reperfused heart[132,169]. This sequence would explain the observation that a PKC activator could rescue hearts experiencing acidic and hypoxic reperfusion[131]. Moreover, chelerythrine, a non-specific PKC antagonist, blocks PostC protection[132-134].
Indeed, it has been reported that ROS can activate PKC in vitro by reacting with thiol groups associated with the zinc finger region of the molecule[173]. Reactive nitrogen species-dependent activation of PKC, possibly via a redox-sensitive S-nitrosylation process, has been also suggested; a process which also occurs within mitochondria[4,175,175]. Recently, we have observed in an ex vivo study that PostC induces downregulation of superoxide dismutase (SOD), whereas catalase activity does not change in the early reperfusion phase. Moreover, PostC reduces 3-nitrotyrosine and increases S-nitrosylated protein levels, thus contributing to cardioprotection triggering[176]. The persistence of acidosis[50,68,131,142,165] and the NO• augmentation (enzymatic and non-enzymatic production)[132] in early reperfusion of postconditioned hearts, together with SOD downregulation may favor nitrosylation and/or may limit protein de-nitrosylation. In fact SOD is a de-nitrosylating enzyme[177]. Intriguingly, exogenous-SOD prevents PostC-triggering, whereas exogenous-catalase does not interfere with PostC protection. That is, the addition of exogenous-SOD during PostC maneuvers does not allow the early reduction in overall SOD activity, usually induced by PostC.
Preconditioning and postconditioning activate cardioprotective pathways that are protective against reperfusion injury via preservation of the functional and morphological integrity of mitochondria. The protection of PostC against apoptosis is mediated by reduced generation of superoxide anions, lowered activity of c-Jun-N-terminal kinases/p38, lowered levels of caspases 3 and 8, reduced release of TNFα, and by the modulation of the Bax/Bcl-2 ratio[178]. PostC increases the levels of anti-apoptotic markers, including the cardioprotective kinase Pim-1, decreases pro-apoptotic markers, e.g. Cyt c, and preserves the mitochondrial structure. In fact, at the onset of reperfusion, mitochondria undergo profound structural alterations. In particular, post-ischemic mitochondria are characterized by disruption of membranes, broken christae and the appearance of dense granules within the mitochondrial matrix, which are caused by massive accumulation of Ca2+, generating insoluble calcium phosphate precipitate[179]. These mitochondrial damages are reduced by PostC[138]. Carbonylation of mitochondrial proteins was prevented and aconitase activity was preserved in the PostC hearts suggesting that mitochondrial integrity was associated with a diminution in oxidative stress[180]. However, PostC does not influence mitochondrial respiration[181]. In particular, PostC does not affect basal state 4 or ADP-stimulated state 3 respiration, excluding uncoupling or inhibition of the respiratory chain as a mechanism of mPTP inhibition[182]. Nevertheless, while basal respiration was not affected, ADP-stimulated respiration was increased after pharmacological PostC with morphine[183]. This is in line with many reports showing that a mild degree of mitochondrial dysfunction confers protection against ischemia/reperfusion injury[175,184].
In summary, ROS signaling before index ischemia, i.e. during brief preconditioning ischemia and/or during the following reperfusion, is clearly involved in the triggering of preconditioning protection. Excessive ROS formation during reperfusion, following infarcting ischemia, enhances cell death, but ROS signaling during early reperfusion is essential for protection of ischemic and some pharmacological preconditioning and postconditioning against reperfusion injury. In early reperfusion, opening of mKATP channels may be upstream of ROS signaling. Cardioprotective procedures delay the post-ischemic recovery of intracellular pH that may prevent mPTP opening directly and indirectly (i.e. by inhibiting calpain activation). In addition, mPTP opening may be further prevented by a ROS signaling that appears to depend on acidosis and by a decrease in intracellular Ca2+. ROS signaling triggers a protective kinase cascade starting from PKC and converging on mPTPs. Thus, mPTP closure may be dependent on ROS signaling effects, both upstream, together with an acidotic effect, and downstream, dependent on kinase effects. Therefore, mitochondria are involved in at least four different steps to limit reperfusion injury: (1) as the target of acidosis (i.e. prevention of mPTP opening); (2) as triggers or signal amplifiers (i.e. activation of mKATP channels and resulting formation of small amounts of ROS); (3) as the target of signaling pathways and end-effectors (i.e. inhibition of mPTP opening and of release of pro-apoptotic factors into the cytosol); and (4) as targets of damage and protection from it (i.e. functional and morphological integrity).
The signaling role of ROS in early reperfusion must be kept in mind for successful treatment in reperfusion. Clearly, the mPTP is a major factor in determining cell death, and mPTP inhibition affords significant cardioprotection[12,32,185,186], a concept that has been successfully translated into the clinical setting[81,82,187,188]. In particular, postconditioning transition to the clinical setting has proven the existence of lethal myocardial reperfusion injury in man, and the clinical studies suggest that 40%-50% of the final reperfused infarct in humans may be attributable to myocardial reperfusion injury[189].
In AMI patients the involved coronary artery may be opened by either angioplasty or thrombolysis, with or without application of a stent. The ischemic postconditioning, though reserved for patients reperfused by primary angioplasty, may provide impressive results when the no-reflow phenomenon, the infarct size and the myocardial contractile function are considered, raising great hopes for potential clinical benefits. Feasible in every patient, the pharmacological postconditioning, including CsA infusion, would allow the expansion of postconditioning protection to almost all ST-elevation AMI patients. Obviously restoring reperfusion to the ischemic myocardium is the definitive strategy in reducing infarct size. However, blood flow may not be restored to all segments of the microvasculature in the post-ischemic myocardium[190], a situation that is associated with the no-reflow phenomenon as a predictor of adverse long-term outcomes in patients[191]. The obvious implication of low- or no-reflow is that the blood supply is inadequate to sustain contractile function, and the decrease may be severe enough to induce cell death of the involved myocardium. Reducing the no-reflow area may lead to smaller infarcts, less adverse remodeling and less severe heart failure. Post-ischemic blood flow in a small group of patients undergoing PCI for AMI was studied by Laskey[192]. Patients were assigned to receive standard care vs an “ischemic conditioning” stimulus. While the control group (standard care) had a 90-s balloon inflation only before withdrawal of the catheter, the conditioned group had a 90-s inflation followed by 3-5 min of balloon deflation, and after that the balloon was advanced distal to the stenosis. These conditioned patients have shown an improved post-ischemic coronary blood flow as revealed by an increased peak coronary blood flow velocity, diastolic/systolic velocity ratio and blood flow velocity reserve (evaluated with Doppler flow wire) compared to the control group. Staat et al[81] used blush grade, the speed by which contrast is washed out of the myocardium at risk, as a marker of myocardial reperfusion after the initial period of reflow in patients subjected to standard angioplasty or postconditioning, which consisted of four cycles of 60-s deflation/inflation of the angioplasty balloon in the target vessel. In postconditioned patients the blush grade was 25% greater than that of control patients. Also Ma et al[193] found that post-ischemic coronary blood flow in the target vessel was greater in AMI patients who received postconditioning treatment. This was associated with lower markers of lipid peroxidation by ROS and lower plasma levels of myocardial creatine kinase. Moreover, brachial arterial endothelium-mediated (flow-dependent) relaxation in response to transient cuff occlusion applied 24 h after PCI was better in the postconditioned patients. Although flow-dependent vasodilator effects in the brachial artery do not directly reflect physiology of the coronary vascular endothelium, it may be reflective of a “remote” protection to the endothelium of other organs, which then becomes a surrogate measure of the coronary vascular endothelium. Actually remote ischemic postconditioning (conditioning stimuli applied to a distant organ during reperfusion of the target organ) induced by transient episodes of ischemia of distant organs, including kidney, arms and legs, is a clinically feasible method for protecting the heart against injury at the time of reperfusion. Recently, it has been observed in rats that remote ischemic perconditioning may reduce infarct size, and that repeated remote postconditioning further reduces adverse remodeling of the left ventricle and may improve survival in a dose-dependent fashion[194]. Indeed, remote postconditioning has been reported experimentally[194,195], and correlated with endothelial protection in humans[196,197]. Thus, although these data suggest that local and remote ischemic postconditioning have favorable effects on recovery of microvascular perfusion following relief of ischemia, further experimental and clinical studies are needed to establish whether postconditioning attenuates microvascular injury and the extent of no-reflow.
As mentioned above, a pharmacological approach may be more suitable. In fact, initial progress has been made with novel approaches for preventing myocardial reperfusion injury by administering drugs in the first minutes of reperfusion; preliminary clinical data indicate that drugs targeting mPTPs or RISK may confer benefits to patients with AMI, with and without comorbidities, above that provided by myocardial reperfusion alone. Very good results are obtained with drugs such as CsA as an mPTP desensitizer[81,187,188], as well as with other drugs targeting RISK, such as erythropoietin and its analogs[198,199]. Importantly, similar cardioprotective effects were obtained with other drugs acting on mPTP, confirming the relevance of this approach. For instance, derivatives of CsA, such as [N-methyl-ala6]CsA, [N-methyl-Val4]CsA, Debio 025, NIM811, or sanglifehrin A[11,12,56,58,59,68,71,136,189] also prevented myocardial ischemia/reperfusion injury in an experimental setting. Importantly, in small, proof-of-concept trials[81,82,187,189], the administration of CsA in patients with AMI at the time of reperfusion has been associated with smaller infarct size. The efficacy of treatment has been assessed measuring the release of the cardiac biomarkers creatine kinase and troponin I and by measuring the area of late hyperenhancement of the reperfused myocardium on magnetic resonance imaging (MRI). In one of this studies[187], the area under the curve for the creatine kinase concentration suggested that the administration of cyclosporine induced a reduction in infarct size of approximately 40%. This result was confirmed by a reduction in the area of late hyperenhancement on MRI. However, the area under the curve for the troponin I concentration was not significantly reduced by the administration of cyclosporine. Mewton et al[188] recently examined whether CsA modified left ventricle remodeling in patients. Cardiac MRI was performed at day 5 and after 6 mo. The authors reported that CsA did not exert any deleterious effect on left ventricle remodeling, and confirmed that infarct size reduction persisted after 6 mo. In addition, infarct size reduction by CsA was associated with a lower left ventricle dilatation at day 5, which was maintained at 6 mo. These data require confirmation in larger clinical trials.
It appears that many different signals can induce (and inhibit) mPTP formation, strictly linking ischemia/reperfusion stress and damage to the mitochondria. This highlights the capacity of mitochondria to function as general cell death sensors and to integrate many lethal signals. Clearly, mitochondria and ROS are attractive mechanistic targets for cardioprotection. Indeed, proof-of-concept studies demonstrated beneficial effects of the mPTP desensitizer CsA during early reperfusion in patients with AMI. Patient-tailored treatment to either prevent mPTP formation or the upstream events leading to mPTP opening may be achievable in the next future.
We thank Professor Donatella Gattullo for her invaluable support.
Peer reviewers: Jesus Peteiro, MD, PhD, Unit of Echocardiography and Department of Cardiology, Juan Canalejo Hospital, A Coruna University, A Coruna, P/ Ronda, 5-4º izda, 15011, A Coruña, Spain; Tommaso Gori, MD, PhD, II Medizinische Klinik, Universitätsmedizin der Johannes Gutenberg Universitats Mainz, 55131 Mainz, Germany
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
| 1. | Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2931] [Cited by in RCA: 2776] [Article Influence: 126.2] [Reference Citation Analysis (1)] |
| 2. | Penna C, Mancardi D, Raimondo S, Geuna S, Pagliaro P. The paradigm of postconditioning to protect the heart. J Cell Mol Med. 2008;12:435-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Penna C, Mancardi D, Rastaldo R, Pagliaro P. Cardioprotection: a radical view Free radicals in pre and postconditioning. Biochim Biophys Acta. 2009;1787:781-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Pagliaro P, Moro F, Tullio F, Perrelli MG, Penna C. Cardioprotective pathways during reperfusion: focus on redox signaling and other modalities of cell signaling. Antioxid Redox Signal. 2011;14:833-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Vinten-Johansen J, Granfeldt A, Mykytenko J, Undyala VV, Dong Y, Przyklenk K. The multidimensional physiological responses to postconditioning. Antioxid Redox Signal. 2011;14:791-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 666] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 7. | Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 631] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 8. | Takagi H, Matsui Y, Sadoshima J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid Redox Signal. 2007;9:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Grover GJ, Atwal KS, Sleph PG, Wang FL, Monshizadegan H, Monticello T, Green DW. Excessive ATP hydrolysis in ischemic myocardium by mitochondrial F1F0-ATPase: effect of selective pharmacological inhibition of mitochondrial ATPase hydrolase activity. Am J Physiol Heart Circ Physiol. 2004;287:H1747-H1755. [PubMed] [DOI] [Full Text] |
| 10. | Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1161] [Cited by in RCA: 1121] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 11. | Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 860] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 12. | Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009;1787:1402-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 13. | Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233-249. [PubMed] |
| 14. | Kutala VK, Khan M, Angelos MG, Kuppusamy P. Role of oxygen in postischemic myocardial injury. Antioxid Redox Signal. 2007;9:1193-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Ladilov Y, Efe O, Schäfer C, Rother B, Kasseckert S, Abdallah Y, Meuter K, Dieter Schlüter K, Piper HM. Reoxygenation-induced rigor-type contracture. J Mol Cell Cardiol. 2003;35:1481-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Piper HM, Abdallah Y, Schäfer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res. 2004;61:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 242] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Piper HM, Kasseckert S, Abdallah Y. The sarcoplasmic reticulum as the primary target of reperfusion protection. Cardiovasc Res. 2006;70:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Ruiz-Meana M, Abellán A, Miró-Casas E, Garcia-Dorado D. Opening of mitochondrial permeability transition pore induces hypercontracture in Ca2+ overloaded cardiac myocytes. Basic Res Cardiol. 2007;102:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Di Lisa F, Bernardi P. A CaPful of mechanisms regulating the mitochondrial permeability transition. J Mol Cell Cardiol. 2009;46:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Boengler K, Heusch G, Schulz R. Mitochondria in postconditioning. Antioxid Redox Signal. 2011;14:863-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Di Lisa F, Canton M, Carpi A, Kaludercic N, Menabò R, Menazza S, Semenzato M. Mitochondrial injury and protection in ischemic pre- and postconditioning. Antioxid Redox Signal. 2011;14:881-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976;251:5069-5077. [PubMed] |
| 24. | Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1801] [Cited by in RCA: 1762] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 25. | Baines CP. The mitochondrial permeability transition pore as a target of cardioprotective signaling. Am J Physiol Heart Circ Physiol. 2007;293:H903-H904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, Sollott SJ, Forte M, Bernardi P, Rasola A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS One. 2008;3:e1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 27. | Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 815] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 28. | Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1253] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 29. | Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 778] [Cited by in RCA: 743] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 30. | Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558-18561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 778] [Cited by in RCA: 743] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 31. | Eliseev RA, Malecki J, Lester T, Zhang Y, Humphrey J, Gunter TE. Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J Biol Chem. 2009;284:9692-9699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 33. | Di Lisa F, Canton M, Menabò R, Kaludercic N, Bernardi P. Mitochondria and cardioprotection. Heart Fail Rev. 2007;12:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Di Lisa F, Kaludercic N, Carpi A, Menabò R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol. 2009;104:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 486] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 36. | Nicolli A, Petronilli V, Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by matrix pH. Evidence that the pore open-closed probability is regulated by reversible histidine protonation. Biochemistry. 1993;32:4461-4465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529 Pt 1:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 938] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 38. | Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1065] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 39. | Bodyak N, Rigor DL, Chen YS, Han Y, Bisping E, Pu WT, Kang PM. Uncoupling protein 2 modulates cell viability in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H829-H835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Sack MN. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovasc Res. 2006;72:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Hoerter J, Gonzalez-Barroso MD, Couplan E, Mateo P, Gelly C, Cassard-Doulcier AM, Diolez P, Bouillaud F. Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation. 2004;110:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Kristian T, Bernardi P, Siesjö BK. Acidosis promotes the permeability transition in energized mitochondria: implications for reperfusion injury. J Neurotrauma. 2001;18:1059-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Hearse DJ, Humphrey SM, Chain EB. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol. 1973;5:395-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 272] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Lemasters JJ, Bond JM, Chacon E, Harper IS, Kaplan SH, Ohata H, Trollinger DR, Herman B, Cascio WE. The pH paradox in ischemia-reperfusion injury to cardiac myocytes. EXS. 1996;76:99-114. [PubMed] |
| 45. | Piper HM, García-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 376] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 46. | Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995;486:1-13. [PubMed] |
| 47. | Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R, Scorrano L, Pagano F, Prato M, Di Lisa F, Bernardi P. Arachidonic acid released by phospholipase A(2) activation triggers Ca(2+)-dependent apoptosis through the mitochondrial pathway. J Biol Chem. 2004;279:25219-25225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha aopototic signaling. J Biol Chem. 2001;276:12035-12040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Shulga N, Pastorino JG. Acyl coenzyme A-binding protein augments bid-induced mitochondrial damage and cell death by activating mu-calpain. J Biol Chem. 2006;281:30824-30833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Inserte J, Ruiz-Meana M, Rodríguez-Sinovas A, Barba I, Garcia-Dorado D. Contribution of delayed intracellular pH recovery to ischemic postconditioning protection. Antioxid Redox Signal. 2011;14:923-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030-12034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 373] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 52. | Di Lisa F, Ziegler M. Pathophysiological relevance of mitochondria in NAD(+) metabolism. FEBS Lett. 2001;492:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Bernardi P, Petronilli V. The permeability transition pore as a mitochondrial calcium release channel: a critical appraisal. J Bioenerg Biomembr. 1996;28:131-138. [PubMed] |
| 54. | Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 568] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 55. | Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M. Superoxide flashes in single mitochondria. Cell. 2008;134:279-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 626] [Cited by in RCA: 577] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 56. | Hausenloy DJ, Ong SB, Yellon DM. The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol. 2009;104:189-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 57. | Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 58. | Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 432] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 59. | Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93-98. [PubMed] |
| 60. | Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 494] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 61. | Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 62. | Di Lisa F, Menabò R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 519] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 63. | Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem. 2004;279:17197-17204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 519] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 64. | Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2418] [Cited by in RCA: 2546] [Article Influence: 121.2] [Reference Citation Analysis (0)] |
| 65. | Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 66. | Vinten-Johansen J, Zhao ZQ, Zatta AJ, Kin H, Halkos ME, Kerendi F. Postconditioning--A new link in nature’s armor against myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2005;100:295-310. [PubMed] |
| 67. | Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J Biol Chem. 2008;283:26307-26311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 68. | Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 69. | Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 254] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 70. | Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol. 2005;289:H237-H242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, Scalfaro P, Derumeaux G, Ovize M. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H1654-H1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 72. | Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 73. | Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579-H588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1468] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 74. | Iliodromitis EK, Downey JM, Heusch G, Kremastinos DT. What is the optimal postconditioning algorithm? J Cardiovasc Pharmacol Ther. 2009;14:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Skyschally A, van Caster P, Iliodromitis EK, Schulz R, Kremastinos DT, Heusch G. Ischemic postconditioning: experimental models and protocol algorithms. Basic Res Cardiol. 2009;104:469-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 76. | Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 705] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 77. | Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 372] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 78. | Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal. 2011;14:893-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [PubMed] |
| 80. | Heusch G. Postconditioning: old wine in a new bottle? J Am Coll Cardiol. 2004;44:1111-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 81. | Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L’Huillier I, Aupetit JF, Bonnefoy E, Finet G, André-Fouët X. Postconditioning the human heart. Circulation. 2005;112:2143-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 717] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 82. | Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF. Long-term benefit of postconditioning. Circulation. 2008;117:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 83. | Luo W, Li B, Chen R, Huang R, Lin G. Effect of ischemic postconditioning in adult valve replacement. Eur J Cardiothorac Surg. 2008;33:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 84. | Ludman AJ, Yellon DM, Hausenloy DJ. Cardiac preconditioning for ischaemia: lost in translation. Dis Model Mech. 2010;3:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 85. | Manintveld OC, Hekkert ML, van der Ploeg NT, Verdouw PD, Duncker DJ. Interaction between pre- and postconditioning in the in vivo rat heart. Exp Biol Med (Maywood). 2009;234:1345-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Grech ED, Ramsdale DR. Termination of reperfusion arrhythmia by coronary artery occlusion. Br Heart J. 1994;72:94-95. [PubMed] |
| 87. | Na HS, Kim YI, Yoon YW, Han HC, Nahm SH, Hong SK. Ventricular premature beat-driven intermittent restoration of coronary blood flow reduces the incidence of reperfusion-induced ventricular fibrillation in a cat model of regional ischemia. Am Heart J. 1996;132:78-83. [PubMed] |
| 88. | Airaksinen KE, Huikuri HV. Antiarrhythmic effect of repeated coronary occlusion during balloon angioplasty. J Am Coll Cardiol. 1997;29:1035-1038. [PubMed] |
| 89. | Galagudza M, Kurapeev D, Minasian S, Valen G, Vaage J. Ischemic postconditioning: brief ischemia during reperfusion converts persistent ventricular fibrillation into regular rhythm. Eur J Cardiothorac Surg. 2004;25:1006-1010. [PubMed] |
| 90. | Kloner RA, Dow J, Bhandari A. Postconditioning markedly attenuates ventricular arrhythmias after ischemia-reperfusion. J Cardiovasc Pharmacol Ther. 2006;11:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Downey JM, Cohen MV. We think we see a pattern emerging here. Circulation. 2005;111:120-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Heusch G. No risk, no cardioprotection? A critical perspective. Cardiovasc Res. 2009;84:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Penna C, Cappello S, Mancardi D, Raimondo S, Rastaldo R, Gattullo D, Losano G, Pagliaro P. Post-conditioning reduces infarct size in the isolated rat heart: role of coronary flow and pressure and the nitric oxide/cGMP pathway. Basic Res Cardiol. 2006;101:168-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Penna C, Mancardi D, Rastaldo R, Losano G, Pagliaro P. Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res. 2007;75:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 772] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 96. | Sivaraman V, Mudalagiri NR, Di Salvo C, Kolvekar S, Hayward M, Yap J, Keogh B, Hausenloy DJ, Yellon DM. Postconditioning protects human atrial muscle through the activation of the RISK pathway. Basic Res Cardiol. 2007;102:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 97. | Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 98. | Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 99. | Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2253] [Cited by in RCA: 2367] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 100. | Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 653] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 101. | Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2999] [Cited by in RCA: 3306] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 102. | Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6483] [Cited by in RCA: 6353] [Article Influence: 276.2] [Reference Citation Analysis (0)] |
| 103. | Głab M, Lojek A, Wrzosek A, Dołowy K, Szewczyk A. Endothelial mitochondria as a possible target for potassium channel modulators. Pharmacol Rep. 2006;58 Suppl:89-95. [PubMed] |
| 104. | Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6484] [Cited by in RCA: 6089] [Article Influence: 380.6] [Reference Citation Analysis (0)] |
| 105. | Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3404] [Cited by in RCA: 3374] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 106. | Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys. 1996;328:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 529] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 107. | Sarkela TM, Berthiaume J, Elfering S, Gybina AA, Giulivi C. The modulation of oxygen radical production by nitric oxide in mitochondria. J Biol Chem. 2001;276:6945-6949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Lizasoain I, Moro MA, Knowles RG, Darley-Usmar V, Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem J. 1996;314:877-880. [PubMed] |
| 109. | Beltrán B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S. The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death. Proc Natl Acad Sci USA. 2000;97:14602-14607. [PubMed] |
| 110. | Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 762] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 111. | Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 914] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 112. | Haynes V, Elfering SL, Squires RJ, Traaseth N, Solien J, Ettl A, Giulivi C. Mitochondrial nitric-oxide synthase: role in pathophysiology. IUBMB Life. 2003;55:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424-C1437. [PubMed] |
| 114. | Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2074] [Cited by in RCA: 2072] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 115. | Lemoine S, Buléon C, Rouet R, Ivascau C, Babatasi G, Massetti M, Gérard JL, Hanouz JL. Bradykinin and adenosine receptors mediate desflurane induced postconditioning in human myocardium: role of reactive oxygen species. BMC Anesthesiol. 2010;10:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 116. | Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 926] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 117. | Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279:25689-25695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 118. | Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 390] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 119. | Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1143] [Cited by in RCA: 1332] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 120. | Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 1045] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 121. | Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 270] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 122. | Zhang YY, Xu AM, Nomen M, Walsh M, Keaney JF, Loscalzo J. Nitrosation of tryptophan residue(s) in serum albumin and model dipeptides. Biochemical characterization and bioactivity. J Biol Chem. 1996;271:14271-14279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 123. | Zou M, Martin C, Ullrich V. Tyrosine nitration as a mechanism of selective inactivation of prostacyclin synthase by peroxynitrite. Biol Chem. 1997;378:707-713. [PubMed] |
| 124. | Clayton DA. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573-594. [PubMed] |
| 125. | Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 482] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 126. | Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240-H2246. [PubMed] |
| 127. | Kupatt C, Hinkel R, Horstkotte J, Deiss M, von Brühl ML, Bilzer M, Boekstegers P. Selective retroinfusion of GSH and cariporide attenuates myocardial ischemia-reperfusion injury in a preclinical pig model. Cardiovasc Res. 2004;61:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 128. | Robin E, Guzy RD, Loor G, Iwase H, Waypa GB, Marks JD, Hoek TL, Schumacker PT. Oxidant stress during simulated ischemia primes cardiomyocytes for cell death during reperfusion. J Biol Chem. 2007;282:19133-19143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 129. | Bolli R. Mechanism of myocardial “stunning”. Circulation. 1990;82:723-738. [PubMed] |
| 130. | Guth BD, Schulz R, Heusch G. Time course and mechanisms of contractile dysfunction during acute myocardial ischemia. Circulation. 1993;87:IV35-IV42. [PubMed] |
| 131. | Cohen MV, Yang XM, Downey JM. Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning’s success. Basic Res Cardiol. 2008;103:464-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 132. | Penna C, Rastaldo R, Mancardi D, Raimondo S, Cappello S, Gattullo D, Losano G, Pagliaro P. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol. 2006;101:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 133. | Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 134. | Zatta AJ, Kin H, Lee G, Wang N, Jiang R, Lust R, Reeves JG, Mykytenko J, Guyton RA, Zhao ZQ. Infarct-sparing effect of myocardial postconditioning is dependent on protein kinase C signalling. Cardiovasc Res. 2006;70:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 135. | Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 549] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 136. | Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 137. | Hausenloy DJ, Lim SY, Ong SG, Davidson SM, Yellon DM. Mitochondrial cyclophilin-D as a critical mediator of ischaemic preconditioning. Cardiovasc Res. 2010;88:67-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 138. | Penna C, Perrelli MG, Raimondo S, Tullio F, Merlino A, Moro F, Geuna S, Mancardi D, Pagliaro P. Postconditioning induces an anti-apoptotic effect and preserves mitochondrial integrity in isolated rat hearts. Biochim Biophys Acta. 2009;1787:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 139. | Yao YT, Li LH, Chen L, Wang WP, Li LB, Gao CQ. Sevoflurane postconditioning protects isolated rat hearts against ischemia-reperfusion injury: the role of radical oxygen species, extracellular signal-related kinases 1/2 and mitochondrial permeability transition pore. Mol Biol Rep. 2010;37:2439-2446. [PubMed] |
| 140. | Tsutsumi YM, Yokoyama T, Horikawa Y, Roth DM, Patel HH. Reactive oxygen species trigger ischemic and pharmacological postconditioning: in vivo and in vitro characterization. Life Sci. 2007;81:1223-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 141. | Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 142. | Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291:H2067-H2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 143. | Sasaki N, Sato T, Ohler A, O’Rourke B, Marbán E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439-445. [PubMed] |
| 144. | Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD, Cohen MV, Downey JM. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol. 2004;286:H468-H476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 180] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 145. | Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 146. | Costa AD, Pierre SV, Cohen MV, Downey JM, Garlid KD. cGMP signalling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res. 2008;77:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 147. | Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, García-Dorado D, Di Lisa F, Schulz R, Heusch G. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res. 2005;97:583-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 148. | Das M, Parker JE, Halestrap AP. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J Physiol. 2003;547:893-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 149. | Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 150. | Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089-2102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 151. | Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 152. | Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, Heusch G, Schulz R. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol. 2009;104:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 153. | Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 154. | Rodriguez-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, Konietzka I, Miró E, Totzeck A, Heusch G. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circ Res. 2006;99:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 155. | Miro-Casas E, Ruiz-Meana M, Agullo E, Stahlhofen S, Rodríguez-Sinovas A, Cabestrero A, Jorge I, Torre I, Vazquez J, Boengler K. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc Res. 2009;83:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 156. | Rottlaender D, Boengler K, Wolny M, Michels G, Endres-Becker J, Motloch LJ, Schwaiger A, Buechert A, Schulz R, Heusch G. Connexin 43 acts as a cytoprotective mediator of signal transduction by stimulating mitochondrial KATP channels in mouse cardiomyocytes. J Clin Invest. 2010;120:1441-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 157. | Li X, Heinzel FR, Boengler K, Schulz R, Heusch G. Role of connexin 43 in ischemic preconditioning does not involve intercellular communication through gap junctions. J Mol Cell Cardiol. 2004;36:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 158. | Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol. 2002;283:H1740-H1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 159. | Totzeck A, Boengler K, van de Sand A, Konietzka I, Gres P, Garcia-Dorado D, Heusch G, Schulz R. No impact of protein phosphatases on connexin 43 phosphorylation in ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2008;295:H2106-H2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 160. | Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am J Physiol. 1999;276:H935-H943. [PubMed] |
| 161. | Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566-H574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 162. | Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 353] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 163. | Dost T, Cohen MV, Downey JM. Redox signaling triggers protection during the reperfusion rather than the ischemic phase of preconditioning. Basic Res Cardiol. 2008;103:378-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 164. | Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem. 2006;281:20801-20808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 165. | Fujita M, Asanuma H, Hirata A, Wakeno M, Takahama H, Sasaki H, Kim J, Takashima S, Tsukamoto O, Minamino T. Prolonged transient acidosis during early reperfusion contributes to the cardioprotective effects of postconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H2004-H2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 166. | Hausenloy DJ, Wynne AM, Yellon DM. Ischemic preconditioning targets the reperfusion phase. Basic Res Cardiol. 2007;102:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 167. | Cohen MV, Downey JM. Ischemic postconditioning: from receptor to end-effector. Antioxid Redox Signal. 2011;14:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 168. | Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207-216. [PubMed] |
| 169. | Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res. 2000;87:460-466. [PubMed] |
| 170. | Das DK, Maulik N, Sato M, Ray PS. Reactive oxygen species function as second messenger during ischemic preconditioning of heart. Mol Cell Biochem. 1999;196:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 171. | Yue Y, Qin Q, Cohen MV, Downey JM, Critz SD. The relative order of mK(ATP) channels, free radicals and p38 MAPK in preconditioning’s protective pathway in rat heart. Cardiovasc Res. 2002;55:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 172. | Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 258] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 173. | Korichneva I. Zinc dynamics in the myocardial redox signaling network. Antioxid Redox Signal. 2006;8:1707-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 174. | Sun J, Steenbergen C, Murphy E. S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal. 2006;8:1693-1705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 175. | Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2009;106:10764-10769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 176. | Penna C, Perrelli MG, Tullio F, Moro F, Parisella ML, Merlino A, Pagliaro P. Post-ischemic early acidosis in cardiac postconditioning modifies the activity of antioxidant enzymes, reduces nitration, and favors protein S-nitrosylation. Pfl¨¹gers Arch. 2011;In press. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 177. | Okado-Matsumoto A, Fridovich I. Putative denitrosylase activity of Cu,Zn-superoxide dismutase. Free Radic Biol Med. 2007;43:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 178. | Sun HY, Wang NP, Halkos M, Kerendi F, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ. Postconditioning attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Apoptosis. 2006;11:1583-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 179. | Shen AC, Jennings RB. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972;67:417-440. [PubMed] |
| 180. | Correa F, García N, Robles C, Martínez-Abundis E, Zazueta C. Relationship between oxidative stress and mitochondrial function in the post-conditioned heart. J Bioenerg Biomembr. 2008;40:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 181. | Paillard M, Gomez L, Augeul L, Loufouat J, Lesnefsky EJ, Ovize M. Postconditioning inhibits mPTP opening independent of oxidative phosphorylation and membrane potential. J Mol Cell Cardiol. 2009;46:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 182. | Argaud L, Gateau-Roesch O, Augeul L, Couture-Lepetit E, Loufouat J, Gomez L, Robert D, Ovize M. Increased mitochondrial calcium coexists with decreased reperfusion injury in postconditioned (but not preconditioned) hearts. Am J Physiol Heart Circ Physiol. 2008;294:H386-H391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 183. | Obame FN, Plin-Mercier C, Assaly R, Zini R, Dubois-Randé JL, Berdeaux A, Morin D. Cardioprotective effect of morphine and a blocker of glycogen synthase kinase 3 beta, SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione], via inhibition of the mitochondrial permeability transition pore. J Pharmacol Exp Ther. 2008;326:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 184. | Elz JS, Nayler WG. Calcium gain during postischemic reperfusion. The effect of 2,4-dinitrophenol. Am J Pathol. 1988;131:137-145. [PubMed] |
| 185. | Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104:181-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 186. | Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: new strategies for cardioprotection. Diabetes Obes Metab. 2008;10:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 187. | Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473-481. [PubMed] |
| 188. | Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet G. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 189. | Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121-1135. [PubMed] |
| 190. | Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, Higashino Y, Fujii K, Minamino T. Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223-228. [PubMed] |
| 191. | Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 404] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 192. | Laskey WK. Brief repetitive balloon occlusions enhance reperfusion during percutaneous coronary intervention for acute myocardial infarction: a pilot study. Catheter Cardiovasc Interv. 2005;65:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 193. | Ma XJ, Zhang XH, Li CM, Luo M. Effect of postconditioning on coronary blood flow velocity and endothelial function in patients with acute myocardial infarction. Scand Cardiovasc J. 2006;40:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 194. | Wei M, Xin P, Li S, Tao J, Li Y, Li J, Liu M, Li J, Zhu W, Redington AN. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res. 2011;108:1220-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 195. | Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 196. | Loukogeorgakis SP, Panagiotidou AT, Yellon DM, Deanfield JE, MacAllister RJ. Postconditioning protects against endothelial ischemia-reperfusion injury in the human forearm. Circulation. 2006;113:1015-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 197. | Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM, Deanfield JE, MacAllister RJ. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation. 2007;116:1386-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 198. | Binbrek AS, Rao NS, Al Khaja N, Assaqqaf J, Sobel BE. Erythropoietin to augment myocardial salvage induced by coronary thrombolysis in patients with ST segment elevation acute myocardial infarction. Am J Cardiol. 2009;104:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 199. | Bullard AJ, Govewalla P, Yellon DM. Erythropoietin protects the myocardium against reperfusion injury in vitro and in vivo. Basic Res Cardiol. 2005;100:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |