INTRODUCTION
The primary task of the cardiovascular system is to deliver enough oxygen to meet the metabolic demands of the body. When it fails to satisfy metabolic demands adequately, shock and tissue hypoxia occur. Sustained tissue hypoxia is one of the most important contributory factors in the pathophysiology of organ dysfunction[1]. Therefore, assessment of the adequacy of global systemic and tissue oxygenation in critically ill patients is indispensable in their treatment[2].
Unfortunately, traditional clinical signs of tissue perfusion that have been used over the last century (capillary refilling, mental status, heart rate, pulse pressure, systemic blood pressure and urine output) are now recognized to be limited in their ability to act as sensitive indicators of tissue perfusion[3-5]. Therefore, detection of compensated shock (the most common presentation of shock) remains a serious challenge. Normalization of these traditional clinical indices after initial resuscitation does not exclude ongoing inadequate tissue perfusion[6]. Over the last two decades, an intense search for a more sensitive monitoring technology has continued[7]. The ideal monitoring modality should provide an indication for the treatment of low flow states and should also act as an ideal assessor of the altered state of oxygen delivery at its earliest stage, even before clinical signs are evident.
Maintenance of adequate oxygen delivery (DO2) is essential to preserve organ function, and sustained low DO2 is a path to organ failure and death[8,9]. DO2 does not influence oxygen consumption (VO2) until it reaches critically low values (DO2crit), when VO2 starts to decrease. It is at this point that VO2 becomes directly dependent on DO2. Tissue extraction of oxygen cannot be increased further to meet tissue demands, and cells begin to convert to mainly anerobic metabolism, as manifested by significant increases in metabolic byproducts, and other cellular entities reflective of this state, such as lactate, NADH, and reduced cytochrome oxidase. Each individual organ has its own biphasic relationship with differing points of DO2crit, which can vary significantly based on the severity of the insult, the system’s metabolic activity, vascular responsiveness to a myriad of mediators, and the type of insult.
Low cardiac output states (cardiogenic, hypovolemic and obstructive types of shock) and anemic and hypoxic hypoxemia are characterized by a decreased DO2 but preserved oxygen extraction ratio (OER = VO2/DO2) so that DO2crit remains normal. In distributive shock (septic shock), the oxygen extraction capability is altered so that the critical OER is typically decreased. These situations are typically associated with an increased DO2crit, and VO2 can become dependent on DO2 even when the latter is normal or elevated. Central venous (ScvO2), and mixed venous oxygen saturation (SvO2) are used to estimate the OER as an indicator of global tissue oxygenation.
Regional perfusion changes can occur significantly earlier than traditional global indices[10]. Examples of technologies which may take advantage of regional changes, and which may help identify these states, include transcutaneous pO2 and pCO2, subcutaneous and interstitial pH, pCO2, and pO2 measurements, gastric and sublingual tonometry, and near-infrared absorption spectroscopy (NIRS)[11].
The technique of NIRS is exciting because it potentially provides noninvasively-derived information concerning all of the major components of oxygen transport, ranging from bulk transport of oxygen to its cellular utilization at the level of the mitochondria. This paper will review the principles of NIRS and its application in assessing global systemic and tissue (skeletal muscle) oxygenation in heart failure/cardiogenic shock and septic shock.
PRINCIPLES OF NEAR INFRARED SPECTROSCOPY
NIRS research for measuring tissue properties is not new[11]. The technique was pioneered by Millikan who developed a dual wavelength oximeter for muscle, and Jobsis who was the first to note the differential spectral absorption of hemoglobin (Hb) and the mitochondrial enzyme cytochrome oxidase or cytochrome a, a3 (CtOx) in vivo with NIR transillumination[12,13]. Visible light (450-700 nm) penetrates tissue only a short distance because of strong attenuation by various tissue components. In the NIR spectrum (700-1100 nm), however, photons are capable of deeper penetration (several centimeters or more), even through bone. It is also within this spectral region that oxygen-dependent electronic transitions of the metalloproteins Hb, myoglobin and CtOx can be detected. These metalloproteins act as chromophores and absorb NIR radiation differently based on their concentration and interaction with oxygen. The Beer-Lambert law provides the physical and mathematical basis for NIRS. This law states that light passing through a solution of a colored compound (chromophore) is absorbed by the compound, resulting in a reduction in the intensity of the emerging light[14].
It should be noted now that NIRS differs from pulse oximetry in several important ways. These include the use of different (red) and fewer wavelengths of light in pulse oximetry and the need for pulsatile flow in pulse oximetry. Pulse oximetry is based on the assumption that the only pulsatile absorbance between the photodetector and light source is arterial blood.
HEMOGLOBIN
It is the electronic transition of the heme molecule of Hb and its response to the presence of oxygen that is responsible for its differential absorption of NIR radiation.
The basis for the use of NIRS to monitor changes in Hb and HbO2 to monitor states of tissue oxygenation lies in the tissue compartmentalization of blood volume, which in most organ systems is believed to be in a ratio of 10:20:70 among the arteriolar, capillary, and venular compartments, respectively[15,16]. Consequently, the majority of the NIRS signal is believed to reflect the venous or post-extraction compartment of any particular tissue. This phenomenon provides valuable information on the tissue oxygen consumption or extraction in much the same way as mixed venous Hb oximetry is used with the pulmonary artery catheter. The NIR value of Hb oxygen saturation from the tissue (StO2) thus represents spatially integrated information from arterioles, capillaries, and venules, which are normally weighted towards the venous compartment. Larger vessels (> 1 mm) are assumed to be excluded from StO2 determination[17].
CYTOCHROME OXIDASE
Mitochondrial cytochrome oxidase (CtOx) is responsible for more than 90% of cellular oxygen consumption (reduction) in its role in producing ATP. Although the enzyme contains four metal centers (CuA, heme a, CuB, and heme a3), it is the CuA center that has the strongest absorbance in the NIR region (830 nm), and is responsible for over 80% of the spectral changes in this region[18]. CuA is a unique Cu-Cu dimer, which is a one-electron acceptor - a donor that accepts an electron from cytochrome c and mediates its transfer[18]. Hb NIR absorbance is directly proportional to oxygen binding to its metal centers, but NIR changes in the CtOx signal are due to a decrease in electron flow, a consequence of insufficient oxygen in the environment. Reduction of CtOx results in the disappearance of its 830 nm absorption band. These facts are largely responsible for the significant challenges in monitoring and quantifying changes in the CtOx redox state, except from a baseline state. This overall absorption signal will represent only the immediate steady-state contribution of both the oxidized and reduced form of the enzyme within the volume of tissue being sampled, but again, can only be compared with the changes preceding or following it (trend monitoring) without knowing if the baseline state was normal to begin with. Thus to date, it has only been possible to calculate the percentage of the oxidized vs reduced form of the enzyme in animal models where the enzyme is transiently totally reduced (anoxia), the total enzyme concentration is known, and the optical path length is measured. More NIRS studies coupled with the use of nuclear magnetic resonance (which can determine the onset of intracellular dysoxia and the oxygenation status of Hb and myoglobin) should be performed to enhance our understanding of the ability of NIRS to accurately perform as a precise monitor of tissue oxygen transport[19].
CLINICAL EVALUATION
The use of NIRS is an attempt to move beyond the common physical examination in order to avoid tissue dysoxia. Thus, determination of regional StO2 might provide an early warning of global hypoperfusion prior to significant alterations in vital signs or DO2crit. Additionally, it would allow clinicians to guide resuscitations better and reduce periods with occult tissue hypoxia. Changes in StO2 would provide an earlier warning sign of impending dysoxia and would help the clinician ensure that oxygen delivery to the tissue had been restored to a level well above that required to simply reverse dysoxia. However, it may not be obvious of what value the monitoring of the CtOx redox state should be. As expected, the regional CtOx redox state has been shown to correlate strongly with regional VO2 (r2 = 0.9) only when VO2 becomes dependent on DO2 (below DO2crit)[20]. When both StO2 and CtOx redox statuses have been studied together in organs not containing myoglobin, changes in StO2 occurred first in response to reductions in DO2, and were restored last on restitution of regional DO2[21].
NIRS OF SKELETAL MUSCLE IN HEART FAILURE AND CARDIOGENIC SHOCK WITH OR WITHOUT SEVERE SEPSIS/SEPTIC SHOCK
Measurement of SvO2 from the pulmonary artery is used for calculations of oxygen consumption and has been advocated as an indirect index of tissue oxygenation and a prognostic predictor in critically ill patients[22-25]. However, catheterization of the pulmonary artery is costly, has inherent risks and its usefulness remains under debate[26,27]. In a recently published paper, we studied skeletal muscle StO2 in severe left heart failure with or without additional severe sepsis/septic shock, and compared it with SvO2[28]. The hypothesis was that skeletal muscle StO2 could estimate SvO2 in patients with severe left heart failure and preserved oxygen extraction capability (without severe sepsis/septic shock), because blood flowing through upper limb muscles could make an important contribution to flow through the superior vena cava. On the other hand, in patients with a decreased oxygen extraction capability (with severe sepsis/septic shock), we expected disagreement between StO2 and SvO2, because in these patients, higher oxygen extraction can probably take place in other organs different from skeletal muscles.
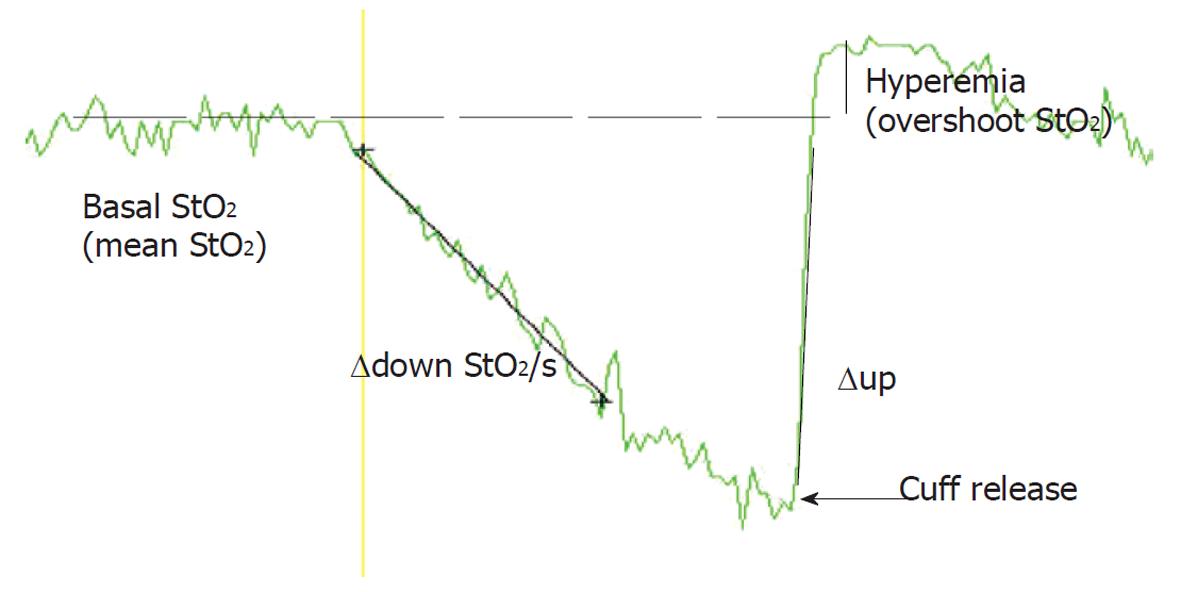
The results confirmed the hypothesis that skeletal muscle StO2 does not estimate SvO2 in patients with severe left heart failure and additional severe sepsis or septic shock. However, in patients with severe left heart failure without additional severe sepsis or septic shock, StO2 values could be used for fast non-invasive SvO2 estimation; and the trend of StO2 may be substituted for the trend of SvO2. The results were in concordance with our previous report of high StO2 and the slow deceleration rate of StO2 during stagnant ischemia in septic patients[29]. In this study we used the vascular occlusion test to study dynamic changes in skeletal muscle StO2. Upper limb ischemia was induced by rapid automatic pneumatic cuff inflation around the upper arm (Figure 1). During the vascular occlusion test, several StO2 parameters can be studied: mean StO2 before arterial cuffing/occlusion; StO2 downslope during cuffing-the deoxygenation rate (∆down StO2/s); StO2 upslope (∆up StO2/s); hyperemia (overshoot of StO2 above baseline). The deoxygenation rate is a surrogate for tissue oxygen consumption.
Figure 1 Vascular occlusion test: An original thenar tissue oxygen saturation (StO2) recording after arterial upper arm cuffing, and cuffing release (upper arm ischemia reperfusion test).
During the upper arm ischemia reperfusion test, several StO2 parameters can be studied: average StO2 before arterial cuffing/occlusion; StO2 downslope during cuffing-the deoxygenation rate (∆down StO2)/s); StO2 upslope (∆up StO2)/s); hyperemia (overshoot of StO2 above baseline).
Our group confirmed that thenar muscle tissue deoxygenation during stagnant ischemia at admission and after hemodynamic stabilization was significantly slower in septic shock compared with that in severe sepsis, localized infection and healthy controls. The rate of StO2 decrease correlated strongly with the severity of septic shock (Sequential Organ Failure Assessment score) and weakly with norepinephrine requirement, plasma lactate and C-reactive protein concentrations. The muscle tissue deoxygenation rate increased with improvement in sepsis in the septic shock and severe sepsis group.
Our results were in accordance with those reported in a baboon septic shock model[30]. In these primates, the NIRS-determined rate of skeletal muscle enzyme CtOx a, a3 reduction during stagnant ischemia was decreased in Gram-negative septic shock. These data were interpreted as being consistent with the presence of a defect in the ability of the enzyme to accept electrons from oxygen or a limitation in the availability of the reducing equivalent. Similar results were reported in the dog gracilis muscle preparation after treating the animals with endotoxin[31].
The high StO2/low SvO2 seen in severe sepsis and septic shock, suggest blood flow redistribution. Thenar muscle StO2 probably correlates with ScvO2 which is measured in the mixture of blood from head and both arms. In healthy resting individuals ScvO2 is slightly lower than SvO2[32]. Blood in the inferior vena cava has a high oxygen content because the kidneys do not utilize much oxygen but receive a high proportion of the cardiac output[33]. As a result, inferior vena cava blood has a higher oxygen content than blood from the upper body, and SvO2 is greater than ScvO2.
This relationship changes in periods of cardiovascular instability. Scheinman and co-workers performed the earliest comparison of ScvO2 and SvO2 in both hemodynamically stable and shocked patients[34]. In stable patients, ScvO2 was similar to SvO2. In patients with a failing heart, ScvO2 was higher than SvO2, and in patients with shock the ratio of SvO2 to ScvO2 was greater (47.5% ± 15.11% vs 58.0% ± 13.05%, respectively, P < 0.001). Lee and co-workers described similar findings[35]. Other more detailed studies in mixed groups of critically-ill patients designed to test if the ScvO2 measurements could substitute for SvO2 showed problematically large confidence limits[36], and poor correlations between the two values[37].
Most writers attributed this pattern to changes in the distribution of cardiac output that occur in periods of hemodynamic instability. In shock states, blood flow to the splanchnic and renal circulations drops, while the flow to the heart and brain is maintained[38]. This results in a drop in oxygen content of blood in the inferior vena cava. As a consequence, in shock states the normal relationship is reversed and ScvO2 is greater than SvO2[34-36]. Consequently, when using ScvO2 or probably StO2, as a treatment goal, relative oxygen consumption of the superior vena cava system may remain stable at a time when oxidative metabolism of vital organs, such as the splanchnic region, may reach a level where flow-limited oxygen consumption is achieved, together with a marked decrease in oxygen saturation. In this situation, StO2 provides a false favorable impression of adequate body perfusion, because of the inability to detect organ ischemia in the lower part of the body. A recent paper confirmed our hypothesis of the relationship between StO2 and invasive oxygen delivery measurements in early under-resuscitated septic shock[39].
The hypothesis that the skeletal muscle StO2 deoxygenation rate correlates (or is inversely proportional) to the ScvO2-SvO2 difference in patients with severe heart failure with additional sepsis/septic shock was confirmed by our recent study[40]. We also showed that these patients had a clinically considerable ScvO2-SvO2 discrepancy. Monitoring ScvO2 is a simpler and cheaper method of assessing the global DO2 to oxygen consumption ratio, but its use as a treatment monitor in patients with severe heart failure with additional sepsis/septic shock is questionable.
Our data in patients with severe heart failure/cardiogenic shock without severe sepsis/septic shock are supported by previous work of Boekstegers et al[41], who measured the oxygen partial pressure distribution in the biceps muscle. They found low peripheral oxygen availability in cardiogenic shock compared with sepsis. In cardiogenic shock, skeletal muscle oxygen partial pressure correlated with systemic oxygen delivery (r = 0.59, P < 0.001) and systemic vascular resistance (r = 0.74, P < 0.001). No correlation was found between systemic oxygen transport variables and skeletal muscle partial oxygen pressure in septic patients. These measurements were performed in the most common cardiovascular state of sepsis in contrast to hypodynamic shock, which is only present at the very final stage of sepsis or in patients without adequate volume replacement[42]. In the following study, the same authors showed that even in the final state of hypodynamic septic shock, leading to death, mean muscle partial oxygen pressure did not decrease to < 4.0 kPa before a circulatory standstill[41].
In a human validation study, a significant correlation between NIRS-measured StO2 and venous oxygen saturation (r = 0.92, P < 0.05) was reported, where the venous effluent was obtained from a deep forearm vein that drained the exercising muscle[43]. StO2 was minimally affected by skin blood flow. Changes in limb perfusion affect StO2: skeletal muscle StO2 decreases during norepinephrine infusion and increases during nitroprusside infusion.
StO2 overestimated SvO2 (bias -2.5%) in severe heart failure without severe sepsis/septic shock in our study[28]. This may be due to the NIRS method, which does not discriminate between compartments. It provides a global assessment of oxygenation in all vascular compartments (arterial, venous and capillary) in the sample volume of underlining tissue. The non-invasive measurement of only venous oxygen saturation is complicated by the fact that the isolation of the contribution of the venous compartment to the non-invasive optical signal is not straightforward. New methods, such as NIR spiroximetry, which measures venous oxygen saturation in tissue from the NIR spectrum of the amplitude of respiration-induced absorption oscillations, may lead to the design of a non-invasive optical instrument capable of providing simultaneous and real-time measurements of local arterial, tissue and venous oxygen saturation[1].
In low flow states such as heart failure/cardiogenic shock, where there are still controversies on how to monitor them[45], it appears logical to combine data of both macro- and micro-circulation parameters to guide resuscitation[46]. A large prospective study is being performed now to evaluate the possibility of additional StO2 regional monitoring for tissue oxygenation guidance on top of the early goal directed therapy suggested by Rivers et al[47].
CONCLUSION
The present review provides a foundation to understand and evaluate the potential value and limitations of NIRS as a tool in the assessment of critically ill patients. Despite continuing technical controversies concerning signal derivation, accuracy, precision, and quantitative ability, skeletal muscle NIRS clearly demonstrates promise in being able to monitor the balance of oxygen delivery and consumption at the end-organ level in severe heart failure or cardiogenic shock. It is also possible to estimate global oxygenation in low cardiac output states without additional severe sepsis or septic shock. However, a significant amount of additional work may be required to determine: (1) the contribution of myoglobin to the StO2 signal in humans, if skeletal muscle is the end-organ being monitored; (2) quantification of the CtOx redox signal; (3) definitive determination of the etiology of decoupling of the StO2 and CtOx redox NIRS signals in multisystem organ failure; (4) interpretation of the ischemia reperfusion test; and (5) assessment of whether there are significant advantages in monitoring one organ system over another in cases of shock. Many of these issues should be addressed before NIRS comes into widespread use as a tool in the initial evaluation and treatment of these severely ill patients.
ACKNOWLEDGMENTS
We thank the doctors, the nurses, and the technical support staff at the Centre for Internal Intensive Care Medicine, University Medical Centre, Ljubljana, and Department for Internal Intensive Care Medicine, General and Teaching Hospital, Celje, for their assistance in different studies which have resulted in the current review.
Peer reviewer: Alberto Dominguez-Rodriguez, MD, PhD, FESC, Department of Cardiology, University Hospital of Canarias, Ofra s/n La Cuesta, La Laguna, E-38320, Tenerife, Spain
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM