Revised: November 17, 2010
Accepted: November 24, 2010
Published online: January 26, 2011
There has been significant progress in our understanding of the pathobiology, epidemiology and prognosis of pulmonary vascular disease and, over the past few years, there has been an explosion of clinical therapeutic trials for pulmonary arterial hypertension (PAH). The increasing number of different conditions now associated with PAH and the appearance of new diagnostic techniques have led to a need for a systematic diagnostic approaches and a new disease classification, which has resulted in notable improvements in the quality and efficacy of clinical care. We appreciate traditional resting right heart catheterization techniques (which still remain the gold standard for diagnosing PAH and managing patients on therapy) and look forward to novel invasive techniques (e.g. intravascular ultrasound) that add greatly to our understanding of right ventricle and pulmonary circulation, and for the interpretation of data from clinical trials as well.
- Citation: Grignola JC. Hemodynamic assessment of pulmonary hypertension. World J Cardiol 2011; 3(1): 10-17
- URL: https://www.wjgnet.com/1949-8462/full/v3/i1/10.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i1.10
We have experienced significant progress in our understanding of pulmonary hypertension (PH) including elucidation of the pathobiology of PH and the development of diagnostic approaches, treatments and prognostic abilities[1]. Many cardiac and pulmonary diseases are associated with an abnormal increase in pulmonary artery pressures (PAP). The most common causes of PH are left heart failure and chronic hypoxemic lung diseases. PH is the third most common cardiovascular condition, after coronary heart disease and systemic hypertension. A resting mean PAP (mPAP) of 8 to 20 mmHg should be considered normal, based on available evidence. According to the last World Symposium on PH held in DanaPoint (2008), PH is a hemodynamic and pathophysiological condition defined as an increase in mPAP ≥ 25 mmHg at rest as assessed by right heart catheterization (RHC, Table 1). Further studies are needed to better determine the natural history of patients with mPAP of 21 to 24 mmHg. Currently, the normal behavior of pulmonary pressure during exercise remains unknown, and it presents wide variability according to age and the degree of physical fitness in the healthy individuals. Thus, a definition of PH during exercise as a mPAP > 30 mmHg is not supported by published data. PH can be found in multiple clinical conditions, which have been classified into 6 clinical groups with different pathological, pathophysiological, prognostic and therapeutic features[1].
| Definition | Characteristics | Clinical group(s)1 |
| PH | mPAP ≥ 25 mmHg | All |
| Pre-capillary PH | mPAP ≥ 25 mmHg | 1 Pulmonary arterial hypertension |
| PAOP ≤ 15 mmHg | 3 PH due to lung diseases | |
| CO N or reduced2 | 4 Chronic thromboembolic PH | |
| 5 PH with unclear and/or multi- factorial mechanisms | ||
| Post-capillary PH | mPAP ≥ 25 mmHg | 2 PH due to left heart disease |
| PAOP > 15 mmHg | ||
| CO N or reduced2 | ||
| Passive | TPG ≤ 12 mmHg | |
| Reactive ("out of proportion") | TPG > 12 mmHg |
The subgroup of PH known as pulmonary arterial hypertension (PAH, group 1) is a clinical condition characterized by the presence of precapillary PH (pulmonary arterial wedge pressure ≤ 15 mmHg) in the absence of other causes of precapillary PH, such as PH due to lung diseases, chronic thromboembolic PH or other rare diseases.
PH is a life threatening disease characterized by a progressive increase of pulmonary blood pressure that often leads to right ventricular (RV) failure and death. In fact, PAH is a disease of the arterial vessel wall (obliterative proliferation and remodelling) that affects both steady and pulsatile components of the pulmonary arterial hemodynamics. The increase of the pulmonary vascular resistance (PVR), the intrinsic wall arterial stiffness and the magnitude and timing of the reflection wave (secondary to the increase pulse wave velocity), determine a progressive dynamic afterload increase, which in turn leads to RV hypertrophy, dilatation and failure[2].
The maintenance of stroke volume or ventricular flow output in the presence of an increase of the afterload depends on systolic function adaptation, with secondary diastolic changes and altered RV-left ventricle (LV) interactions. The insufficient homeometric adaptation to afterload (Anrep effect) leads to heterometric adaptation (Starling effect), given the increased preload is secondary to the RV dilatation. Finally, the exhaustion of these mechanisms plus the ventricular contractility decrease, extended relaxation time constant and increased stiffness, determines RV failure[3].
Taking into account that RV functional status is a strong predictor of survival and that the evolution of RV function parallels the evolution of the pulmonary vascular anatomo-functional pathology, it is becoming apparent that a comprehensive approach to the RV, pulmonary circulation and their interactions as a unit (ventricular-pulmonary vascular coupling) will be beneficial in both clinical management of PH patients and clinical research. Here we discuss standard and evolving invasive parameters that have the ability to better assess pulmonary circulation and RV function as a unit[2].
Right heart catheterization (RHC) remains the gold standard for diagnosing PH, assessing disease severity, and determining the prognosis and response to therapy (Table 2)[4]. The procedure has been shown to be safe, with no deaths reported in the NIH registry study. In addition, a recent study reported a procedure-related mortality of 0.055% and morbidity of 1.1% when conducted in specialized centers. The most critical aspects of RHC are that it is performed appropriately and the data are interpreted accurately. Since end-expiratory intrathoracic pressure most closely correlates with atmospheric pressure, it is important that all RV, pulmonary artery (PA), PA occlusion pressure (PAOP), and left ventricular pressures be measured at end-expiration (specially in obese patients and patients with intrinsic lung disease in whom there can be significant variation between inspiration and end-expiratory vascular pressures)[2-5].
| Statement | Class of re-commendation | Level of evidence |
| RHC is indicated in all patients with pulmonary arterial hypertension to confirm the diagnosis, to evaluate the severity and when PAH specific drug is considered | I | C |
| RHC should be performed for confirmation of efficacy of pulmonary arterial hypertension specific drug therapy | IIa | C |
| RHC should be performed for confirmation of clinical deterioration and as baseline for the evaluation of the effect of treatment escalation and/or combination therapy | IIa | C |
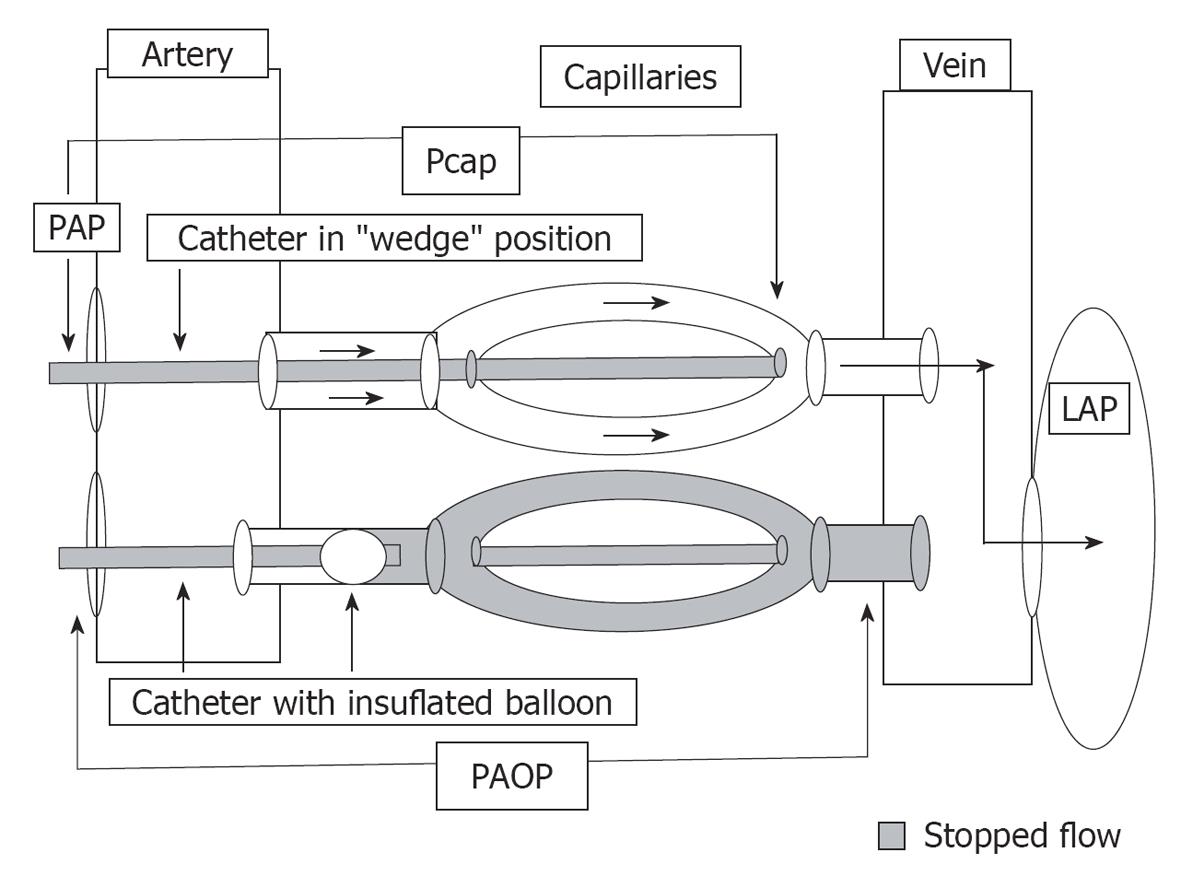
After determination of the presence of PH, pulmonary venous pressure should be evaluated by the PAOP. Inflation of the balloon at the tip of a PA catheter to measure PAOP creates a downstream stop-flow phenomenon extending to the same diameter veins. Therefore, PAOP generally gives a satisfactory estimate of left atrial (LA) or end-diastolic LV pressure (Figure 1)[6]. In order to obtain retrograde transmission of LA events through the pulmonary capillary bed (i.e. PAOP), the PA catheter tip must be located in a lung segment where pulmonary venous pressure exceeds the alveolar pressure (physiologic zone 3). Conditions such as hypovolemia, advanced parenchymal lung disease, or positive pressure ventilation make the alveolar pressure exceed the pulmonary venous pressure, therefore creating zones 1 or 2. In this case, PAOP becomes a measure of alveolar pressure rather than LA pressure[7].
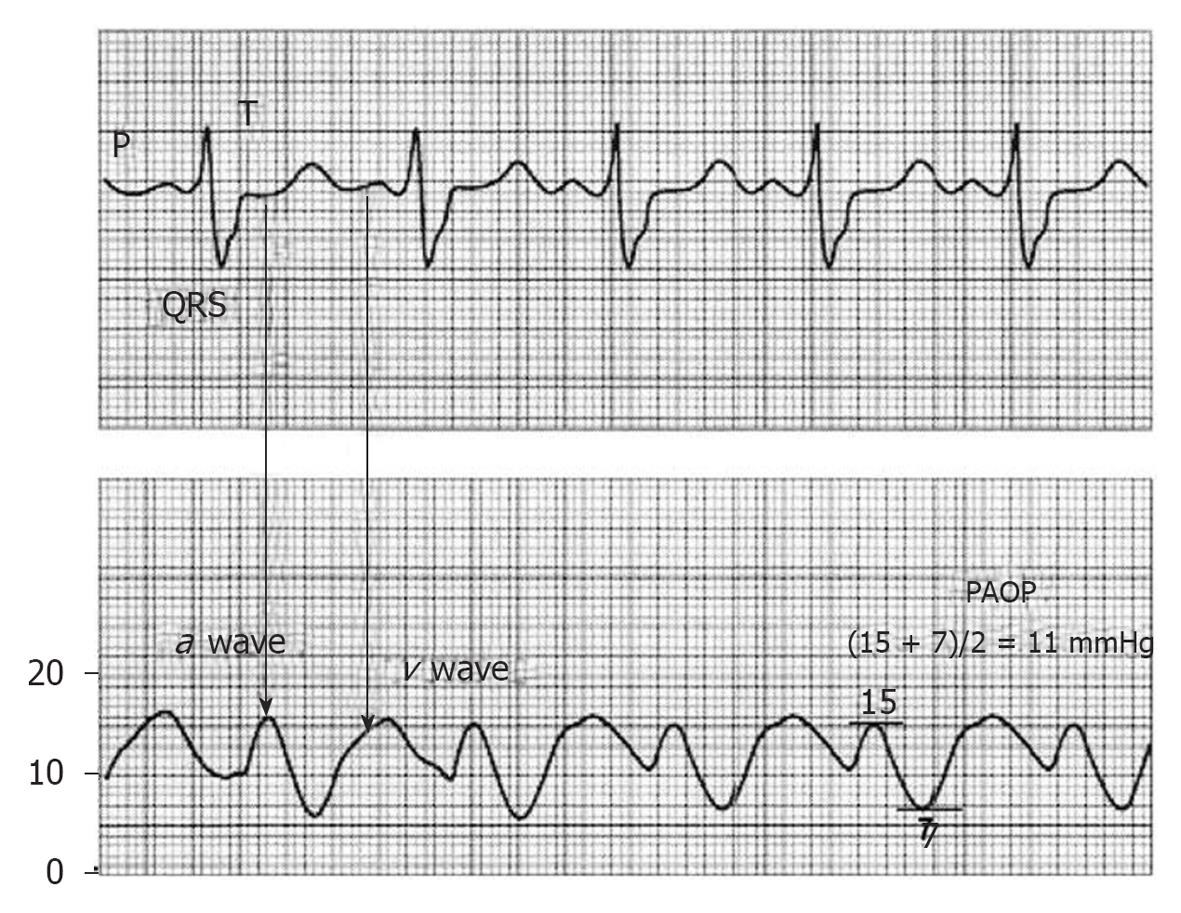
Because the PAOP is a backward reflection of the LA, the timing of the waves with the ECG is slightly delayed: the a wave occurs with atrial contraction and is found near the end or after the QRS and the v wave occurs when blood fills the atria and the mitral valve is closed. This is observed well after the T wave. A very prominent v wave can hinder the accurate measurement of PAOP and tends to appear due to severe mitral regurgitation or severe alterations in LV distensibility[6,7].
To measure the mean PAOP value, we must locate the a wave (near or after the QRS complex) and measure the maximum and minimum a wave values, and then these values are averaged (Figure 2).
Wedging a PA catheter without balloon inflation yields a PA wedge pressure, sometimes called a pulmonary capillary wedge pressure (Pcwp) or (wrongly) a pulmonary capillary pressure (Pcap), which measures the pressure of the same diameter veins (Figure 1). The measurement of an effective Pcap, requires the analysis of a PAP decay curve after balloon occlusion, as will be described later[8,9].
A hemodynamic study was recently conducted in 3920 patients with PH, and the reliability of PAOP to distinguish PAH and PH associated with LV disease compared to LV end-diastolic pressure (the gold standard of LV preload) was analyzed. Approximately half of the patients classified as having PAH based on PAOP < 15 mmHg, actually had PH associated with LV disease when based on the criterion of an LV end-diastolic pressure < 15 mmHg. Thus, if the patient presents a clinical profile compatible with PH associated with LV disease (age > 65 years, obesity, metabolic syndrome, coronary artery disease, hypertension, diabetes mellitus, LA enlargement, LV hypertrophy), a direct measurement of LV end-diastolic pressure is recommended to confirm the diagnosis of PAH if PAOP is < 15 mmHg[10].
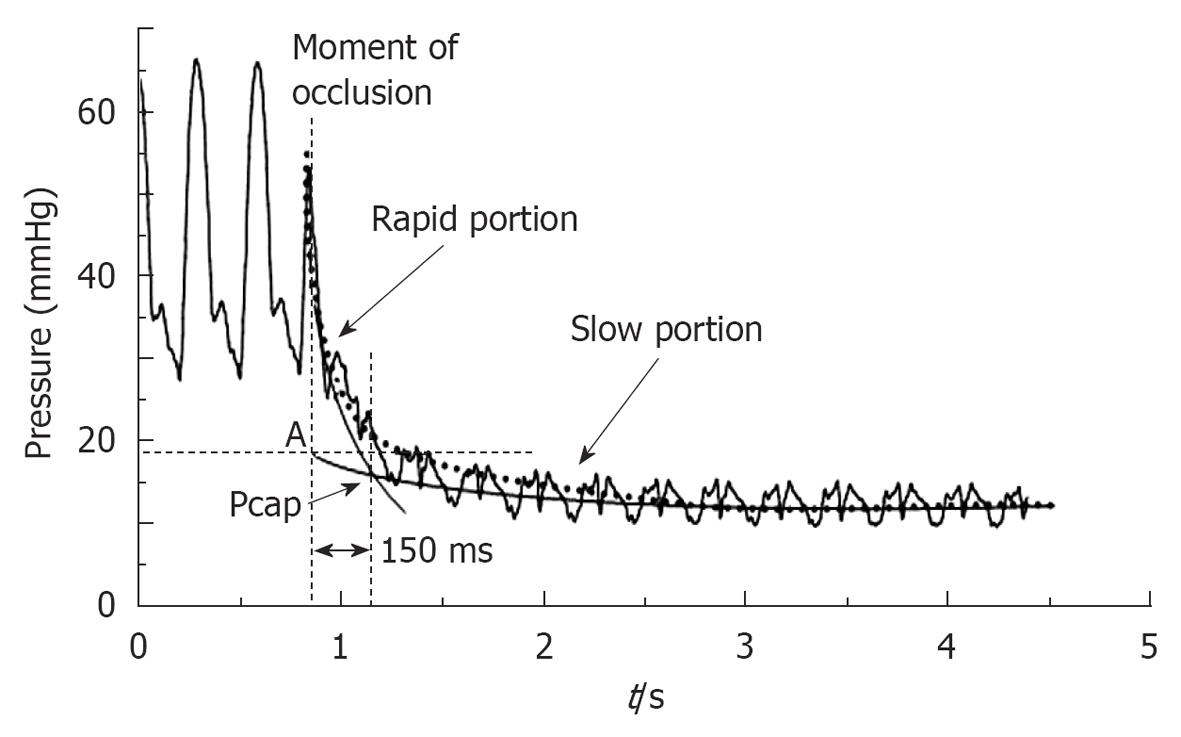
The normal value of PAOP or LV end diastolic pressure is less than 8 mmHg and no more than 15 mmHg, since approximately 14 mmHg is 2 standard deviations from a normal PAOP. In clinical practice, Pcap is seldom assessed, and PAOP (confusingly called Pcwp or Pcap) is commonly used to estimate PVR and to guide fluid therapy. Under numerous pathological conditions the longitudinal distribution of the precapillary arterial and the postcapillary venous resistance and subsequently the relationship between Pcap and PAOP varies greatly. Hence, Pcap can no longer be predicted from PAOP. The measurement of the effective Pcap requires the analysis of a PAOP decay curve after balloon occlusion through different methods: (1) the visual inspection method, where the inflection point is designated as the capillary pressure; (2) a single exponential curve is fitted to the average arterial occlusion pressure decay in the segment between 0.3 s and 2 s after time 0 (occlusion); and (3) a better fit of PAOP decay curves after balloon occlusion is obtained with a bi-exponential function. This method of calculation led to lower Pcap values than found using a mono-exponential fitting (Figure 3)[6,8,11].
When the PA is occluded, there is a rapid decrease in blood flow as the occluded downstream PA discharges its blood volume sequentially into the pulmonary capillaries across the arterial resistance and then into the pulmonary veins across the venous resistance. This two-part pressure discharge is reflected in the PAOP decay curve. The initial rapid pressure drop approaches the pressure in the capillaries as the blood trapped in the downstream pulmonary capillaries equilibrates with pulmonary capillary pressure. This is followed by a slower pressure decrease approaching the PAOP as pulmonary capillary pressure equilibrates with pulmonary venous pressure. The initial pressure drop reflects the proximal arterial resistance, and the slower pressure drop reflects the distal, venous resistance. Normally two-thirds of the transpulmonary gradient pressure drop occurs over the arterial resistance, with approximately one-third of the pressure drop occurring over the venous resistance, as calculated from Gaar’s equation: Pcap = PAOP + 0.4 (mPAP - PAOP). An increase in the pulmonary venous resistance increases the Pcap. Under these conditions the PAOP or LA pressure underestimates the Pcap[6-9].
In patients with PAH, Pcap measured with the occlusion technique is higher than normal and helps to locate the site of predominantly increased PVR in severe PH. The increased Pcap may be due to a previously assumed unimportant venous involvement in PAH. The results of Fesler et al[8], showed that compared to PAH, the arterial segment of the PVR (PVRa) is increased in chronic thromboembolic PH and decreased in pulmonary veno-occlusive disease, but isolated measurement of PVRa does not allow a differential diagnosis between these three types of severe PH.
The term PVR describes, in part, the forces opposing the flow across the pulmonary vascular bed. The equation traditionally used is based on the assumption that the pulmonary capillaries, as well as some others vessels in series, behave like a Poiseuille resistance, assuming a laminar type flow of a homogeneous Newtonian fluid. A single point measurement of mPAP, PAOP and cardiac output (CO), and derived PVR calculation may be misleading because the inherent assumptions of linearity and zero crossing of the (mPAP-PAOP)/CO relationship are not met. Therefore, a single point PVR determination at variable flow may underestimate or overestimate changes in the functional state of the pulmonary circulation. These errors or approximations can be limited by the definition of PVR as a multipoint pressure/flow line[2,3].
In clinical practice, determination of CO and cardiac index (CI) is typically done by either the thermodilution method or Fick method (using the Fick principle). Normal values are: CO: 4 to 8 L/min; CI: 2.6 to 4.2 L/m2 per minute. Although a low CI (≤ 2 L/m2 per minute) has been shown to offer prognostic value for patients with PAH, the stroke volume index (SVI: CI/heart rate) should also be calculated in order to assessed the impact of heart rate on CO/CI values. An increased SVI with the same CI suggests better RV myocardial performance[7].
There is no technique that can be expected to provide flawless results for CO in the clinical setting. In the thermodilution method, cold or room-temperature solution is injected in the right atrium through the proximal port of the PAC. A curve generated by plotting the decline in PA temperature (°C) vs time (s). The area under the curve is inversely related to the CO because the injected solution is diluted by body temperature blood flow (e.g. higher area, lower CO). At least 3 measurements should be obtained and they should be within 10% of each other to improve accuracy. The physiological factors affecting accuracy of thermodilution CO determinations are: dysrhythmias, congenital heart defects (e.g. atrial septal defect), and severe tricuspid regurgitation (> 33% of right atrium area). The accuracy of the thermodilution technique in patients with low CO (overestimate) or severe tricuspid regurgitation (underestimate) has been questioned.
The Fick method measures pulmonary blood flow using principles described by Adolph Fick in 1870. This is obligatory when systemic-to-pulmonary shunt is present. It requires documentation of both O2 consumption (VO2) and the arteriovenous oxygen difference (∆a-vO2). Ideal determination of VO2 can be done by collecting the patient’s exhaled air over several minutes, or by metabolic carts at bedside using indirect calorimetry or assuming a basal O2 consumption of 3.5 mL/kg or 125 mL/m2. Calculation of ∆a-vO2 requires simultaneous determination of arterial and mixed venous O2. When comparing changes in CO/CI by this method (e.g. before and after PH therapy), one must remember that significant changes in hemoglobin levels could account for differences in results.
Hoeper et al[12] showed, from 105 CO measurements in 35 patients, that thermodilution was equally accurate over a broad spectrum of CO values ranging from as low as 1.7 L/min to as high as 7.8 L/min. In addition, the agreement between the Fick method and thermodilution was not affected by the severity of tricuspid regurgitation.
Vasoreactivity testing is indicated in patients with idiopathic PAH, heritable PAH and associated anorexigen use to detect patients who can be treated with high doses of calcium channel blockers (class I-level C)[1,5]. A positive response to vasoreactivity testing is defined as a reduction of mPAP ≥ 10 mmHg to reach an absolute value of mPAP ≤ 40 mmHg with an increased or unchanged CO (class I-level C)[13]. Vasoreactivity testing should be performed only in referral centers and using nitric oxide (class IIa-level C), iv epoprostenol, iv adenosine or inhaled iloprost (class IIb-level C)[1].
In those patients with risk factors for LV diastolic dysfunction, acute vasoreactivity testing can lead to a significant increase in both LV end-diastolic pressure and PAOP, which unmasks the presence of impaired relaxation of the LV, resulting in acute pulmonary edema. A dramatic v wave pressure increase of the PAOP during vasoreactivity testing alerts the occurrence of this situation.
Some patients with pulmonary vascular disease are not symptomatic at rest, but have symptoms with exertion. This observation provides the potential for exercise or volume challenge during RHC to better diagnose early pulmonary vascular disease. These procedures have not been standardized and each cardiac catheterization laboratory has its own protocol. A typical amount of infused fluid varies between 500 to 1000 mL of normal saline, taking measurements every 250 mL. Challenge is interrupted when PAOP is > 18 mmHg or symptoms appear. An increase in PAOP to greater than 15 mmHg in response to exercise or fluid challenge suggests the presence of pulmonary venous hypertension, a condition with dramatically different management than PAH[1,4,7].
Calculation of PVR is essential in the management of patients with suspected PH. However, arterial pressure and ventricular load are also dependent on total arterial compliance (Cp). A simple approximation of Cp is the ratio of stroke volume to pulse pressure. In a prospectively obtained cohort of 104 patients with primary PH, Mahapatra et al[14] analyzed Cp as a predictor of mortality after adjusting for other modifiers of risk. During 4-year follow-up, 21 patients died and they demonstrated that Cp is a strong independent predictor of mortality in patients with PAH (ROC area of 0.91).
The hemodynamic prognostic parameters used in PAH are based on patient cohorts and have included: right atrium pressure > 12 mmHg, CI ≤ 2 L/m2 per minute, mixed venous O2 saturation < 63% and in exercise RHC, the inability to augment CO and to reduce PVR. A Cp < 0.81 mL/mmHg predicted a < 40% probability of survival at 4 years, and a Cp > 2 mL/mmHg predicted a 100% survival. The presence of angina, presyncopal symptoms or frank syncope in response to exercise are also poor prognosis factors[1,14,15].
The PA occlusion technique can be used in intact animals and patients for the determination of an effective Pcap, and for the partitioning of PVR into an arterial segment and a capillary-venous segment[6-9].
Recently, Kim et al[16], based on PVR being partitioned into large arterial (upstream, Rup) and small arterial plus venous (downstream) components, determined the risk of surgery in chronic thromboembolic pulmonary hypertension (CTEPH) patients with a highly elevated PVR who underwent pulmonary endarterectomy (PEA) through preoperative assessment of microvascular disease. Using a standard Swan-Ganz catheter, the pulmonary pressure signal was filtered using a two-pole digital low-pass filter with a cutoff at 18 Hz. A bi-exponential fitting of the pressure decay curve was then performed, which allowed estimation of the derived occlusion pressure (Poccl = Pcap). Rup (mPAP-Pcap/mPAP-PAOP) could identify CTEPH patients at high risk for residual PH (PVR > 3 wu) and poor outcome after PEA. In patients with small-vessel arteriopathy the Poccl pressure was higher (a longer time is required for the pressure to reach PAOP), and therefore the Rup was lower. In this cohort, the only postoperative deaths occurred in patients with Rup preoperatively estimated at less than 60%, indicating significant down-stream, inoperable, small-vessel involvement. Thus, patients with lower Rup appear to be at high risk for persistent PH and death after PEA. They concluded that a Rup < 60% appears to be at highest risk secondary to a large microvascular disease[16].
Chronic pulmonary hypertension results from an increase in PVR, which is a simple measure of the opposition to the mean component of flow. However, given the low resistance/high compliance nature of the pulmonary circulation, the pulsatile component of hydraulic load is also critical to consider. The fact that the mean and the pulsatile components of flow are dependent on different portions of the pulmonary circulation suggests that they can be controlled separately, without much overlap. Pulmonary arterial pulse pressure (pPAP) indicates the amplitude of pulsatile stress. pPAP is mainly determined by both the characteristics of ventricular ejection and arterial compliance, so that the lower the compliance, the higher the pPAP. It has been reported that pulsatility as the ratio of pPAP to mPAP [i.e. fractional pulse pressure (fPp)] in CTEPH was larger than in PAH and that in patients with severe haemodynamic impairment (PVR > 1000-1100 dyne s/cm5), fPp in addition to PVR might be useful in predicting the outcome of PEA. fPp might be low in CTEPH with inaccessible distal thrombi and/or secondary pulmonary hypertensive change[17].
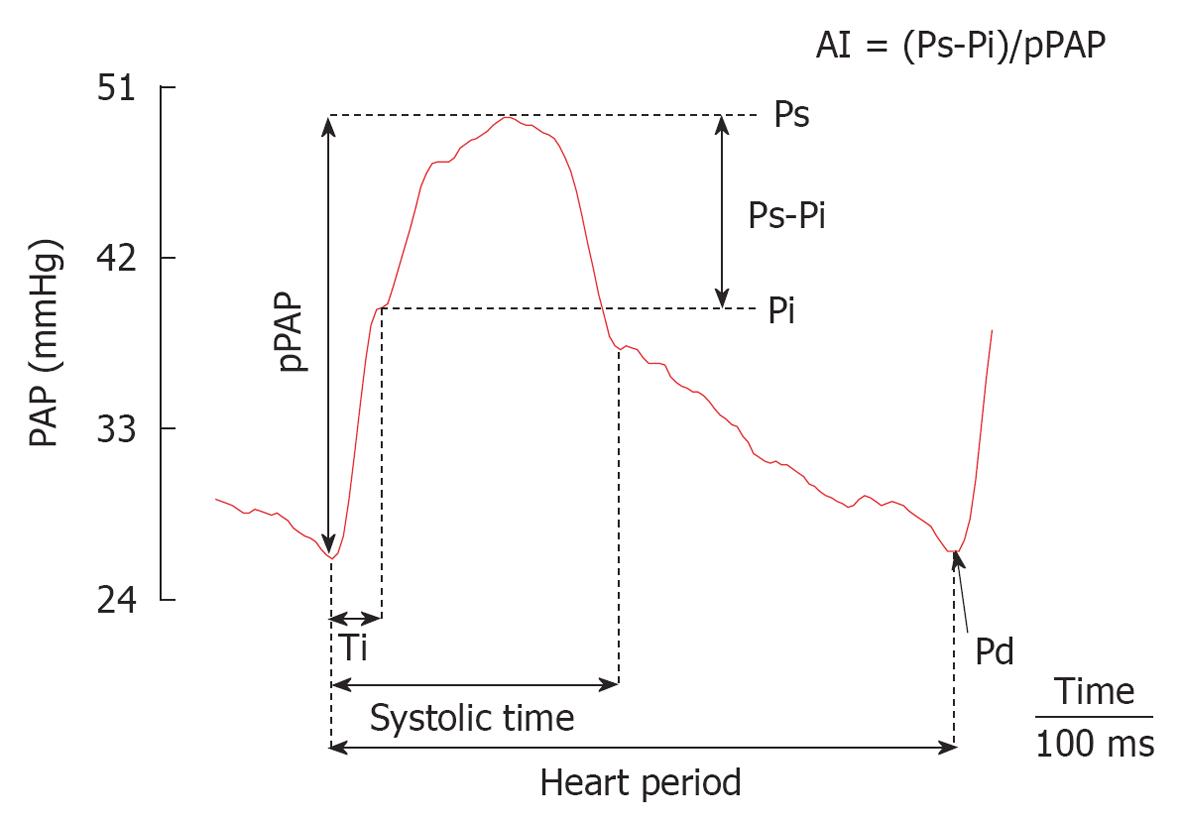
The main pulmonary arterial pressure waveform (PAPW) qualitative morphology analysis may be of diagnostic and prognostic value in patients with PH. A main PAPW is the result of an interaction between the heart and the arterial system[18]. We can estimate the amplitude of the forward (Pi-dPAP) and reflected (sPAP-Pi) pressure waves depending on the inflection point (Pi), where dPAP and sPAP are diastolic and systolic PAP. Since, in most cases, the inflection in pressure was smooth, the Pi is defined as the time at which the first derivative of PAP (dPAP/dt) reached its first minimum. The augmentation index (AI) can be estimated by the ratio of sPAP-Pi to pPAP and the timing of wave reflection was quantified by the inflection time (Ti, Figure 4). Therefore, PAPW with amplitude sPAP-dPAP is composed of a forward traveling wave, with amplitude Pi-dPAP generated by the RV ejection and a later arriving reflected wave, with amplitude sPAP-Pi from the periphery. The forward wave is dependent largely upon elastic properties of the main PA and is not influenced by wave reflections. The reflected wave is dependent upon the elastic properties of the entire arterial tree, pulse wave velocity, the round-trip travel time of the wave from the heart to the periphery and back, and the distance to the major reflecting sites[18,19].
Castelain et al[20] analyzed high-fidelity PA pressure in 14 patients with CTEPH (n = 7) and primary PH (n = 7). They showed that CTEPH patients had shorter Ti and higher AI, with an increased and anticipated wave reflection as compared with primary PH, suggesting differences in the pulsatile component of the RV afterload.
Finally, the extra workload due to wave reflection (wasted pressure or energy the RV must generate during ejection) can also be estimated as EW = [(Ts-Ti)(sPAP-Pi)π/2], where Ts corresponds to systolic time. We have shown that under steady isobaric condition, RV pulsatile load (AI) is attenuated during active PH by maintaining main PA stiffness (Pi-dPAP) and reducing the extent of the reflected wave (sPAP-Pi, EW)[21].
In clinical settings, PAPW analysis might help in evaluating the severity of different forms of PH. Vasculopathy of PAH involves both distal resistive arteries and proximal conduit arteries, which would determine different dynamic RV afterload with different RV adaptation.
As was mentioned previously, the PAPW mainly depends on both the characteristics of ventricular ejection and the properties of the arterial circulation, which determine the compliance (cushioning capacity) and the transmission properties of the arterial system (timing and intensity of arterial wave). The extent of vascular obstruction and associated vasculopathy are the major determinants of the mPAP. However, a more proximal occlusive site and a higher PA stiffness determine an earlier and greater wave reflection, which in turn increases sPAP and decreases dPAP pressures (“ventricularization” of the PA pressure curve), with non significant changes in mPAP and PAOP[22,23]. Pulmonary vascular capacitance (Cp) reflects the ability of the pulmonary vessels to dilate during systole and recoil during diastole. By storing blood during systole, a high capacitance tree dampens the sPAP, and by recoiling during diastole, a high Cp increases dPAP.
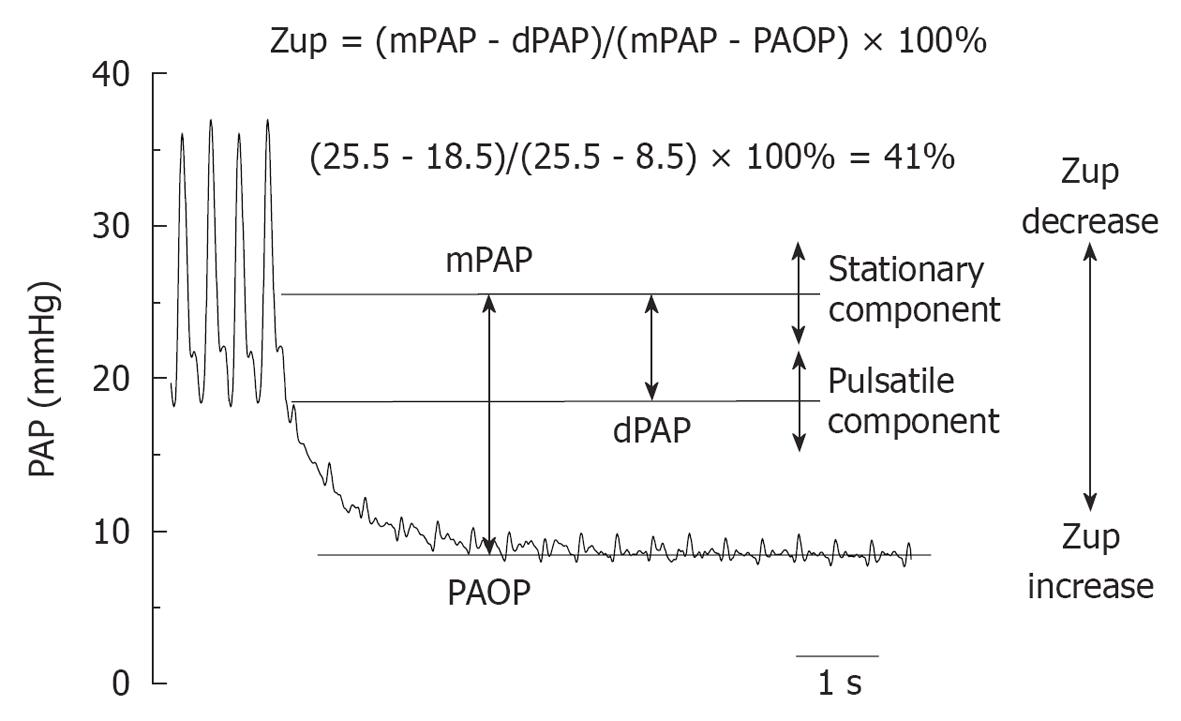
Taking into account that the difference between mPAP and PAOP is in proportion with PVR, and that dPAP depends on PA recoil during diastole and the timing and degree of wave reflection, as well as heart rate, mPAP - PAOP is a measure of the steady component of afterload, while mPAP - dPAP is an indirect measure of the pulsatile component of the afterload. The ratio between both (mPAP-dPAP/mPAP-PAOP, named upstream pulmonary vascular impedance, Zup) would be evaluated pulsatile and steady afterload components simultaneously (Figure 5). Therefore, Zup is in proportion to the upstream impedance (a higher upstream impedance determines lower dPAP and vice versa)[24].
We recently analyzed preoperative Zup in operable (I, n = 32) and inoperable (II, n = 31) CTEPH patients to compare it with PVR, Cp and fPp. In I, 5 patients died during the first 30 d after surgery and had higher basal PVR and lower basal Zup, Cp index and fPp than survivors (P < 0.05). Patients with residual pulmonary hypertension (RPH) had significantly lower Zup and lower improvement of Cp index and pulse pressure 1 year after PEA, than patients without residual PH. Although, operable (I) and non-operable (II) patients showed the same mPAP, basal Zup in I was significantly higher than in II (58 ± 15; 50 ± 14%). Zup showed the highest ROC area for discriminating poor outcome (0.923) with a cut-off value of 47%. We concluded that early mortality and RPH after PEA may be better discriminated by preoperative Zup than by steady (PVR) or pulsatile (fPp) pulmonary afterload alone. Among operable patients with good evolution 1 year after PEA, a lower Zup could be predictive of RPH, which is associated with a lower improvement of capacitance index and pulse pressure[25].
The lower Zup in inoperable CTEPH (II) and in idiopathic PAH patients (isobaric steady component analysis) with respect to operable CTEPH (I), is in accordance with a more diffuse distribution of RV afterload and would be related to different vascular wall remodeling (thrombus organization and small vessel arteriopathy)[25,26]. This could also explain why CTEPH lung is virtually indistinguishable from IPAH lung at the histopathologic level at autopsy and in biopsy studies.
PAH is a disease of the arterial vessel wall that affects both steady and pulsatile components of the pulmonary arterial hemodynamics. PA stiffness is an important factor governing dynamic afterload. It has also been reported that proximal PA stiffness may increase early in the course of PH, suggesting a potential contributory role of PA stiffness in the development and progression of PH[27]. Increased stiffness observed in PA hypertension may be secondary to elevated distending pressures and/or to structural changes of the PA wall.
Intravascular ultrasound (IVUS) assessment of the pulmonary circulation has been proposed as a technique to evaluate the arterial wall changes observed in the elastic pulmonary vessels of patients diagnosed with PAH. There has been preliminary data on in vivo PA pulsatility evaluation in PA hypertension[28]. The arterial pulsatility depends on the the intraluminal pressure and on the intrinsic viscoelastic properties of the arterial wall. The absence of the relation between pulsatility and hemodynamic variables, described in some studies, suggests that the changes in pulmonary vessel remodeling may be responsible for the functional alterations shown by IVUS. It has already been reported that only indexes relating pressure and diameter followed qualitative changes of the elastic incremental modulus (a true evaluator of the elastic status of the vessel wall from the stress-strain relationship) in an animal model of acute PH[29]. Rodés-Cabau et al[30] demonstrated PA wall abnormalities in all patients with primary PH, mostly eccentric. Although the severity of the impaired PA functional state did not correlate with hemodynamic variables, it was associated with mortality at follow up of these patients.
We recently evaluated local PA stiffness in a cohort of patients with PAH and its relation with dynamic afterload. This study showed that only local PA stiffness indexes normalized by pulse pressure correlated with global capacitance and resistance properties of the pulmonary arterial tree. Neither the hemodynamic capacitance index nor pulmonary vascular resistance correlated with IVUS pulsatility[31].
Elastic modulus, local and dynamic compliance and distensibility may be an expression of the intrinsic PA wall remodeling and viscoelastic properties, and should be used to better understand the histopathological changes and response to treatment of the pulmonary circulation in PAH.
Instead of trying to quantify the RV afterload by different approaches, it might be more appropriate to quantify the RV function directly and to couple to the pulmonary circulation. Sagawa et al[32] showed that this can be done graphically using a ven-tricular pressure-volume diagram. The diagram allows for the determination of maximal or end-systolic ventricular elastance (Ees, end-systolic pressure on end-systolic volume), which is the best possible load-independent measurement of contractility, of arterial elastance (Ea, end-systolic pressure on stroke volume), as a measurement of afterload as it is ‘seen’ by the ventricle, and of the calculation of an Ees/Ea ratio as a measurement of the coupling of ventricular to arterial function.
The complex geometry of the RV makes functional evaluations with measurement of instantaneous volume changes technically difficult, and the determination of Ees may be unreliable because of the triangular shape of the RV pressure-volume loop resulting from the fact that RV ejection continues after end-systole. This difficulty in measuring instantaneous RV volume is overcome using a single-beat method, which derives a systolic pressure-volume relationship from instantaneous RV pressure and an integration of pulmonary arterial flow[3]. On such a pressure-volume curve, it is easy to determine graphically Ees and Ea. The optimal value of the Ees/Ea ratio, compatible with flow output at a minimal energy cost, is between 1 and 2. Patients with severe PH present with a decreased elastance ratio, in spite of an adaptative increase in systolic function, which underscores that RV failure in the face of increased afterload is a relative notion. Recently, Kuehne et al[33] used magnetic resonance imaging together with RV pressure measurements to generate pressure-volume loops and to determine Ees and Ea values in patients with PAH. As compared with controls, RV Ees was increased from 5.2 ± 0.9 mmHg/mL per 100 g to 9.2 ± 1.2 mmHg/mL per 100 g (P < 0.05), but RV Ees/Ea was decreased from 1.9 ± 0.4 to 1.1 ± 0.3 (P < 0.05), indicating an increased RV contractility in response to increased afterload that was, however, insufficiently coupled to its hydraulic load, with inefficient mechanical work production.
Further studies are needed to confirm that right ventriculo-arterial decoupling accounts for a decreased aerobic exercise capacity by a limitation of cardiac output adaptation to peripheral demand.
While traditional resting RHC techniques still remain the gold standard for diagnosing PH and managing patients on therapy, there are novel invasive techniques that do not add significant time or risk to the procedure that add greatly to our understanding of the RV, pulmonary circulation, and the interaction between them, and for the interpretation of data from clinical trials as well.
Peer reviewers: Mashio Nakamura, MD, PhD, Department of Cardiology, Mie University Graduate School of Medicine, 2-174, Edobashi, Tsu 514-8507, Japan; Serafino Fazio, Associate Professor of Internal Medicine, Department of Internal Medicine, Cardiovascular and Immunologic Sciences, University Federico II, Via S. Pansini 5, 80131 Naples, Italy
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM
| 1. | Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55-S66. |
| 2. | Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992-1007. |
| 3. | Naeije R, Huez S. Right ventricular function in pulmonary hypertension: physiological concepts. Eur Heart J. 2007;9 Suppl:H5-H9. |
| 4. | Chemla D, Castelain V, Hervé P, Lecarpentier Y, Brimioulle S. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J. 2002;20:1314-1331. |
| 5. | Escribano Subias P, Barberà Mir JA, Suberviola V. Current diagnostic and prognostic assessment of pulmonary Hypertension. Rev Esp Cardiol. 2010;63:583-596. |
| 6. | Takala J. Pulmonary capillary pressure. Intensive Care Med. 2003;29:890-893. |
| 7. | Soto FJ, Kleczka JF. Cardiopulmonary hemodynamics in pulmonary hypertension: pressure tracings, waveforms, and more. Adv Pulm Hypertension. 2008;7:386-393. |
| 8. | Fesler P, Pagnamenta A, Vachiéry JL, Brimioulle S, Abdel Kafi S, Boonstra A, Delcroix M, Channick RN, Rubin LJ, Naeije R. Single arterial occlusion to locate resistance in patients with pulmonary hypertension. Eur Respir J. 2003;21:31-36. |
| 9. | Ganter CC, Jakob SM, Takala J. Pulmonary capillary pressure. A review. Minerva Anestesiol. 2006;72:21-36. |
| 10. | Halpern SD, Taichman DB. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest. 2009;136:37-43. |
| 11. | Cope DK, Grimbert F, Downey JM, Taylor AE. Pulmonary capillary pressure: a review. Crit Care Med. 1992;20:1043-1056. |
| 12. | Hoeper MM, Maier R, Tongers J, Niedermeyer J, Hohlfeld JM, Hamm M, Fabel H. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:535-541. |
| 13. | Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105-3111. |
| 14. | Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799-803. |
| 15. | Barberà JA, Escribano P, Morales P, Gómez MA, Oribe M, Martínez A, Román A, Segovia J, Santos F, Subirana MT. [Standards of care in pulmonary hypertension. Consensus statement of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Spanish Society of Cardiology (SEC)]. Rev Esp Cardiol. 2008;61:170-184. |
| 16. | Kim NH, Fesler P, Channick RN, Knowlton KU, Ben-Yehuda O, Lee SH, Naeije R, Rubin LJ. Preoperative partitioning of pulmonary vascular resistance correlates with early outcome after thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Circulation. 2004;109:18-22. |
| 17. | Tanabe N, Okada O, Abe Y, Masuda M, Nakajima N, Kuriyama T. The influence of fractional pulse pressure on the outcome of pulmonary thromboendarterectomy. Eur Respir J. 2001;17:653-659. |
| 18. | Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543-551. |
| 19. | Nakayama Y, Nakanishi N, Sugimachi M, Takaki H, Kyotani S, Satoh T, Okano Y, Kunieda T, Sunagawa K. Characteristics of pulmonary artery pressure waveform for differential diagnosis of chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol. 1997;29:1311-1316. |
| 20. | Castelain V, Hervé P, Lecarpentier Y, Duroux P, Simonneau G, Chemla D. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol. 2001;37:1085-1092. |
| 21. | Grignola J, Devera L, Ginés F. Main pulmonary artery pressure waveform analysis: a time-domain approach for complete hemodynamic evaluation during pulmonary hypertension. Circulation. 2008;118:e379. |
| 22. | Grant BJ, Lieber BB. Clinical significance of pulmonary arterial input impedance. Eur Respir J. 1996;9:2196-2199. |
| 23. | Chemla D, Castelain V, Simonneau G, Lecarpentier Y, Hervé P. Pulse wave reflection in pulmonary hypertension. J Am Coll Cardiol. 2002;39:743-744. |
| 24. | Grignola JC, Ruiz-Cano MJ, Escribano P, Cortina J, Velazquez T, Gomez-Sanchez MA, Delgado J, Saenz De La Calzada C. Assessment of early outcome and residual pulmonary hypertension after pulmonary endarterectomy by preoperative analysis of dynamic right ventricular afterload. Eur Heart J. 2009;30 Suppl:110. |
| 25. | Ruiz-Cano MJ, Grignola JC, Escribano P, Cortina J, Velázquez T, Gómez Sánchez MA, Delgado JF, Sáenz de la Calzada C. Preoperative partitioning of pulmonary vascular impedance: a novel hemodynamic index for operable and inoperable chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2010;55:A171. |
| 26. | Grignola JC, Ruiz-Cano MJ, Escribano P, Gomez-Sanchez MA, Tello De Meneses R, Delgado J, Jimenez Lopez-Guarch C, Saenz De La Calzada C. Isobaric analysis of pulmonary arterial compliance of idiopatic and chronic thromboembolic pulmonary hypertension: correlation with right ventricular remodeling. Eur Heart J. 2009;30 Suppl:108. |
| 27. | Sanz J, Kariisa M, Dellegrottaglie S, Prat-González S, Garcia MJ, Fuster V, Rajagopalan S. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2:286-295. |
| 28. | Bressollette E, Dupuis J, Bonan R, Doucet S, Cernacek P, Tardif JC. Intravascular ultrasound assessment of pulmonary vascular disease in patients with pulmonary hypertension. Chest. 2001;120:809-815. |
| 29. | Santana DB, Barra JG, Grignola JC, Ginés FF, Armentano RL. Pulmonary artery smooth muscle activation attenuates arterial dysfunction during acute pulmonary hypertension. J Appl Physiol. 2005;98:605-613. |
| 30. | Rodés-Cabau J, Domingo E, Román A, Majó J, Lara B, Padilla F, Anívarro I, Angel J, Tardif JC, Soler-Soler J. Intravascular ultrasound of the elastic pulmonary arteries: a new approach for the evaluation of primary pulmonary hypertension. Heart. 2003;89:311-315. |
| 31. | Grignola JC, Domingo E, Bravo C, Aguilar R, Lopez-Messeguer M, Vazquez M, Roman A. Local pulmonary artery stiffness indexes are correlated with steady and pulsatile components of right ventricular afterload in pulmonary arterial hypertension. J Am Coll Cardiol. 2010;55:A172. |
| 32. | Sagawa K, Maughan L, Suga H, Sunagawa K. Cardiac contraction and the pressure-volume relationship. New York: Oxford University Press 1988; . |
| 33. | Kuehne T, Yilmaz S, Steendijk P, Moore P, Groenink M, Saaed M, Weber O, Higgins CB, Ewert P, Fleck E. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: in vivo validation and clinical application in patients with pulmonary hypertension. Circulation. 2004;110:2010-2016. |