INTRODUCTION
Cell damage that occurs by insults such as oxidative stress and toxicants may contribute to atherosclerosis, coronary artery disease, stroke, myocardial infarction, Alzheimer’s disease, Parkinson’s disease and cancer[1-5]. Of these diseases, excessive oxidative stress and chronic inflammation are both major characteristics of the pathology seen in type 2 diabetes (T2D), cardiovascular diseases (CVD) and the aging process[1,6]. Specifically, T2D and CVD are associated with increased production of reactive oxygen species (ROS) and compromised endogenous anti-oxidant defense. Oxidative stress is tightly regulated by a balance between production and removal of ROS. ROS are natural by-products of metabolism and these molecules play important roles in cell signaling. However, excessive levels of ROS can be toxic to cells, i.e. whenever the expression of anti-oxidant enzymes, including superoxide dismutases (SODs), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO-1), catalase and thioredoxin are not sufficient to control ROS and minimize ROS-induced damage[3]. A compromised anti-oxidant defense system can lead to excessive oxidative stress and ultimately result in cell damage[7-9].
Recent work has indicated that chronic inflammation is an important pathophysiological factor in the development of T2D and CVD, with increased circulating levels of pro-inflammatory cytokines, such as circulating C-reactive protein (CRP), tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β[10-14]. Opposing the pro-inflammatory cytokines, anti-inflammatory cytokines, such as IL-10 and adiponectin, are inversely correlated with the incidence of these diseases. These anti-inflammatory cytokines play a role in inhibiting the action of TNF-α on endothelial cell adhesion, reducing nuclear factor (NF)-κB activation, and delaying macrophage foam-cell development[15-18]. T2D and CVD are associated with aging and a sedentary lifestyle; however, emerging evidence suggests that the anti-inflammatory effects of exercise and/or physical activity can reduce mortality and morbidity of these patients[19-22]. However, the mechanism(s) that confer anti-inflammatory effects following an exercise training regimen have not been clearly identified.
This review addresses the effects of interventions, such as nutrition, pharmacology, genetics and exercise on anti-oxidant systems and on inflammation.
ROLE OF INTERVENTIONS IN ENDOGENOUS ANTIOXIDANT SIGNALING
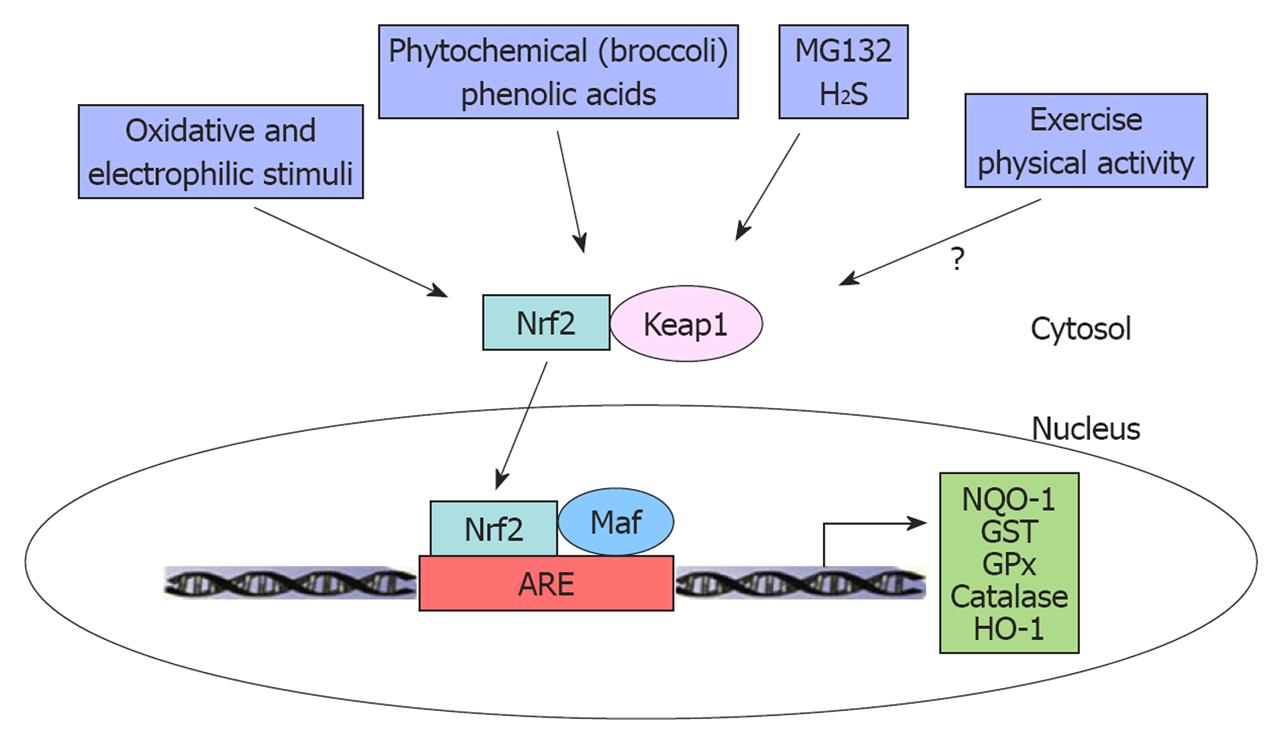
The anti-oxidant defense system is regulated, in large part, by a transcription factor termed nuclear factor erythroid 2-related factor 2 (Nrf2), which is a member of the cap ‘n’ collar subfamily of the basic leucine zipper transcription factors[5]. Under normal physiological conditions, Nrf2 is bound to a cytoplasmic repressor, termed Kelch-like ECH-associated protein 1 (Keap1)[23]. Keap1 functions as a substrate adaptor for a Cullin3-dependent ubiquitin ligase and targets Nrf2 for degradation by the proteasome[24-26]. The substrate adaptor function of Keap1 is inactivated in response to a range of oxidative and electrophilic stimuli such as ROS, diethyl malonate and certain disease processes, resulting in the accumulation of Nrf2, which enters the nucleus and activates expression of anti-oxidant genes[5,9]. Although most investigators believe that Keap1-mediated repression occurs in the cytoplasm, several studies have shown that Nrf2 and Keap1 can shuttle between the nucleus and the cytoplasm[27-29]. In the nucleus, Nrf2 forms a heterodimer with members of the small musculo-aponeurotic fibrosarcoma (Maf) transcription factor family. These Nrf2/Maf heterodimers bind to antioxidant response elements present in the promoters of numerous anti-oxidant genes, including NQO-1, glutathione S-transferase, glutathione peroxidase (GPx), catalase and HO-1[5,9,30-32] (Figure 1). Nrf2 is widely expressed and it has been studied in many different tissues[7,33,34]. In the cardiovascular system, it has been shown that ischemia/reperfusion (I/R) down-regulates Nrf2 protein expression in rat heart and that aging decreases glutathione synthesis via diminished Nrf2 signaling in rat vascular endothelial and smooth muscle cells, suggesting that Nrf2 may play a critical role in the development of CVD in the aged population[6,35]. He et al[30] have shown a functionally decreased contractility when Nrf2 is genetically deleted from cardiomyocytes due to a marked increase in high-glucose oxidative stress and apoptosis.
Figure 1 The role of interventions in nuclear factor erythroid 2-related factor 2-Kelch-like ECH-associated protein 1 signaling pathway.
Nuclear factor erythroid 2-related factor 2 (Nrf2) can be activated by interventions such as nutrition (phytochemical, phenolic acids), pharmacology (MG132, H2S) and oxidative and electrophilic stimuli. Under basal conditions, Nrf2 is sequestered in the cytosol by binding with Kelch-like ECH-associated protein 1 (Keap1). On activation, Nrf2 can be released from Keap1 and translocated into the nucleus. Nrf2 forms a heterodimer with musculo-aponeurotic fibrosarcoma (Maf) and antioxidant response element (ARE) and regulates phase II anti-oxidant enzymes. NQO-1: NAD(P)H quinone oxidoreductase-1; GST: Glutathione S-transferase; GPx: Glutathione peroxidase; HO-1: Heme oxygenase-1.
Role of nutrition in antioxidant signaling
Numerous studies have indicated that increased oxidative stress may be involved in the pathogenesis of CVD. Several animal models suggest that when endogenous anti-oxidant systems are overwhelmed, exogenous anti-oxidant supplementation can be used for preventive and/or therapeutic intervention of oxidative cardiovascular disorders[35,36]. Phenolic acids are a group of compounds that are widely distributed in natural plant foods including fruits, vegetables and whole grains[36]. Yeh et al[36] have shown that 14 d of oral gavage (100 mg/kg) of phenolic acids in male rats increased anti-oxidant capacity via SOD-1, GPx and catalase, while HO-1 mRNA increased via Nrf2 signaling in the heart. Other phytochemicals, such as those found in broccoli sprouts may confer protection against cancer, although little is known about these effects on the cardiovascular system[37,38]. Recently, Mukherjee et al[35] have tested if daily consumption of broccoli, which contains sulforaphane and selenium for 1 mo could be beneficial to the heart. They have found that broccoli induced cardio-protection in I/R through the induction of HO-1[35].
Role of pharmacology and genetics in antioxidant signaling
The proteasome system uses a ubiquitin tag to activate the major intracellular protein degradation in eukaryotic cells[39]. The ubiquitin-proteasome system is critical for degradation of proteins related to the cell cycle and apoptosis[40,41]. In this sense, proteasome inhibition has been highlighted as a promising therapeutic target for treatment of human diseases. For instance, proteasome inhibitors have been proposed as an anti-inflammatory treatment via inhibition of NF-κB[42]. As steady-state levels of Nrf2 increase following proteasome inhibition, Dreger et al[39] have suggested that non-toxic inhibition of the ubiquitin-proteasome system by MG132 (0.5 μmol/L for 48 h) may contribute to protection of rat cardiomyocytes against oxidative stress via Nrf2-mediated transcriptional activation of anti-oxidants. Calvert et al[43] showed that hydrogen sulfide (H2S) may be an attractive pharmacological agent for the treatment of CVD by up-regulating anti-oxidants and anti-apoptogens. They showed that 100 μg/kg precondition by H2S in the form of sodium sulfide resulted in protection against myocardial I/R injury in a mouse model by increasing endogenous anti-oxidant defenses via an Nrf2-dependent manner. In this study, Nrf2 deficient mice showed an exacerbated injury in response to I/R, suggesting that Nrf2 may play an important cardio-protective role in heart disease[43]. On the other hand, Sussan et al[44] have shown that disruption of Nrf2 in apolipoprotein E (ApoE) knockout mice significantly decreased atherosclerotic plaque after 20 wk of high-fat diet. However, Nrf2 knock-out mice showed increased susceptibility to pulmonary emphysema, asthma and sepsis due to increased oxidative stress and inflammation[44]. This study suggested that Nrf2 might promote atherosclerotic plaque development through a mechanism separate from oxidative stress. More studies are required to fully understand the contribution of Nrf2 signaling in regards to atherosclerosis.
Role of exercise and physical activity in antioxidant processes
A sedentary lifestyle is a risk factor for T2D and CVD with several clinical studies illustrating a reduction of mortality and morbidity in physically active individuals compared to sedentary individuals[45-47]. Exercise or physical activity may contribute to improvement of insulin resistance via improved insulin action, improved vascular function via increase of nitric oxide (NO) bioavailability, and by increasing ROS-detoxification and decreasing ROS generation[48-53]. Since generation of ROS is a normal result of aerobic metabolism, it is efficiently removed by cellular anti-oxidant systems under physiological conditions. Several studies have shown that chronic exercise training increases SOD gene expression in vascular systems. Exercise training increased SOD-3 gene expression in mice aorta in NO-dependent manner and up-regulated SOD-1 in Yucatan miniature pig aortas[50,51]. Recently, Moien-Afshari et al[54] have suggested that low intensity exercise training increased SOD-1 protein expression, whereas moderate intensity increased SOD-2 gene expression in diabetic mice aorta with improved NO availability. Even though many studies have shown that exercise and physical activity up-regulated anti-oxidants such as SODs in cardiovascular systems, little is known about how exercise and physical activity may increase phase II anti-oxidant systems via the Nrf2-Keap1 signaling pathway[50,51,54,55]. Even though there are no clear studies to determine if exercise training may alter Nrf2 signaling, Niess et al[56] have shown that leukocytes from endurance trained athletes down-regulate the baseline expression of HO-1, presumably due to the adaptation mechanism of exercise training. Since HO-1 is an anti-oxidant protein that is mainly induced through the Nrf2-Keap1 signaling pathway, exercise training may down-regulate Nrf-2 signaling in humans. However, more studies are needed to further elucidate the effect of exercise on Nrf2 mechanisms in the cardiovascular system.
ROLE OF EXERCISE IN INFLAMMATION
Effect of acute exercise on inflammation
The effect of acute exercise on pro-inflammatory cytokines release has been a matter of considerable debate, since although a majority of studies have reported that acute exercise simulates release of inflammatory cytokine[57-61], some studies have also shown that acute exercise did not change levels of the pro-inflammatory cytokines TNF-α and IL-1β[58,61-63]. These discrepant findings suggest that the level of pro-inflammatory cytokines during and following exercise is dependent on several factors including the pathological condition, intensity and duration of exercise, and timing of sampling[64]. For example, plasma concentration and muscle mRNA expression of TNF-α are elevated in chronic obstructive pulmonary disease patients during continuous moderate-intensity exercise (for 11 min at 40% VO2max) whereas no change occurs in normal individuals[64]. Although the circulating level of TNF-α is not altered during low intensity and long duration of two-leg knee extensor exercise, short duration and high intensity of cycling exercise, approximately 80% VO2max, increases the circulating level of pro-inflammatory cytokines, IL-4, IL-6, TNF-α, interferon (IFN)-γ and anti-inflammatory cytokine such as IL-1β and IL-10[59,64]. Ostrowski et al[57] found that IL-6 and IL-1 receptor antagonist (IL-1ra) levels were enhanced during 2 h of continuous exercise (measured at every 30 min for 2 h) and following exercise, despite no change in the TNF-α level. Of these multiple pro-inflammatory cytokines, IL-6 is the most responsive cytokine that is increased during and following exercise and it is related to exercise intensity, duration, and muscle mass recruited[65,66]. Contracting skeletal muscle is one of the major sources of IL-6 produced during exercise. For example, during even moderate intensity of exercise (50% of maximal power output), 3 h of dynamic two-legged knee-extensor, muscle IL-6 mRNA expression and plasma concentration of IL-6 is increased 16-fold and 20-fold, respectively[67]. An even greater amount of IL-6 is produced in higher intensity and longer duration of exercise[66]. More interestingly, Petersen et al[66] suggest that IL-6 produced from working muscle can play a hormone-like role that stimulates lipolysis and fat oxidation in adipose tissue and induces gluconeogenesis in liver that may enhance exercise capacity. Moreover, IL-6 has been suggested as an anti-inflammatory cytokine because some studies have shown that an infusion of IL-6 decreases TNF-α production in healthy humans and stimulates the release of anti-inflammatory cytokines, IL-1ra and IL-10[68,69]. However, IL-6 is a well-established pro-inflammatory cytokine that is closely linked to various CVD and morbidity and mortality of several diseases. One possible explanation of a paradoxical role of IL-6 as an inflammatory cytokines and as a mediator of beneficial adaptation to exercise is the location of IL-6 production. Muscle contracting-induced local production of IL-6 may play a positive role in lipid and carbohydrate metabolism during exercise whereas systemic IL-6 may result in a negative consequence of tissue injury, chronic infection and diseases.
Effect of chronic exercise on inflammation
Exercise training and/or a high level of physical activity has a beneficial effect on inflammation through a reduction of pro-inflammatory cytokines and an increase in anti-inflammatory cytokines. Cross-sectional studies show lower plasma levels of IL-6, TNF-α and CRP while higher plasma levels of IL-10 and adiponectin occur in physically active individuals compared to physically inactive groups[16,70-72]. Exercise decreases pro-inflammatory cytokines and indicators of systemic inflammation. For example, long-term exercise (for 6 mo) significantly attenuates the production of pro-inflammatory cytokines, TNF-α and IFN-γ, and enhances the anti-inflammatory cytokine IL-10 in individuals at risk of developing ischemic heart disease[73]. Participation in an exercise training program for 6 mo in patients with stable chronic heart failure (CHF) significantly decreases the mRNA expression of TNF-α, IL-6, IL-1β in skeletal muscle, compared to the healthy individuals[74]. On the other hand, some studies demonstrate that the levels of pro-inflammatory cytokines are not significantly altered after exercise training in the obese individuals and healthy elderly[75-77]. This discrepancy may be derived from differences in experimental design and disease status of the subjects. The studies showing the effectiveness of exercise training on pro-inflammatory cytokines investigated the patients with severe disease conditions such as CHF and ischemic heart disease where basal levels of cytokines were already elevated compared to the healthy individuals before the exercise training[73,74]. In contrast, no apparent change in pro-inflammatory cytokines is shown in relatively less severe conditions, such moderate obesity (approximately 40% of % body fat) and aging (approximately 66 years old)[75-77]. Moreover, local change in inflammation after exercise training is an important factor to be considered. For example, mRNA expression of pro-inflammatory cytokines, TNF-α, IL-6, IL-1β in skeletal muscle are reduced after exercise training although the circulating levels of those cytokines are not changed[74]. This finding suggests that exercise training does not play a role in reducing systemic inflammation and is not effective enough to reduce the circulating levels of cytokines. However, regional expression of cytokines in skeletal muscle are affected. This regional reduction of pro-inflammatory cytokines in skeletal muscle may have a beneficial effect in skeletal muscles homeostasis despite the lack of effect on systemic inflammation.
Mechanisms of anti-inflammatory effect of exercise
As previously described, acute exercise stimulates production of pro-inflammatory cytokines and superoxide (O2-•) that can cause the tissue injury. Interestingly, exercise induced pro-inflammatory cytokines are triggers to generate the anti-inflammatory cytokines such as IL-10, IL-1ra and transforming growth factor β and the anti-oxidant, SOD-2 that have protective functions[58,60,63]. The major role of these cytokines is to recruit neutrophils and monocytes into injured tissue for repair[78]. During this process, anti-inflammatory cytokines and anti-oxidant mechanisms can be initiated and limit the inflammatory reaction in response to exercise. It is suggested that this stimulated anti-inflammatory mechanism, in turn, may down-regulate production of pro-inflammatory cytokines during and following exercise.
CONCLUSION
Oxidative stress plays a critical role in the pathology of CVD. Exogenous anti-oxidant supplementations such as broccoli, curcumin and phenolic acids as well as stimulators of endogenous pathways such as MG132, H2S and exercise seemed to be effective in providing cellular protection. However, large discrepancies are noted among several studies. For example, Sussan et al[44] have shown that double deletions of ApoE and Nrf2 genes in mice aortas showed a decrease in plaque area compared with ApoE knock-out mice in spite of the anti-oxidant effect of Nrf2. This suggests that upregulation of Nrf2 may play a detrimental role in generation of atherosclerosis. On the other hand, several studies have suggested beneficial roles of Nrf2 in the cardiovascular systems. These contradictory findings based on narrowly focused studies indicate that a broader understanding of Nrf2 is needed to understand the role of the oxidant/anti-oxidant system in cardiovascular disease. Physical activity and/or exercise training provides an ideal experimental context for further study of Nrf2 and other cytokines because acute exercise induces an increase in pro-inflammatory cytokine production that eventually stimulates anti-inflammatory responses to achieve an overall beneficial anti-inflammatory effect. The elucidation of the mechanisms governing exercise-induced protection from disease in the cardiovascular system is needed to devise more effective therapies.