Published online Oct 26, 2011. doi: 10.4330/wjc.v3.i10.315
Revised: September 23, 2011
Accepted: September 30, 2011
Published online: October 26, 2011
Both ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndromes (ACS) are the result of an acute thrombotic lesion obstructing blood flow in the coronary vasculature. Percutaneous treatment has shown to improve clinical outcome in this clinical setting by resolving coronary obstruction with different devices directed to restore coronary blood flow. In comparison with balloon alone angioplasty, implantation of bare metal stents reduced the rate of restenosis and cardiac events, but high rates of restenosis remained, leading to further investigations to develop drug-eluting stents with different pharmacological coatings that reduced restenosis rates and clinical events. In this review, we discuss the current treatment of ACS, reviewing recent randomized clinical trials and advances in medical treatment, including new antiplatelet agents and recent guideline recommendations.
- Citation: Alegría-Barrero E, Moreno R. Percutaneous treatment in acute coronary syndromes. World J Cardiol 2011; 3(10): 315-321
- URL: https://www.wjgnet.com/1949-8462/full/v3/i10/315.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i10.315
Myocardial revascularization is the key therapy for acute coronary syndromes (ACS). Accordingly, it is in this clinical setting when the expected benefits (increased survival, relief of symptoms, and improvement of quality of life) exceed the potential negative consequences of the procedure[1].
ACS include both ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation (NSTE) ACS. However, coronary vasospastic angina (10%-15% presenting with ST-segment elevation) is commonly included in the NSTE-ACS group. Both STEMI and NSTE-ACS are associated with high morbidity and mortality rates. Risk assessment is crucial in contemporary clinical practice and should hinge on developed risk scores[1] to predict mortality, with EuroSCORE for percutaneous and surgical treatment and SYNTAX for percutaneous coronary interventions (PCI).
Recent guidelines[1] have highlighted that patients should take an active role in the decision-making process especially when offered different types of revascularization procedures, so it is necessary to provide clinical information. This strategy has shown to improve outcomes[1]. A multidisciplinary team (Heart Team) should meet and discuss each patient’s characteristics and optimize the objective decision-making process, with consideration of sex, race, availability, technical skills, local results, referral patterns, and patient preference. Coronary artery bypass graft (CABG) surgery may be considered in some patients according to their clinical characteristics, and number and location of coronary lesions.
NSTE-ACS is the most frequent manifestation of ACS and represents the largest group of patients with ACS undergoing PCI. Despite continuous advances in medical and interventional treatments, mortality and morbidity remain high and are frequently equivalent to those of patients with STEMI after the initial month[1].
Patients with NSTE-ACS are very heterogeneous with a highly variable prognosis. Therefore, early risk stratification is essential for selection of the best treatment strategy.
Randomized clinical trials have shown that an early invasive strategy reduces ischemic endpoints mainly by reducing severe recurrent ischemia and the clinical need for further rehospitalization and revascularization. These trials have also shown a clear reduction in the rate of mortality or myocardial infarction (MI) in the medium term, while the reduction in mortality in the long term has been moderate and MI rates during the initial hospital stay have even been increased (early hazard) (Table 1). The most recent meta-analysis confirms that an early invasive strategy reduces the rate of cardiovascular death or MI at up to 5 years of follow-up[2]. These benefits were more evident in patients at higher risk. Troponin elevation and ST-segment depression at baseline appear to be the most powerful individual predictors of benefit from invasive treatment. Recently published European Society of Cardiology Guidelines on Coronary Revascularization[1] recommend the use of the Global Registry of Acute Coronary Events (GRACE risk score)[3] to guide clinical management[4,5]. Predictors of high thrombotic risk or of high risk for progression to MI, which constitute indications for emergency coronary angiography are[1,6]: (1) ongoing or recurrent ischemia; (2) dynamic spontaneous ST changes (> 0.1 mV depression or transient elevation); (3) deep ST-segment depression in anterior leads V2-V4 indicating ongoing posterior transmural ischemia; (4) hemodynamic instability; and (5) major ventricular arrhythmia.
| Situation | Class of recommendation | Level of evidence |
| An invasive strategy is indicated in patients with: | I | A |
| GRACE score > 140 or at least one high-risk criterion | ||
| Recurrent symptoms | ||
| Inducible ischemia at stress test | ||
| An early invasive strategy (< 24 h) is indicated in patients with GRACE score > 140 or multiple other high-risk criteria | I | A |
| A late invasive strategy (within 72 h) is indicated in patients with GRACE score < 140 or absence of multiple other high-risk criteria but with recurrent symptoms or stress-inducible ischemia | I | A |
| Patients at very high ischemic risk (refractory angina, with associated heart failure, arrhythmias or hemodynamic instability) should be considered for emergent coronary angiography (< 2 h) | IIa | C |
| An invasive strategy should not be performed in patients: | III | A |
| At low overall risk | ||
| At a particular high-risk for invasive diagnosis or intervention |
The 2009 American College of Cardiology/American Heart Association Guidelines on coronary revascularization included a new class IIa recommendation to perform coronary angiography within the first 12-24 h after the onset of symptoms for patients with high risk (GRACE score > 140)[7,8]. In lower risk patients, revascularization can be delayed without increased risk but should be performed during the same hospital stay, preferably within 72 h of admission. Although subgroups of patients, such as women and the elderly, may be at higher risk of bleeding and other complications, they should not be treated differently from other patients included in clinical trials.
Aims of pharmacologic treatment in patients with NSTE-ACS undergoing coronary angiography and PCI are: (1) to prevent coronary clot formation or progression; (2) to stabilize atherosclerotic plaques; and (3) to relieve ischemia. Treatment should be decided with consideration of both ischemic (ST-segment changes, elevated troponin, diabetes, GRACE score > 140) and bleeding risk (female sex, age > 75 years, bleeding history, glomerular filtration rate < 30 mL/min and use of femoral access), as they both worsen short- and long-term prognosis.
Angiotensin converting enzyme inhibitors should be initiated as part of the treatment of ACS as they have been shown to reduce left ventricular dilatation and to improve left ventricular ejection fraction. High-dose statin treatment has been shown to improve in-hospital and long-term outcomes in patients presenting with ACS. Up-titration of β-blocker therapy on admission is of critical value for these patients.
Antiplatelet therapy: Dual antiplatelet therapy (DAPT) includes aspirin (ASA) 150-300 mg po or 250-500 mg iv bolus, followed by 75-100 mg daily, and either clopidogrel (600 mg as loading dose, followed by 75 mg daily), or prasugrel (60 mg as loading dose, followed by 10 mg daily), or ticagrelor (180 mg as loading dose, followed by 90 mg twice daily). A higher clopidogrel maintenance dose for 1 or 2 wk immediately following stent implantation has shown some benefit in terms of reduced major adverse cardiac event rates without a significant increase in bleeding[9], but additional studies are necessary in order to confirm preliminary results.
In the TRITON TIMI 38 trial, prasugrel has been tested against a 300 mg loading dose of clopidogrel, with both started in the catheterization laboratory after diagnostic angiography, and proved to be beneficial with respect to a combined thromboembolic-ischemic outcome[10]. Recurrent cardiovascular events were significantly reduced in patients allocated to prasugrel patients. Severe bleeding complications increased with prasugrel, specifically in patients with a history of stroke and transient ischemic attack, in the elderly (≥ 75 years), and in patients with body weight < 60 kg. Bleeding was also increased in prasugrel-treated patients referred for early CABG. Excluding those patients at higher risk of bleeding, prasugrel offers significant benefit over clopidogrel with respect to cardiovascular events without increasing severe bleeding. In diabetic patients presenting with ACS, prasugrel confers a significant advantage over clopidogrel without increased bleeding[11].
Ticagrelor, a non-thienopyridine ADP receptor blocker which reversibly inhibits platelet function, has been compared with clopidogrel. The PLATO study confirmed a significant improvement in combined clinical endpoints, including mortality, in favor of ticagrelor[12]. The rate of severe non-CABG-related bleeding was similar to that of prasugrel in the TRITON-TIMI 38 trial, while CABG-related bleeding was lower than for clopidogrel, most probably a consequence of the faster inactivation of the agent after stopping intake.
The greatest benefit of GPIIb-IIIa inhibitors vs placebo was demonstrated in earlier recent clinical trials when ADP receptor blockers were not routinely used[5]. The usefulness of upstream eptifibatide, with or without clopidogrel, was not confirmed in the EARLY-ACS trial. This lack of benefit was associated with a higher bleeding risk[13]. The selective “downstream administration” of abciximab in the catheterization laboratory, in combination with a 600 mg clopidogrel loading dose, has been shown to be effective in troponin-positive NSTE-ACS patients in some studies[14] and may therefore be preferred over upstream use.
Anticoagulation: The golden rule is to avoid crossover especially between unfractionated heparin (UFH) and low molecular weight heparin[5] and to discontinue antithrombinic agents after PCI except in specific individual situations (e.g., thrombotic complications).
Risk stratification in NSTE-ACS patients determines the use of specific agents and doses. Patients at very high ischemic risk (e.g., persistent angina, hemodynamic instability, refractory arrhythmias) should immediately be referred to the catheterization laboratory and receive UFH, combined with DAPT. In patients at high risk of bleeding, bivalirudin (0.75 mg/kg bolus followed by 1.75 mg/kg per hour) can be used instead of UFH.
In patients at intermediate or high risk (e.g. troponin positive, recurrent angina, dynamic ST changes) for whom an invasive strategy is planned within 24-48 h, options for anticoagulation are: (1) in patients < 75 years, either UFH (60 IU/kg iv bolus, then infusion until PCI, controlled by activated partial thromboplastin time) or enoxaparin (1 mg/kg sc twice daily until PCI) or fondaparinux (2.5 mg daily sc until PCI) or bivalirudin (0.1 mg/kg iv bolus followed by infusion of 0.25 mg/kg per hour until PCI); and (2) in patients ≥ 75 years, either UFH (60 IU/kg iv bolus, then infusion until PCI) or enoxaparin (0.75 mg/kg sc twice daily until PCI) or fondaparinux (2.5 mg daily sc) or bivalirudin (0.1 mg/kg iv bolus followed by infusion of 0.25 mg/kg per hour until PCI).
Management during catheterization: The initial therapy should be maintained, avoiding switching between different anti-thrombotic drugs (with the exception of adding UFH to fondaparinux). The management during PCI depends on the treatment administered prior to the procedure. (1) Previous treatment with UFH: continue infusion, activated clotting time measurement should be used during PCI with the following target range: 200-250 s with GPIIb-IIIa inhibitors, 250-350 s without GPIIb-IIIa inhibitors; (2) Previous treatment with enoxaparin: In patients with less than 8 h since last sc dose, no additional bolus is needed. In contrast, in patients within 8-12 h of the last sc dose, a 0.30 mg/kg iv bolus should be added, and in those with > 12 h since the last sc dose, a 0.75 mg/kg iv bolus should be administered; (3) Previous treatment with fondaparinux: it is indicated that UFH 50-80 IU/kg be added when PCI is performed. Fondaparinux, an indirect factor Xa inhibitor, has been tested against enoxaparin in the OASIS-5 trial[15]. The combined ischemic event rate was similar, but severe bleeding complications were highly significantly reduced with fondaparinux. This favourable net clinical outcome with fondaparinux included lower long-term mortality and stroke rates. Because of a higher rate of catheter thrombosis when fondaparinux alone was used, UFH should be added for patients referred for angiography and PCI[16]; and (4)Previous treatment with bivalirudin: An additional iv bolus of 0.5 mg/kg should be given and the infusion rate increased to 1.75 mg/kg per hour before PCI. Bivalirudin, a direct antithrombin, alone or in combination with GPIIb-IIIa inhibition, was compared with UFH/enoxaparin + GPIIb-IIIa inhibition. Bivalirudin monotherapy was superior to either regimen with respect to reduced bleeding, without increased ischemic events[17].
Primary PCI performed within the first 6-12 h after symptom onset has shown to provide more effective restoration of vessel patency, less re-occlusion, improved residual left ventricular function and better clinical outcome compared with fibrinolysis[18-20].
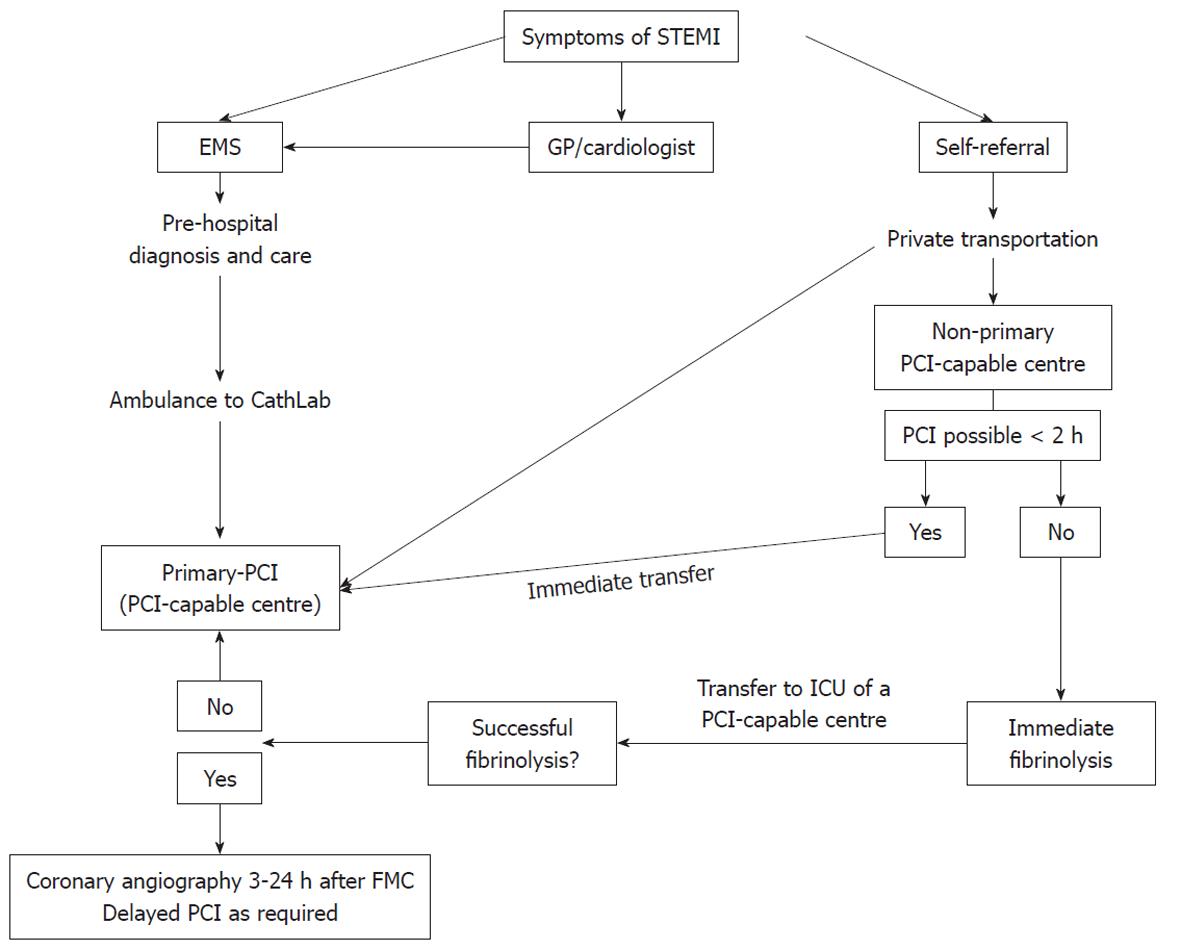
It is essential to minimize all time delays. When the expected delay is > 2 h, patients admitted to a non-PCI centre should receive fibrinolysis and then be transferred to a PCI-capable centre. In cases of persistence of ST-segment elevation after fibrinolysis (more than a half of the maximal initial elevation in the worst ECG lead) and/or persistent ischemic chest pain, rescue PCI should be considered. In the case of successful fibrinolysis, patients may be referred for PCI within 24 h (Figure 1)[7].
In patients presenting > 3 d after the acute event with a fully developed Q-wave MI, revascularization is indicated in those with recurrent angina and/or documented ischemia and viability[1,7].
Cardiogenic shock is the leading cause of in-hospital death for MI patients, even in those treated with primary PCI[21]. Echocardiography should always be performed in the setting of acute heart failure to assess left ventricular function and to rule out life-threatening mechanical complications that may require surgery (mitral regurgitation), ventricular septal defect, free wall rupture or cardiac tamponade[1]. In those patients complete PCI of non-infarcted vessels (i.e. PCI performed in all critically stenosed large epical coronary arteries) should be considered. In the presence of hemodynamic impairment, intra-aortic balloon pumping is recommended[21].
In patients with multivessel disease and STEMI but without cardiogenic shock, early PCI should focus on the coronary artery responsible for the ACS[22,23]. Staged PCI for a complete revascularization is the recommended strategy as it encounters less morbidity and mortality.
DAPT consists of ASA 150-300 mg po or 250-500 mg bolus iv, followed by 75-100 mg daily, and either prasugrel (60 mg as loading dose, followed by 10 mg daily), ticagrelor (180 mg as loading dose, followed by 90 mg twice daily), or clopidogrel (600 mg as loading dose, followed by 75 mg daily)[24,25].
Increasing the maintenance dose of clopidogrel to 150 mg/d for 1-2 wk might be effective in STEMI patients, as shown in NSTE-ACS. Prasugrel is superior to clopidogrel (300 mg loading dose, 75 mg maintenance dose) in reducing combined ischemic endpoints and stent thrombosis in STEMI patients without increasing the risk of severe bleeding[24]. A predefined subgroup analysis has demonstrated that STEMI or NSTE-ACS patients referred for PCI significantly benefit from ticagrelor vs clopidogrel, with similar bleeding rates[8,26]. Most studies of GPIIb-IIIa inhibitors in STEMI have evaluated abciximab (0.25 mg/kg iv bolus followed by infusion of 0.125 mg/kg per minute up to a maximum of 10 mg/min for 12 h) but more recent trials have also been performed with tirofiban[27]. Findings are mixed regarding the effectiveness of facilitation (early administration) with GPIIb-IIIa inhibitors before catheterization. While the only available clinical trial[28] showed no benefit, registries, meta-analyses, and post hoc analyses of the APEX-AMI[29] show positive results. The controversial literature data, the negative outcome of the only prospective clinical trial[28], and the beneficial effects of faster acting and more efficacious ADP receptor blockers in primary PCI do not support pre-hospital or pre-catheterization use of GPIIb-IIIa inhibitors.
Options for anticoagulation include mainly UFH (60 IU/kg iv bolus with GPIIb-IIIa inhibitor or 100 IU/kg iv bolus without GPIIb-IIIa inhibitor under monitoring with ACT), and bivalirudin (0.75 mg/kg bolus followed by 1.75 mg/kg per hour). Antithrombins can be stopped after PCI for STEMI with few exceptions such as left ventricular aneurysm and/or thrombus, atrial fibrillation, and prolonged bed rest.
A recent study suggested bivalirudin monotherapy as an alternative to UFH plus a GPIIb-IIIa inhibitor[30]. Significantly lower severe bleeding rates let to a beneficial net clinical outcome, indicating that bivalirudin may be preferred in STEMI patients at high risk of bleeding. The 1-year outcome of the HORIZONS clinical trial confirmed the beneficial effect of bivalirudin monotherapy vs UFH plus a GPIIb-IIIa inhibitor. Uncertainty remains in the early phase of primary PCI, when thrombotic complications seem to be higher with bivalirudin monotherapy. Fondaparinux was inferior to UFH in the setting of primary PCI in patients with STEMI (OASIS-6 trial)[31].
Bare metal stents (BMS) were initially designed to treat major dissections, avoid acute vessel closure and prevent restenosis. However, due to a 20%-30% rate of recurrence of angiographic stenosis within 6-9 mo after implantation, restenosis with BMS has often been considered the Achilles’ heel of PCI. In native vessels, drug-eluting stents (DES) significantly reduce angiographic restenosis and ischemia-driven target vessel revascularization[32,33]. In recent clinical trials, no significant differences were observed in the long-term rates of death or MI after DES or BMS use for either off-label or on-label indications[33,34]. First-generation DES are safe and efficacious for both on-label and off-label use, when implanted in the native circulation, in spite of a slightly increased propensity for late and very late stent thrombosis[32].
DES with proven efficacy should be considered by default in nearly all clinical conditions and lesion subsets, except if there are concerns or contraindications for prolonged DAPT. Indications for DES in a few specific patient or lesion subsets remain a matter of debate[35]. In selected STEMI patients[36-38], SES and PES were shown to be safe and effective in follow-up extending from 2 to 4 years. Studies based on angiographic endpoints favor the use of DES with strong antiproliferative properties (late lumen loss ≤ 0.2 mm)[39-42].
ACS are a common manifestation of atherosclerotic disease. Continuous advances have reduced morbidity and mortality risks, but there remain elevated rates of complications and mortality. Risk assessment is crucial in the setting of NSTE-ACS. Coronary revascularization is the major treatment of patients presenting with ACS. Optimal medical treatment including dual or triple antiplatelet therapy and anticoagulation are mandatory in this clinical setting. BMS have been used to alleviate coronary stenosis but high rates of restenosis developed. DES are the state-of-the-art treatment for coronary stenosis, excluding patients with elevated bleeding risk with prolonged DAPT. Further investigations will help us determine better pharmacologic regimens to minimize bleeding risk and thrombotic events.
Peer reviewers: Federico Lombardi, MD, FESC, Professor of Cardiology, University of Milan, Director of Cardiology Division, DMCO, San Paolo Hospital, Via A. di Rudinì 8, 20147, Milan, Italy; Ming-Jui Hung, MD, Cardiology Section, Department of Medicine, Chang Gung Memorial Hospital at Keelung, Chang Gung University College of Medicine, 222 Mai-Chin Road, Keelung City 20401, Taiwan, China; Paul Vermeersch, MD, Antwerp Cardiovascular Institute Middelheim, AZ Middelheim, Lindendreef 1, B-2020 Antwerp, Belgium
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
| 1. | Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-2555. [PubMed] |
| 2. | Fox KA, Clayton TC, Damman P, Pocock SJ, de Winter RJ, Tijssen JG, Lagerqvist B, Wallentin L. Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol. 2010;55:2435-2445. [PubMed] |
| 3. | Yan AT, Yan RT, Tan M, Eagle KA, Granger CB, Dabbous OH, Fitchett D, Grima E, Langer A, Goodman SG. In-hospital revascularization and one-year outcome of acute coronary syndrome patients stratified by the GRACE risk score. Am J Cardiol. 2005;96:913-916. [PubMed] |
| 4. | Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961-972. [PubMed] |
| 5. | Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández-Avilés F, Fox KA, Hasdai D, Ohman EM, Wallentin L. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598-1660. [PubMed] |
| 6. | Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345-2353. [PubMed] |
| 7. | Kushner FG, Hand M, Smith SC, King SB, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205-2241. [PubMed] |
| 8. | Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909-2945. [PubMed] |
| 9. | Mehta SR, Bassand JP, Chrolavicius S, Diaz R, Fox KA, Granger CB, Jolly S, Rupprecht HJ, Widimsky P, Yusuf S. Design and rationale of CURRENT-OASIS 7: a randomized, 2 x 2 factorial trial evaluating optimal dosing strategies for clopidogrel and aspirin in patients with ST and non-ST-elevation acute coronary syndromes managed with an early invasive strategy. Am Heart J. 2008;156:1080-1088.e1. [PubMed] |
| 10. | Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015. [PubMed] |
| 11. | Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, Goodman SG, Corbalan R, Purdy DA, Murphy SA. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118:1626-1636. [PubMed] |
| 12. | Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057. [PubMed] |
| 13. | Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, van 't Hof A, Berdan LG, Lee KL, Strony JT. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. 2009;360:2176-2190. [PubMed] |
| 14. | Kastrati A, Mehilli J, Neumann FJ, Dotzer F, ten Berg J, Bollwein H, Graf I, Ibrahim M, Pache J, Seyfarth M. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA. 2006;295:1531-1538. [PubMed] |
| 15. | Mehta SR, Granger CB, Eikelboom JW, Bassand JP, Wallentin L, Faxon DP, Peters RJ, Budaj A, Afzal R, Chrolavicius S. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol. 2007;50:1742-1751. [PubMed] |
| 16. | Steg PG, Jolly SS, Mehta SR, Afzal R, Xavier D, Rupprecht HJ, López-Sendón JL, Budaj A, Diaz R, Avezum A. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA. 2010;304:1339-1349. [PubMed] |
| 17. | Stone GW, Ware JH, Bertrand ME, Lincoff AM, Moses JW, Ohman EM, White HD, Feit F, Colombo A, McLaurin BT. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. JAMA. 2007;298:2497-2506. [PubMed] |
| 18. | Weaver WD, Simes RJ, Betriu A, Grines CL, Zijlstra F, Garcia E, Grinfeld L, Gibbons RJ, Ribeiro EE, DeWood MA. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review. JAMA. 1997;278:2093-2098. [PubMed] |
| 19. | Moreno R, López-Sendón J, García E, Pérez de Isla L, López de Sá E, Ortega A, Moreno M, Rubio R, Soriano J, Abeytua M. Primary angioplasty reduces the risk of left ventricular free wall rupture compared with thrombolysis in patients with acute myocardial infarction. J Am Coll Cardiol. 2002;39:598-603. [PubMed] |
| 20. | Moreno R, García E, Soriano J, Abeytua M, Martínez-Sellés M, Acosta J, Elízaga J, Botas J, Rubio R, López de Sá E. [Coronary angioplasty in the acute myocardial infarction: in which patients is it less likely to obtain an adequate coronary reperfusion?]. Rev Esp Cardiol. 2000;53:1169-1176. [PubMed] |
| 21. | Moreno R, Garcia E, Abeytua M, Soriano J, Acosta J, Perez De Isla L, Lopez De Sa E, Rubio R, Lopez-Sendon J. Early coronary angioplasty for acute myocardial infarction complicated by cardiogenic shock: have novel therapies led to better results? J Invasive Cardiol. 2000;12:597-604. [PubMed] |
| 22. | Moreno R, García E, Elízaga J, Abeytua M, Soriano J, Botas J, López-Sendón JL, Delcán JL. [Results of primary angioplasty in patients with multivessel disease]. Rev Esp Cardiol. 1998;51:547-555. [PubMed] |
| 23. | Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, Barbato E, Hamilos M, Mangiacapra F, Heyndrickx GR. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274-1281. [PubMed] |
| 24. | Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723-731. [PubMed] |
| 25. | Biondi-Zoccai G, Lotrionte M, Gaita F. Alternatives to clopidogrel for acute coronary syndromes: Prasugrel or ticagrelor? World J Cardiol. 2010;2:131-134. [PubMed] |
| 26. | Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, Husted S, Katus H, Keltai M, Khurmi NS. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375:283-293. [PubMed] |
| 27. | Valgimigli M, Campo G, Percoco G, Bolognese L, Vassanelli C, Colangelo S, de Cesare N, Rodriguez AE, Ferrario M, Moreno R. Comparison of angioplasty with infusion of tirofiban or abciximab and with implantation of sirolimus-eluting or uncoated stents for acute myocardial infarction: the MULTISTRATEGY randomized trial. JAMA. 2008;299:1788-1799. [PubMed] |
| 28. | Ellis SG, Tendera M, de Belder MA, van Boven AJ, Widimsky P, Janssens L, Andersen HR, Betriu A, Savonitto S, Adamus J. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205-2217. [PubMed] |
| 29. | Huber K, Holmes DR, van 't Hof AW, Montalescot G, Aylward PE, Betriu GA, Widimsky P, Westerhout CM, Granger CB, Armstrong PW. Use of glycoprotein IIb/IIIa inhibitors in primary percutaneous coronary intervention: insights from the APEX-AMI trial. Eur Heart J. 2010;31:1708-1716. [PubMed] |
| 30. | Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218-2230. [PubMed] |
| 31. | Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA. 2006;295:1519-1530. [PubMed] |
| 32. | Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937-948. [PubMed] |
| 33. | Daemen J, Simoons ML, Wijns W, Bagust A, Bos G, Bowen JM, Braunwald E, Camenzind E, Chevalier B, Dimario C. ESC Forum on Drug Eluting Stents European Heart House, Nice, 27-28 September 2007. Eur Heart J. 2009;30:152-161. [PubMed] |
| 34. | Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198-3206. [PubMed] |
| 35. | Moreno R, Martin-Reyes R, Jimenez-Valero S, Sanchez-Recalde A, Galeote G, Calvo L, Plaza I, Lopez-Sendon JL. Determining clinical benefits of drug-eluting coronary stents according to the population risk profile: a meta-regression from 31 randomized trials. Int J Cardiol. 2011;148:23-29. [PubMed] |
| 36. | Moreno R, Spaulding C, Jan Laarman G, Tierala I, Kaiser CA, Lopez-Sendon JL. Effectiveness and safety of paclitaxel-eluting stents in patients with ST-segment elevation acute myocardial infarction. EuroIntervention. 2007;3:386-391. [PubMed] |
| 37. | Nordmann AJ, Bucher H, Hengstler P, Harr T, Young J. Primary stenting versus primary balloon angioplasty for treating acute myocardial infarction. Cochrane Database Syst Rev. 2005;CD005313. [PubMed] |
| 38. | Kastrati A, Dibra A, Spaulding C, Laarman GJ, Menichelli M, Valgimigli M, Di Lorenzo E, Kaiser C, Tierala I, Mehilli J. Meta-analysis of randomized trials on drug-eluting stents vs. bare-metal stents in patients with acute myocardial infarction. Eur Heart J. 2007;28:2706-2713. [PubMed] |
| 39. | Moreno R, Fernandez C, Sanchez-Recalde A, Galeote G, Calvo L, Alfonso F, Hernandez R, Sánchez-Aquino R, Angiolillo DJ, Villarreal S. Clinical impact of in-stent late loss after drug-eluting coronary stent implantation. Eur Heart J. 2007;28:1583-1591. [PubMed] |
| 40. | Kaltoft A, Kelbaek H, Thuesen L, Lassen JF, Clemmensen P, Kløvgaard L, Engstrøm T, Bøtker HE, Saunamäki K, Krusell LR. Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: 3-year follow-up of the randomized DEDICATION (Drug Elution and Distal Protection in Acute Myocardial Infarction) Trial. J Am Coll Cardiol. 2010;56:641-645. [PubMed] |
| 41. | Nakagawa Y. What is the effectiveness of drug-eluting stents in the treatment of ST-elevation myocardial infarction? – should drug-eluting stents be indicated for patients with acute coronary syndrome? (Pro) –. Circ J. 2010;74:2225-2231. [PubMed] |
| 42. | Li Y, Han YL, Zhang QY, Guan SY, Wang XZ, Jing QM, Ma YY, Wang G, Wang B, Deng J. Comparison of drug-eluting stents with bare metal stents implantation for the treatment of acute ST-elevation myocardial infarction: 2-year clinical outcomes from single-center registry. Chin Med J (Engl). 2011;124:825-830. [PubMed] |