Published online Nov 26, 2011. doi: 10.4330/wjc.v3.i11.367
Revised: September 11, 2011
Accepted: September 17, 2011
Published online: November 26, 2011
AIM: To investigate the impact of dual antiplatelet therapy (DAT) in patients on anti-vitamin K (AVK) regimen requiring percutaneous coronary intervention (PCI).
METHODS: Between February 2006 and February 2008, 138 consecutive patients under chronic AVK treatment were enrolled in this registry. Of them, 122 received bare metal stent implantation and 16 received drug eluting stent implantation. The duration of DAT, on top of AVK treatment, was decided at the discretion of the clinician. Adequate duration of DAT was defined according to type of stent implanted and to its clinical indication.
RESULTS: The baseline clinical characteristics of patients reflect their high risk, with high incidence of comorbid conditions (Charlson score ≥ 3 in 89% of the patients). At a mean follow-up of 17 ± 11 mo, 22.9% of patients developed a major adverse cardiac event (MACE): 12.6% died from cardiovascular disease and almost 6% had an acute myocardial infarction. Major hemorrhagic events were observed in 7.4%. Adequate DAT was obtained in only 44% of patients. In the multivariate analysis, no adequate DAT and Charlson score were the only independent predictors of MACE (both P = 0.02).
CONCLUSION: Patients on chronic AVK therapy represent a high risk population and suffer from a high MACE rate after PCI. An adequate DAT regimen and absence of comorbid conditions are strongly associated with better clinical outcomes.
- Citation: Brugaletta S, Martin-Yuste V, Ferreira-González I, Cola C, Alvarez-Contreras L, Antonio MD, Garcia-Moll X, García-Picart J, Martí V, Balcells-Iranzo J, Sabaté M. Adequate antiplatelet regimen in patients on chronic anti-vitamin K treatment undergoing percutaneous coronary intervention. World J Cardiol 2011; 3(11): 367-373
- URL: https://www.wjgnet.com/1949-8462/full/v3/i11/367.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i11.367
Currently, the optimal antiplatelet regimen after stent implantation consists of a combination of aspirin and ADP-receptor antagonists, such as ticlopidine or clopidogrel, for the prevention of stent thrombosis[1,2]. The recommended duration of dual antiplatelet therapy (DAT) ranges from 4 wk following bare metal stent (BMS) implantation during elective angioplasty to at least 6 to 12 mo after drug eluting stent (DES) implantation. However, in some clinical situations, such as acute coronary syndromes (ACS), it should be extended to up to 12 mo with either BMS or DES[3].
The antiplatelet regimen of patients on long-term anti-vitamin K (AVK) treatment and who receive percutaneous coronary intervention (PCI) remains a challenge for the interventional cardiologist. This group of patients, indeed, includes a rather old population with comorbidities and a high risk of bleeding and cardiovascular events. Moreover, the risk of bleeding in these patients is increased by the addition of DAT but, on the other hand, a temporary discontinuation of anticoagulation may be associated with a high risk of thromboembolism[4].
The aim of this study was to evaluate the outcomes of patients under AVK therapy receiving PCI and to assess the relationship between duration of DAT, in addition to an AVK regimen, and clinical outcomes.
Between February 2006 and February 2008 consecutive patients with an absolute indication for AVK treatment and who required PCI were prospectively included in the registry. There were no specific exclusion criteria. Patients were treated at the discretion of the interventional cardiologist, either with BMS or DES. The duration of DAT was variable, according to the clinician’s prescription. Those patients who discontinued AVK treatment after index PCI were excluded from the analysis. Clinical and angiographic characteristics of all patients were prospectively recorded. In order to determine comorbidity in the patients, the Charlson score was calculated. Briefly, the Charlson score is the sum of 19 pre-defined comorbidities, that were assigned weights of 1, 2, 3 or 6 (Table 1). Overall, a value ≥ 3 defines a high level of comorbidity[5].
| Comorbid condition | Weight |
| Myocardial infarction | 1 |
| Congestive heart failure | 1 |
| Peripheral vascular disease | 1 |
| Cerebrovascular disease | 1 |
| Dementia | 1 |
| Chronic pulmonary disease | 1 |
| Connective tissue disease | 1 |
| Ulcer disease | 1 |
| Mild liver disease | 1 |
| Diabetes | 1 |
| Hemiplegia | 2 |
| Moderate or severe renal disease | 2 |
| Diabetes with end-organ damage | 2 |
| Any tumor | 2 |
| Leukemia | 2 |
| Lymphoma | 2 |
| Moderate or severe liver disease | 3 |
| Metastatic solid tumor | 6 |
| AIDS | 6 |
Stent implantation was performed according to the experience of the interventional cardiologist following standard guidelines. Either direct stent implantation or balloon pre-dilatation was allowed. Glycoprotein IIb/IIIa
inhibitor administration was left to the interventional cardiologist’s discretion. All patients were treated with a therapeutic dose of aspirin at the time of PCI (100 mg daily from at least 5 d before or 500 mg administered intravenously immediately before the procedure). Clopidogrel was administered in a loading 300 mg dose immediately before or after the procedure. As a routine strategy in our center, a radial approach was preferentially used in patients under AVK treatment. AVK therapy was not discontinued as long as the radial artery was patent with good collateral filling of the palmar arch from the ulnar artery (tested by the Allen maneuver or by plethysmography), and the international normalized ratio (INR) was lower than 4.0 at the time of the procedure. No additional anticoagulation was administered during PCI in these patients[6]. In the event that the radial artery could not be used for the above-mentioned reasons, AVK treatment was discontinued and substituted by a weight-adjusted dose of low molecular weight heparin (LMWH). Whenever INR was normalized, the procedure was performed via femoral artery. In such patients, PCI was performed under LMWH. AVK treatment was reinitiated within 24 h after the procedure while LMWH was finally discontinued as soon as the INR level rose above 2.0.
The outcomes of the patients were assessed by measuring the rates of major adverse cardiac events (MACE), including cardiac death, myocardial infarction or target lesion revascularization. Death was classified as cardiac and non-cardiac. Any death was considered cardiac unless a non-cardiac cause could be adjudicated.
Peri-procedural myocardial infarction was defined as a rise in the troponin T level above the upper reference limit. Acute myocardial infarction was defined as appearance of unequivocal ECG changes together with an increase in creatine kinase levels to at least twice the upper normal limit or an increase in troponin levels[7].
Any revascularization clinically indicated performed on the treated segment was defined as target lesion revascularization. Stent thrombosis was defined and categorized according to Academic Research Consortium, into early (within 30 d), late (more than 30 d to 1 year after stent implantation) and very late (more than 1 year after stent implantation) and into definitive, probable and possible[8].
Cerebrovascular events, including stroke (ischemic or hemorrhagic), cerebrovascular hemorrhage, transient ischemic attack, or reversible ischemic neurological deficits were diagnosed by a neurologist and confirmed by computed tomography scanning.
Bleeding complications were divided into minor and major, according to the TIMI scale[9]. Major bleeding was defined as the occurrence of intracranial or retroperitoneal bleeding, hemorrhage at the vascular access site requiring intervention, a reduction in hemoglobin levels of at least 5 g/dL, reoperation for bleeding or transfusion of a blood product (at least 2 U), or bleeding causing substantial hypotension requiring the use of inotropic agents. All other bleeding events were considered minor (e.g., epistaxis).
To specifically determine the relationship between duration of DAT and outcomes in this high risk population, we defined the adequate duration of DAT, according to the clinical setting and the type of stent implanted, as 1 mo for patients with an elective clinical condition who received a BMS, and 1 year for patients admitted for ACS, regardless of the stent implanted[3,10].
Clinical follow-up was performed by clinical visit or telephone interview. The duration of DAT was specifically investigated by interview with the patient or the doctor who was in charge of the patient. Angiographic follow-up was not mandated unless it was clinically indicated by symptoms or a positive non-invasive test of ischemia.
Continuous variables are presented as mean ± SD and categorical variables as counts and percentage. Continuous variables were compared using the independent sample Student t test or Mann-Whitney U test where appropriate. Categorical variables were compared with the Chi-square or Fisher exact test where appropriate.
Cumulative MACE-free survival was illustrated by the Kaplan-Meier method. We explored the adjusted independent predictors of MACE using Cox regression modeling. We specifically considered, as potential predictors of MACE and thus candidates to enter into the model, the following variables: clinical presentation as ACS, no adequate DAT, Charlson Comorbidity Index and type of stent implanted (BMS or DES). The Charlson Comorbidity Index represents a single aggregate measure of a patient’s risk due to comorbid conditions and it has been well validated[11]. The proportional hazards assumption was tested to explore the time-dependence of covariates all at once. The results are presented as hazard ratio (HR) with 95% confidence interval (CI) and P value. Statistical significance was accepted for a two-sided value of P < 0.05. Analysis was performed with SPSS version 13 (SPSS Inc., Chicago, IL, United States).
Baseline clinical characteristics are summarized in Table 2. During the study period, 138 consecutive patients (178 lesions) on chronic AVK treatment were treated by PCI with either BMS (88%) or DES (12%). All patients were maintained on AVK treatment during follow-up. Mean age was 70.7 ± 9.3 years, 30% of patients were diabetics, almost 22% had renal impairment, 33% had previous acute myocardial infarction and 89% had a Charlson score ≥ 3. Of the total, 57% were admitted with ACS.
| n (%) | |
| Age (mean ± SD, yr) | 70.7 ± 9.3 |
| Female | 29 (21.0) |
| Hypertension | 95 (68.8) |
| Diabetes mellitus | 44 (31.8) |
| Dyslipidemia | 73 (52.9) |
| Smoking | 34 (24.6) |
| Renal failure | 30 (21.7) |
| Previous stroke | 25 (18.1) |
| Vasculopathy | 22 (15.9) |
| Previous coronary artery bypass graft | 32 (23.1) |
| Previous percutaneous coronary intervention | 21 (15.2) |
| Previous myocardial infarction | 46 (33.3) |
| Charlson score (mean ± SD) | 4.3 ± 1.6 |
| Clinical presentation | |
| Asymptomatic | 12 (8.7) |
| Stable angina | 45 (32.6) |
| NSTEMI | 58 (42.0) |
| STEMI | 21 (15.2) |
| Indication to AVK treatment | |
| Atrial fibrillation | 93 (67) |
| CHADS2 0 | 0 (0) |
| CHADS2 1 | 69 (75) |
| CHADS2 ≥ 2 | 24 (25) |
| Pulmonary emboli | 4 (2.9) |
| Systemic emboli | 4 (2.9) |
| Isolated cardiomyopathy | 14 (10.1) |
| Mechanical valve prosthesis | 22 (15.2) |
| Thrombus in left ventricle | 2 (1.4) |
| Number of diseased vessels | |
| 1 | 79 (57.2) |
| 2 | 41 (29.7) |
| 3 | 16 (11.6) |
| Number of treated vessels | |
| 1 | 118 (85.5) |
| 2 | 20 (14.5) |
| Vessels diseased | |
| Left main | 13 |
| Left anterior descending | 85 |
| Left circumflex | 52 |
| Right coronary artery | 69 |
| Saphenous vein graft | 4 |
Angiographic and procedural data are summarized in Table 3. A total of 178 lesions were treated with 222 stents implanted (196 BMS; 26 DES). Almost 75% of PCI were performed though a radial approach and 25% of stents were implanted without pre-dilatation. No closure devices were used in patients in whom the procedure was performed through a femoral approach. At the time of PCI, the mean INR value was 1.7 ± 0.6.
| Lesion type classification | |
| A | 40 (23.1) |
| B | 103 (59.5) |
| C | 25 (17.4) |
| Chronic total occlusion | 9 (5.2) |
| Bifurcation | 30 (17.3) |
| Access site | |
| Transradial | 133 (74.8) |
| Transfemoral | 52 (25.2) |
| Direct stenting | 45 (25.3) |
| DES | 26 (14.6) |
| Length of implanted stents (mm, mean ± SD) | 22.3 ± 11.6 |
| Diameter of implanted stents (mm, mean ± SD) | 3.18 ± 1.24 |
| QCA analysis (mean ± SD) | |
| Reference diameter pre (mm) | 2.9 ± 0.67 |
| Reference diameter post (mm) | 3.0 ± 0.55 |
| MLD pre (mm) | 0.74 ± 0.5 |
| MLD post (mm) | 2.6 ± 0.5 |
| % stenosis pre | 74.6 ± 16.1 |
| % stenosis post | 12.3 ± 8.9 |
| Acute gain (mm) | 1.8 ± 0.6 |
At follow-up, 62 patients (44%) had adequate duration of DAT (12% of the patients with ACS and 89% of the patients without ACS). Mean duration of DAT was 3.1 ± 6.2 mo (BMS 2.2 ± 4.0 mo, DES 11.1 ± 10.3 mo). Specifically, in patients receiving BMS (n = 122) implantation, an adequate DAT regimen was obtained in 53 patients (43%). In the remaining 69 patients, 5 patients did not receive DAT at discharge (2 patients received only aspirin, one patient received clopidogrel alone, and 2 patients did not receive any antiplatelet agent at all) and 64 stopped DAT prematurely. In patients receiving DES implantation (n = 16), an adequate DAT regimen was obtained in 9 patients, while the remaining 7 stopped DAT prematurely. Overall, the reasons for stopping DAT prematurely included: medical decision (n = 55), clinical event (n = 8) and own patient decision (n = 8).
During hospitalization, 3 patients (1.7%) died from refractory heart failure related to their baseline clinical condition. One patient (0.07%) suffered from a peri-procedural myocardial infarction, due to occlusion of a side branch. No cases of definitive or probable stent thrombosis were recorded. Five patients (3.6%) suffered from major hemorrhagic events during hospitalization. Specifically, we recorded 2 femoral pseudoaneurysms, 2 gastrointestinal hemorrhages and one patient had red blood cell transfusion for chronic anemia and blood loss during cardiac catheterization. In all these patients a femoral approach was used. No hemorrhagic events were observed in patients with the transradial approach.
Complete follow-up was available for 132 patients (98%). Mean clinical follow-up was 17 ± 11 mo. Long-term outcomes are summarized in Table 4.
| n (%) | |
| Major adverse cardiac events | 31 (22.9) |
| Death | 21 (15.5) |
| Cardiac death | 17 (12.6) |
| Non-cardiac death | 4 (2.9) |
| Myocardial infarction | 8 (5.9) |
| Target lesion revascularization | 15 (11.1) |
| Major hemorrhagic event | 10 (7.4) |
| Stroke | |
| Hemorrhagic stroke | 3 (2.2) |
| Ischemic stroke | 2 (1.5) |
| Definite/probable stent thrombosis | 6 (4.4) |
| Possible stent thrombosis | 8 (5.9) |
Overall, a total of 21 patients (15.5%) died after discharge during clinical follow-up. Of these, cardiovascular death accounted for 17 patients (12.6%). The clinically-driven target lesion revascularization rate was 11.1% and incidence of myocardial infarction was 5.9%. The MACE rate was 3.0% in the first month, 9.8% in the first 3 mo and 14.3% in the first 6 mo after PCI.
We recorded 6 definitive or probable stent thromboses (4.4%). In particular, one of the two definitive stent thrombosies occurred 1 mo after BMS implantation in a patient who was on DAT and AVK treatment. The second definitive stent thrombosis occurred 40 d after the index procedure in a patient who received a BMS and was only on AVK treatment without any antiplatelet agent. The rate of possible stent thrombosis was 5.9%.
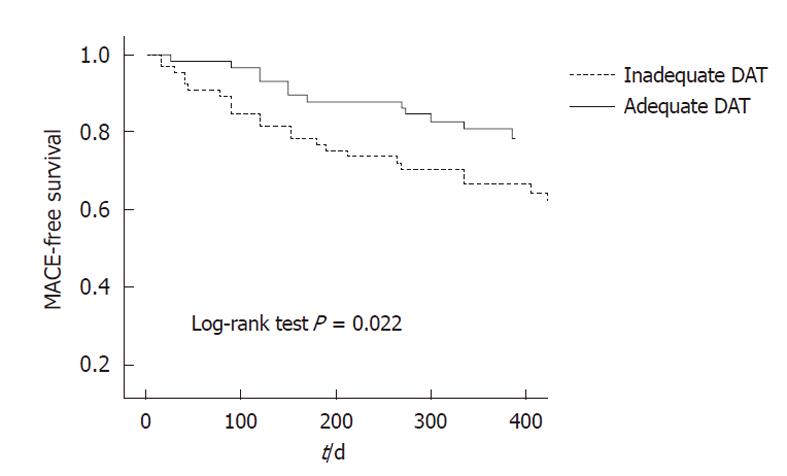
After categorizing the entire population by the duration of DAT, as being adequate or not, the rate of MACE was higher in those patients with no adequate duration of DAT (log-rank test, P = 0.029). In the multivariable analysis, the only variables independently associated with MACE were no adequate DAT (HR, 5.30, 95% CI: 1.19-23.55, P = 0.02) and Charlson Comorbidity Index (HR, 1.6, 95% CI; 1.06-2.42, P = 0.02). Figure 1 presents the cumulative MACE-free survival according to adequate DAT status, adjusted for Charlson Comorbidity Index (P = 0.022).
Regarding hemorrhagic events, there were 10 major hemorrhagic episodes (7.4%) during follow-up. In particular, 3 patients died of a hemorrhagic stroke, 3 required red blood cell transfusion, 3 had a gastrointestinal hemorrhage and one had hematuria, requiring red blood cell transfusion. One of the hemorrhagic strokes occurred in a patient who received a BMS, was on aspirin and the INR was above 9. Only half of major hemorrhagic events (n = 5) occurred in patients on concomitant treatment with DAT and AVK. No difference was found in hemorrhagic event rates between patients categorized according to adequate DAT or not.
The major findings of this analysis are: (1) patients on a chronic anticoagulation regimen represent a highly comorbid population, as shown by a high value of the Charlson score (mean, 4.3) and had a high incidence of MACE after PCI; and (2) no adequate DAT and a high Charlson score seem to be the main independent predictors of events in this population.
Patients who have clear evidence-based indications for long-term AVK treatment represent nearly 10% of patients referred for PCI[12]. This group of patients generally includes a high-risk profile population with comorbid conditions and high risk of ischemic and bleeding events. The Charlson score, an index of their comorbidities, was high in our population (≥ 3 in 89% of the patients) and was one of the independent predictors of MACE at follow-up. The high MACE rate at follow-up (22.9%) was similar to that found by Rogacka et al[13] who compared the long-term outcomes in a similar cohort of patients (23.6%). In another study of 426 patients with atrial fibrillation undergoing PCI, Ruiz-Nodar et al[14] demonstrated a MACE rate of 36.6% and a major bleeding incidence of 12.3% at 2 years.
DAT on top of AVK treatment in this category of patients is known to increase their risk of bleeding[4]. For this reason, in daily practice, various antithrombotic combinations were used in the past[13-15]. Karjalainen et al[12] assessed the safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. The rate of stent thrombosis was highest (15.1%) in patients receiving a warfarin plus aspirin combination without the addition of clopidogrel. This is in agreement with the results of 4 randomized trials (ISAR, FANTASTIC, STARS, and MATTIS) that showed that warfarin plus aspirin combination after PCI is not as effective as DAT in preventing stent thrombosis[16-18]. Recently, Lip et al[10] defined a consensus on the “best practice” antiplatelet regimen of patients who are on concomitant treatment with long-term AVK for atrial fibrillation, according to the clinical indication of PCI (ACS or not) and the type of stent implanted (DES or BMS). Our analysis, applying this definition of adequate DAT to a wider population requiring AVK not only for atrial fibrillation, confirms the importance of ensuring a correct DAT according to the clinical scenario of the patient and to the stent implanted. Of note, for those patients with atrial fibrillation and a CHADS2 score of 1, the benefit of AVK treatment vs aspirin alone is not well established; a large randomized trial would be required to justify withdrawing of the AVK regimen in these patients when they received a PCI.
Most of the concerns about a prolonged duration of DAT on top of AVK treatment come from the occurrence of hemorrhagic events or local complications at the site of arterial access. A femoral approach has been associated with higher hemorrhagic complications in AVK patients, as was confirmed in our study, while a radial approach is safe[19]. In the series from Orford et al[20] and Karjalainen et al[12], the incidence of all major bleeding events while on triple therapy (DAT plus warfarin) ranged from 3.1% to 6.6% during follow-up. In our population, the occurrence of major hemorrhagic events during triple therapy was 7.2%, but it could be underestimated due to a lack of complete compliance to DAT.
The type of stent that has to be implanted in a patient on AVK therapy is another important issue to consider; the clinician should weigh the potential bleeding risk derived from prolonged DAT against the risk and consequences of restenosis of a BMS. In many instances, the balance between risk and benefit may lead the clinician to choose a BMS in order to commit the patient to a short course of DAT in addition to warfarin, as reported in our cohort where 88% of the stents implanted were BMS. Ruiz-Nodar et al[21] showed that the routine use of DES in patients with atrial fibrillation did not seem to be justified because of the higher risk of major bleeding with DES in comparison with BMS: an alternative is to implant a DES for treatment of very high-risk lesions and to accept a small-to-moderate risk of bleeding. Sarafoff et al[22] also showed that use of some clinical and echocardiographic criteria can help to define the antithrombotic/anticoagulation therapy in patients on chronic AVK undergoing DES implantation.
This was a retrospective study. The sample size of this study did not allow demonstration of differences in efficacy between BMS and DES nor the formulation of reliable recommendations. Thus, conclusions regarding the best stent for restenosis prevention in such a population cannot be drawn from this analysis. The single center registries are often not representative and for this reason we included a real-world population as large as possible.
Due to the period of inclusion of the historical cohort, we could not use the European Society of Cardiology/American Heart Association/American College of Cardiology consensus definition of myocardial infarction[23]. Thus, comparison of myocardial infarction rates between groups was based on the classical World Health Organization definition[7]. This definition, however, has been historically used in most of the studies on AVK patients.
In conclusion, patients on AVK requiring PCI and stent implantation represent a high risk population with a high rate of comorbid conditions. Overall, an appropriate DAT regimen according to the type of stent implanted and to its clinical indication, appears crucial in order to avoid MACE.
Patients who receive anti-vitamin K (AVK) treatment have a high risk of bleeding and of ischemic events. For these reasons, every time that a percutaneous coronary intervention (PCI) with stent implantation is needed, the interventional cardiologist has to balance the increased risk of bleeding by adding dual antiplatelet therapy (DAT) on top of AVK with the high rate of event recurrence. Normal clinical practice implies, indeed, that patients with a high risk of event recurrence receive a drug-eluting stent instead of a conventional bare metal stent. However, the 1-year duration of DAT that is needed in the case of a drug-eluting stent could expose those patients to a high risk of bleeding events.
Few recommendations are currently given for PCI in patients under AVK treatment, either for the type of stent to be implanted (drug-eluting or not) or the duration of DAT.
This study represents a real world scenario for the treatment of such patients, where the duration of DAT and the type of stent implanted is left to the discretion of the clinician. No inclusion or exclusion criteria were used. The adequacy of DAT according to the stent implanted and to the baseline clinical condition has been analyzed and related to long-term events.
The major findings of the study are that patients under long-term AVK treatment represent a highly comorbid population with a high incidence of major adverse cardiac and bleeding events at follow-up. Although it is difficult to specify the duration and quality of DAT associated with an AVK regimen, the absence of adequate DAT according to the stent implanted or to the clinical conditions is the main independent predictor of events and should be keep in mind whenever these patients receive PCI.
AVK drugs are a class of drug which inhibit blood coagulation in order to reduce the probability of ischemic events related to thrombi formation. DAT consists of aspirin plus an ADP-inhibitor and is currently prescribed to all patients who receive stent implantation.
The limitations of the analysis are related to the small number of the patients and to its retrospective nature. Nevertheless, it adds an important piece of information to the number of studies that already provide some guidance for avoidance of premature DAT discontinuation.
Peer reviewers: Rajesh Sachdeva, MD, FACC, FSCAI, Assistant Professor of Medicine, Associate Program Director, Interventional Cardiology Fellowship Program, University of Arkansas for Medical Sciences, Director, Cardiac Catheterization Laboratory, Central Arkansas Veterans Healthcare System, 4301 W. Markham street, #532, Little Rock, AR 72205, United States; Olivier F Bertrand, MD, PhD, Interventional Cardiologist, Quebec Heart-Lung Institute, Associate-Professor, Faculty of Medicine, Laval University, Adjunct-Professor, Department of Mechanical Engineering, McGill University, Research-Scholar, Quebec Foundation for Health Research, EASY Research and Education Fund in Transradial PCI, Supported by Cordis, BMS/Sanofi-Aventis, GE Healthcare, Quebec Heart and Lung Institute Foundation, 2725, Chemin Ste Foy, Quebec City, G1V 4G5, Canada; Tim Süselbeck, MD, FESC, Professor, 1 Department of medicine, Unversity Hospital Mannheim, 68167 Mannheim, Germany
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
| 1. | Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting : the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation. 2000;102:624-629. [PubMed] |
| 2. | Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126-2130. [PubMed] |
| 3. | Silber S, Albertsson P, Avilés FF, Camici PG, Colombo A, Hamm C, Jørgensen E, Marco J, Nordrehaug JE, Ruzyllo W. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J. 2005;26:804-847. [PubMed] |
| 4. | Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903-1912. [PubMed] |
| 5. | Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 2005;20:12-19. [PubMed] |
| 6. | Helft G, Dambrin G, Zaman A, Le Feuvre C, Barthélémy O, Beygui F, Favereau X, Metzger JP. Percutaneous coronary intervention in anticoagulated patients via radial artery access. Catheter Cardiovasc Interv. 2009;73:44-47. [PubMed] |
| 7. | Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation. 1979;59:607-609. [PubMed] |
| 8. | Laskey WK, Yancy CW, Maisel WH. Thrombosis in coronary drug-eluting stents: report from the meeting of the Circulatory System Medical Devices Advisory Panel of the Food and Drug Administration Center for Devices and Radiologic Health, December 7-8, 2006. Circulation. 2007;115:2352-2357. [PubMed] |
| 9. | Rao AK, Pratt C, Berke A, Jaffe A, Ockene I, Schreiber TL, Bell WR, Knatterud G, Robertson TL, Terrin ML. Thrombolysis in Myocardial Infarction (TIMI) Trial--phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11:1-11. [PubMed] |
| 10. | Lip GY, Huber K, Andreotti F, Arnesen H, Airaksinen KJ, Cuisset T, Kirchhof P, Marín F. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary intervention/ stenting. Thromb Haemost. 2010;103:13-28. [PubMed] |
| 11. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [PubMed] |
| 12. | Karjalainen PP, Porela P, Ylitalo A, Vikman S, Nyman K, Vaittinen MA, Airaksinen TJ, Niemelä M, Vahlberg T, Airaksinen KE. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J. 2007;28:726-732. [PubMed] |
| 13. | Rogacka R, Chieffo A, Michev I, Airoldi F, Latib A, Cosgrave J, Montorfano M, Carlino M, Sangiorgi GM, Castelli A. Dual antiplatelet therapy after percutaneous coronary intervention with stent implantation in patients taking chronic oral anticoagulation. JACC Cardiovasc Interv. 2008;1:56-61. [PubMed] |
| 14. | Ruiz-Nodar JM, Marín F, Hurtado JA, Valencia J, Pinar E, Pineda J, Gimeno JR, Sogorb F, Valdés M, Lip GY. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol. 2008;51:818-825. [PubMed] |
| 15. | Lip GY, Karpha M. Anticoagulant and antiplatelet therapy use in patients with atrial fibrillation undergoing percutaneous coronary intervention: the need for consensus and a management guideline. Chest. 2006;130:1823-1827. [PubMed] |
| 16. | Schömig A, Neumann FJ, Walter H, Schühlen H, Hadamitzky M, Zitzmann-Roth EM, Dirschinger J, Hausleiter J, Blasini R, Schmitt C. Coronary stent placement in patients with acute myocardial infarction: comparison of clinical and angiographic outcome after randomization to antiplatelet or anticoagulant therapy. J Am Coll Cardiol. 1997;29:28-34. [PubMed] |
| 17. | Bertrand ME, Legrand V, Boland J, Fleck E, Bonnier J, Emmanuelson H, Vrolix M, Missault L, Chierchia S, Casaccia M. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (fantastic) study. Circulation. 1998;98:1597-1603. [PubMed] |
| 18. | Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, Giambartolomei A, Diver DJ, Lasorda DM, Williams DO. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339:1665-1671. [PubMed] |
| 19. | Karjalainen PP, Vikman S, Niemelä M, Porela P, Ylitalo A, Vaittinen MA, Puurunen M, Airaksinen TJ, Nyman K, Vahlberg T. Safety of percutaneous coronary intervention during uninterrupted oral anticoagulant treatment. Eur Heart J. 2008;29:1001-1010. [PubMed] |
| 20. | Orford JL, Fasseas P, Melby S, Burger K, Steinhubl SR, Holmes DR, Berger PB. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J. 2004;147:463-467. [PubMed] |
| 21. | Ruiz-Nodar JM, Marín F, Sánchez-Payá J, Hurtado JA, Valencia-Martín J, Manzano-Fernández S, Roldán V, Pérez-Andreu V, Sogorb F, Valdés M. Efficacy and safety of drug-eluting stent use in patients with atrial fibrillation. Eur Heart J. 2009;30:932-939. [PubMed] |
| 22. | Sarafoff N, Ndrepepa G, Mehilli J, Dörrler K, Schulz S, Iijima R, Byrne R, Schömig A, Kastrati A. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med. 2008;264:472-480. [PubMed] |
| 23. | Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B. Universal definition of myocardial infarction. Circulation. 2007;116:2634-2653. [PubMed] |