Published online Aug 26, 2010. doi: 10.4330/wjc.v2.i8.251
Revised: July 13, 2010
Accepted: July 20, 2010
Published online: August 26, 2010
AIM: To evaluate the prevalence of hypertension and/or left ventricular hypertrophy (LVH) in children with a diagnosis of obstructive sleep apnea (OSA).
METHODS: A cross-sectional case series of consecutive, otherwise healthy children aged > 4 years, with polysomnography-proven OSA [apnea hypopnea index (AHI) > 1.5/h] is described. Echocardiography was performed on all subjects and left ventricular mass was calculated. Study subjects underwent additional investigation with 24-h ambulatory blood pressure (BP) monitoring.
RESULTS: Thirty children (21 males) were studied. Mean age was 8.9 years. Mean body mass index was 19.87 kg/cm2. Mean AHI was 14.3/h. 10/30 (33%) of the study population met criteria for pre-hypertension (n = 3) or masked hypertension (n = 7) based on standard ambulatory monitoring criteria. All 10 children had systolic hypertension throughout the night with 5 of these also having elevated daytime systolic readings. There was a relationship between AHI and BP showing an increase of 1.162 percentile units in mean diastolic night BP (age, gender and height specific) per unit increase in AHI (P = 0.018). There were no subjects with LVH and/or right ventricular hypertrophy.
CONCLUSION: In our population of otherwise healthy Caucasian children, there was a high prevalence of hypertension that would not have been identified using standard office/clinic protocols.
- Citation: Kirk V, Midgley J, Giuffre M, Ronksley P, Nettel-Aguirre A, Al-Shamrani A. Hypertension and obstructive sleep apnea in Caucasian children. World J Cardiol 2010; 2(8): 251-256
- URL: https://www.wjgnet.com/1949-8462/full/v2/i8/251.htm
- DOI: https://dx.doi.org/10.4330/wjc.v2.i8.251
It has been well established that obstructive sleep apnea (OSA) causes increased mortality and significant cardiovascular complications such as pulmonary hypertension, systemic hypertension, and cerebral and coronary artery disease in adults[1,2]. Importantly, Davies et al[3] reported a significant decrease in nocturnal systolic blood pressure (SBP) following treatment of OSA with nasal continuous positive airway pressure in a group of adults with hypertension compared to untreated matched control subjects. These associations have not yet been fully studied in the pediatric population but there are several earlier reports describing pulmonary hypertension, cardiorespiratory failure, coma, and even sudden death associated with adenotonsillar hypertrophy and upper airway obstruction in children[4-6]. More recently, Marcus et al[7] reported elevated diastolic BP during both wakefulness and sleep in a group of 41 children with OSA compared to a control group of 26 with primary snoring. The degree of BP elevation was related to the severity of OSA. Subsequent pediatric studies have reported variable effects of OSA on BP in children, including elevation in either diastolic or systolic pressure alone, in part due to the variability in methodology and patient population studied[2,8,9]. Recently, O'Driscoll et al[10] described minute to minute increases in both heart rate and BP occurring with individual apneic events in children undergoing polysomnography (PSG). Finger photoplethysmography, a surrogate measure of BP, revealed that obstructive events during non-rapid eye movement sleep were associated with the largest increase in mean arterial pressure, particularly when associated with an arousal.
In adults, left ventricular mass (LVM), a surrogate measure of left ventricular hypertrophy (LVH), is a sensitive and specific predictor of clinical events attributed to cardiovascular diseases and it can be accurately measured by echocardiography[11,12]. Recently, Amin et al[8] reported that OSA in children is associated with structural changes of the heart, including increased LVM. They studied 28 children with OSA and identified abnormalities of LVM in 39% (vs 15% in snoring controls). They concluded that the increased LVM was not related to systemic hypertension, as few subjects had evidence of the latter condition. However, only a single BP measurement was obtained on all subjects, and it was recorded during wakefulness[8]. Subsequent papers by the same investigator have reported elevated SBP as measured by ambulatory monitoring as well as some evidence of diastolic dysfunction, both of which were associated with the severity of OSA[2,13]. Only one group to date has reported BP changes consistent with a diagnosis of pediatric hypertension related to OSA in children[9]. Our aim was to confirm a relationship between OSA and hypertension in children and to evaluate the effect of increasing severity of OSA on BP. Our paper adds to the current body of knowledge by confirming a high prevalence of hypertension in otherwise healthy children with PSG-proven OSA that appears to be related to the severity of OSA.
A cross-sectional case series of consecutive, otherwise healthy children aged > 4 years with PSG-proven OSA is described. Children with cardiac or renal disease and those with known risk factors for hypertension were excluded. Eligible subjects were identified by the principal investigator (VK) following confirmation of OSA by PSG. Families were invited to participate when PSG results were discussed with them via telephone. Those indicating an interest in the study were then contacted by one of the study coordinators (AA, PR) and consent was obtained at that time from parents of subjects < 8 years. Both parental and subject consent was obtained in those aged 12 years and older. Assent was obtained in all children aged 8 to 12 years. Echocardiography was performed on all subjects, as per our routine clinical care. Study subjects underwent additional investigation with 24-h ambulatory BP monitoring (see below). Medical charts were reviewed for demographic and medical information in order to confirm that subjects met inclusion criteria. Ethics approval was obtained by the University of Calgary prior to enrollment of the first subject.
Computerized laboratory PSG (Sandman® NT), (Nellcor Puritan Bennett, Ottawa, ON) was performed according to American Thoracic Society guidelines at the Alberta Children’s Hospital sleep laboratory[14]. Monitoring included electroencephalogram (C4-A1, C3-A2, O1-A2, O2-A1), electrooculogram, submental electromyogram, electrocardiogram, oxygen saturation monitoring, chest, abdominal wall, and sum channel movements using respiratory inductance plethysmography, bilateral tibial electromyograms, nasal/oral airflow using a thermistor device, nasal pressure, end-tidal carbon dioxide monitoring, and transcutaneous carbon dioxide monitoring. Sleep architecture was determined using standard criteria[15]. A registered sleep technologist scored sleep architecture and respiratory events using standard scoring criteria. Obstructive apnea was defined as a reduction in airflow of > 80% associated with continued abdominal and chest wall motion lasting 2-3 breaths in duration, obstructive hypopnea was defined as a reduction in airflow of > 50% but < 80% associated with continued abdominal and chest wall motion associated with EEG arousal and/or > 3% drop in oxygen saturation, and central apnea was defined as a reduction in airflow of > 80% associated with no evidence of abdominal or chest wall motion lasting 20 s in duration, or shorter, if associated with > 3% drop in oxygen saturation. Central apneas occurring in association with body movements or sighs were not included in the overall apnea hypopnea index (AHI). Mixed apnea, consisting of both central and obstructive components, was scored using the same criteria as obstructive apneas. OSA was defined as an overall apnea hypopnea index of > 1.5/h of total sleep time[14,16-19].
2D mode echocardiography was used to assess the LVM using the methodology of Devereux et al[20]. Measurements were made with the subject in a supine, resting state for at least 5 min in a quiet, darkened examination room. All studies were interpreted by a blinded study cardiologist (MG). As per the de Simone et al[21] protocol, a LVM index was calculated. This calculation takes into consideration the impact of body size on heart size and improves the accuracy of the measurements. LVM index was calculated by dividing the measured LVM by subject height raised to the power 2.7.
Ambulatory cuff BP was measured using the QuietTrack® instrument (manufacturer Welch Allyn). Measurements were obtained using the right arm and the appropriate cuff size, based on standard criteria[22]. Calibration was performed prior to recording of BP values. Measurements were obtained every 20 min during the day (start at usual wake time) and every 30 min during the night (start at usual bedtime). All subjects were asked to maintain their usual activity but to remain still during daytime measurements. Using the software provided by Welch Allyn, average systolic and diastolic BP and BP load (percentage of BP measurements > 95th percentile) were calculated for each subject and compared to standard normative data for ambulatory BP monitoring (ABPM) in children adjusted for age, gender and height percentiles[22]. Nocturnal, daytime, systolic and diastolic hypertension were defined using standard age, gender and height criteria for children[22]. Briefly, children with a SBP load greater than 25% met criteria for hypertension. If associated with a normal mean SBP but high clinic BP, they were classified as pre-hypertensive. If the mean ambulatory SBP was greater than the 95th percentile, they were classified as having either masked hypertension (normal clinic BP) or hypertension (clinic BP also greater than the 95th percentile)[18]. BP data was also analyzed by converting mean daytime and night-time measurements to body mass index (BMI) and height specific percentiles according to published standards[23,24]. All ambulatory monitoring data were analyzed by the study nephrologist (JM).
A convenience sample size of 30 children with OSA was studied. Based on previous reports[8], we anticipated a prevalence of LVH in children with OSA of approximately 5%. Based on these predictions, 2 study subjects with evidence of LVH were needed to provide an exact binomial confidence interval of 0.8% to 22.1%.
To describe the relationship between hypertension and apnea AHI, apnea arousal index (AAI), BMI, and BMI percentile, logistic regression coefficients were estimated for the slopes and intercept to examine whether there was a relationship between the variables. The relationship between BP as a numerical value (age, gender and height specific percentiles) and simultaneous polysomnographic data (AHI, AAI, and hypoxemia index) were examined via multiple linear regression. Multiple regression was used to examine each of the responses separately (mean systolic day/night BP, mean diastolic day/night BP as age and gender percentiles) vs AHI, AAI and BMI percentiles, hence a conservative significance level of 0.0125 (Bonferroni correction) for the slopes should be considered when interpreting their significance. All BP parameters were analyzed separately for day and night results.
A total of 43 children met inclusion criteria between April 1, 2004 and January 31, 2006. Of these, 30 (21 males) agreed to participate. Children were aged on average 8.9 years (range 4 years and 1 mo to 15 years and 1 mo). The mean BMI was 19.87 kg/cm2 (range 13.6-44.1 kg/cm2). Nine of 30 (26%) subjects were obese based on a BMI percentile > 95. The mean AHI value for the group was 14.3/h (range 1.5-125.2/h). The mean hypoxemia index (percent of total sleep time with oxygen saturation < 90%) was 1.3% (range 0%-19.9%).
10/30 (33%) of the subjects had systolic hypertension (> 25% measurements > 95th percentile for age and height percentiles) with the vast majority (90%) of high measurements occurring during the night. Four of the 10 also had elevated daytime systolic readings and 2 of the 10 had elevated diastolic readings in the night. As per our current protocol, manual BP with ABPM was not done at the same time as clinic baseline BP measurements. Of the polysomnographic variables, there were no significant relationships between the AHI, hypoxemia index or AAI variables and BP status overall (Table 1). A logistic regression of hypertension status on AAI, BMI and AHI was performed. All showed a coefficient of positive numerical value, which suggests an indication of positive relationship with hypertension, but none reached statistical significance.
| Hypertension, OSA (n = 10) | OSA, no hypertension (n = 20) | 95% CI for difference | |
| Age (yr) | 8.61 | 9.01 | -1.65, 2.46 |
| Male, n (%) | 8 (80) | 13 (65) | -54.93, 24.93 |
| Body mass index (kg/cm2) | 19.16 | 20.23 | -2.89, 5.03 |
| Desaturation index (%) | 1.09 | 1.36 | -2.68, 3.22 |
| Oxygen saturation mean (%) | 96.4 | 96.1 | -1.30, 0.71 |
| Oxygen saturation minimum (%) | 87.3 | 85.6 | -6.36, 3.02 |
| Spontaneous arousal index (/h) | 3.5 | 3.7 | -1.20, 1.67 |
| Apnea arousal index (/h) | 10.8 | 3.7 | -24.52, 10.25 |
| Apnea hypopnea index (/h) | 21.5 | 10.6 | -39.13, 17.25 |
The relationship between AHI and hypertension was also analyzed as categorical; that is, for each of the day/night BPs using the variable “percentage of time with systolic (diastolic) BP greater the 95th percentile for day (night)”. Abnormal was defined as > 25%. Although inspection of the dot plots suggested a trend towards higher AHI in those with nocturnal systemic hypertension, they did not meet statistical significance. There were two outliers whose extreme AHI and AAI values set them clinically apart from the rest of the data. We elected to exclude these and re-analyze in the event that they were affecting the results of the others. The conclusions were unchanged.
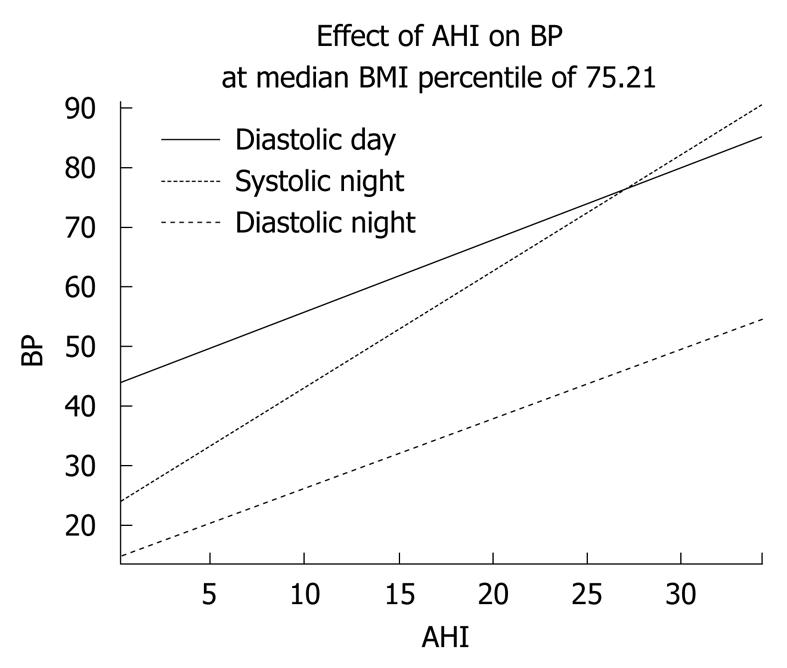
A regression analysis on this subset of 28 subjects, using mean BP values, revealed a linear relationship between AHI and mean daytime diastolic BP, as well as mean systolic night and mean diastolic night BP (Table 2). BMI percentile was identified as a confounder only. The effect of AHI on BP was adjusted for this. As seen in Table 2, for every unit change in AHI, mean systolic night BP (as an age and gender specific percentile) increased by 1.964 percentile points (P = 0.025). The interpretations of the other coefficients shown were similar. This relationship is shown in graphical form in Figure 1.
| BP (percentile) | Severity of OSA | Coefficient (increase of BP in percentual points) | P value |
| Diastolic day | AHI increase of 1/h | 1.211 | 0.046 |
| Systolic night | AHI increase of 1/h | 1.964 | 0.025 |
| Diastolic night | AHI increase of 1/h | 1.162 | 0.018 |
LVM was within normal limits (less than the 95th percentile for age) in all subjects and there was no evidence of LVH in any of the children. Right ventricular pressures were less than one-third systemic pressures in all subjects, indicating absence of right ventricular hypertrophy.
This study provides strong support that Caucasian children with OSA have a significantly increased prevalence of hypertension. We were able to show a linear relationship between the AHI and both nocturnal systolic and diastolic mean BP as well as daytime mean diastolic BP in children with OSA (Table 2). This confirms the recent report of Li et al[9] concluding that OSA in children is an independent risk factor for nocturnal hypertension. Although evidence of target organ damage due to hypertension is most correlated with night time SBP in children[22], we did not identify evidence of increased LVM in any of our 30 subjects. This is not in keeping with previous reports and may be due to differences in demographics. Prior reports have included evaluation of obese and non-Caucasian children. These factors were controlled for in the overall statistical analysis but perhaps they contributed something to the findings of LVH in those populations[8,13]. We did not specifically study mitral valve inflow as part of our protocol for this study but plan to study diastolic dysfunction in a prospective future analysis to verify the previous report by Amin et al[2,13] and to further describe the relationships between OSA, BP and cardiac function.
Our findings are in contrast to a recently published meta-analysis concluding there was no relationship between OSA in childhood and elevated BPs[25]. This analysis included only 5 papers with the vast majority of BP measurements performed during wakefulness. Additionally, as noted above, the populations studied included a significant proportion of obese (12%-29%) and/or African American/Hispanic children (up to 51%). Race and obesity are independent risk factors for hypertension and may act as confounders when specific relationships between OSA and BP are being examined.
Previous pediatric studies using ABPM also failed to show this relationship. Amin et al[26] evaluated 60 snoring children and did not find any evidence of hypertension in the group. However, the normative references used for their analysis were not specific for ambulatory BP measurements, which could have resulted in inappropriate interpretations. A subsequent and more recent publication by the same investigators[13] evaluating morning BP surge (nocturnal dip) and BP load by ABPM reported an independent association between elevation of all these parameters and increasing AHI (2).
The majority (70%) of children with abnormal BP findings in our study met criteria for masked hypertension, defined as normal day time clinic BP but elevated ambulatory levels. The estimated prevalence of masked hypertension in the general pediatric population is approximately 6%[27] and is associated with progression to sustained clinical hypertension and higher LVM[28]. In adults, masked hypertension has been associated with an increased cardiovascular risk[29] and with progression of chronic kidney disease[30]. The greatly increased prevalence of masked hypertension in our study population is a remarkable finding. Ten percent of the children studied had pre-hypertension [elevated clinic BP and BP load (SBP > 95th percentile for 25%-50% of ABPM readings) with a normal mean ambulatory BP]. Pre-hypertension is an indicator of increased BP variability, which is also associated with target organ damage, at least in adults[31].
In summary, 30% of otherwise healthy Caucasian children with a new diagnosis of OSA had evidence of nocturnal systolic hypertension. This study adds further support to the hypothesis that hypertension due to OSA may begin at a much younger age than previously thought suggesting a potentially powerful role in the prevention of adult hypertension and cardiovascular disease. Currently, ABPM is not part of the standard clinical evaluation of children with OSA. Such measurements during a sleep study may, in fact, cause some sleep disruption and affect the overall PSG results. Further evaluation of the effect of ABPM on sleep architecture is warranted, and if shown to not skew PSG results, consideration should be given for inclusion of BP monitoring during PSG in children as part of the standard testing regimen. Alternatively, surrogate measures of BP, such as finger photoplethysmography, could be further studied to identify how the data obtained minute to minute relates to overall BP status. In the interim, ABPM should be considered following PSG diagnosis of OSA in children as part of the evaluation for end-organ involvement.
Obstructive sleep apnea (OSA) is well established as a cause of hypertension and cardiovascular disease in adults. The pediatric literature related to these issues is preliminary and mixed. We were able to show that blood pressure (BP), but not ventricular geometry or function, is influenced by the severity of OSA in otherwise healthy, Caucasian children.
Marcus et al reported elevated diastolic BP during both wakefulness and sleep in children that was related to the severity of OSA. Amin et al reported structural changes of the heart in children with OSA that did not appear to be BP, based on one measurement obtained while awake. Subsequent papers by the same investigator have reported elevated systolic BP, as measured by ambulatory monitoring, as well as some evidence of diastolic dysfunction, both of which were associated with the severity of OSA. Only one previous group to date has reported BP changes consistent with a diagnosis of pediatric hypertension related to OSA in children.
More recent publications have included data related to both wake and sleeping BP measurements in homogeneous groups of children and may be more helpful in identifying true relationships. The inclusion of obese and/or mixed race children in study populations adds complexity to the interpretation of findings related to risk attributed to OSA on BP and cardiac geometry and function.
The relationship between OSA and cardiovascular disease may begin at a very young age. Learning more about the specifics in children will potentially allow for prevention of a significant proportion of adult cardiovascular disease and/or hypertension and related morbidities.
This article on the relation of sleep apnea to hypertension is of great interest. It is unfortunate that there were no subjects with left ventricular hypertrophy, but it is significant that there is a high prevalence of hypertension in the nighttime using 24 h ambulatory BP monitoring.
Peer reviewer: Mashio Nakamura, MD, PhD, Department of Cardiology, Mie University Graduate School of Medicine, 2-174, Edobashi, Tsu 514-8507, Japan
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM
| 1. | Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19-25. |
| 2. | Amin R, Somers VK, McConnell K, Willging P, Myer C, Sherman M, McPhail G, Morgenthal A, Fenchel M, Bean J. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51:84-91. |
| 3. | Davies RJ, Jenkins NE, Stradling JR. Effect of measuring ambulatory blood pressure on sleep and on blood pressure during sleep. BMJ. 1994;308:820-823. |
| 4. | Menashe VD, Farrehiab C, Miller M. Hypoventilation and cor pulmonale due to chronic upper airway obstruction. J Pediatr. 1965;67:198-203. |
| 5. | Ainger LE. Large tonsils and adenoids in small children with cor pulmonale. Br Heart J. 1968;30:356-362. |
| 6. | Yates DW. Adenotonsillar hypertrophy and cor pulmonale. Br J Anaesth. 1988;61:355-359. |
| 7. | Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098-1103. |
| 8. | Amin RS, Kimball TR, Bean JA, Jeffries JL, Willging JP, Cotton RT, Witt SA, Glascock BJ, Daniels SR. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1395-1399. |
| 9. | Li AM, Au CT, Sung RY, Ho C, Ng PC, Fok TF, Wing YK. Ambulatory blood pressure in children with obstructive sleep apnoea: a community based study. Thorax. 2008;63:803-809. |
| 10. | O'Driscoll DM, Foster AM, Ng ML, Yang JS, Bashir F, Nixon GM, Davey MJ, Anderson V, Walker AM, Trinder J. Acute cardiovascular changes with obstructive events in children with sleep disordered breathing. Sleep. 2009;32:1265-1271. |
| 11. | Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345-352. |
| 12. | Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561-1566. |
| 13. | Amin RS, Kimball TR, Kalra M, Jeffries JL, Carroll JL, Bean JA, Witt SA, Glascock BJ, Daniels SR. Left ventricular function in children with sleep-disordered breathing. Am J Cardiol. 2005;95:801-804. |
| 14. | Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153:866-878. |
| 15. | Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute 1968; . |
| 16. | Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872-878. |
| 17. | Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, Keens TG, Ward SL. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235-1239. |
| 18. | Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. |
| 19. | Verhulst SL, Schrauwen N, Haentjens D, Van Gaal L, De Backer WA, Desager KN. Reference values for sleep-related respiratory variables in asymptomatic European children and adolescents. Pediatr Pulmonol. 2007;42:159-167. |
| 20. | Devereux RB. Detection of left ventricular hypertrophy by M-mode echocardiography. Anatomic validation, standardization, and comparison to other methods. Hypertension. 1987;9:II19-II26. |
| 21. | de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251-1260. |
| 22. | Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433-451. |
| 23. | Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;1-27. |
| 24. | National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555-576. |
| 25. | Zintzaras E, Kaditis AG. Sleep-disordered breathing and blood pressure in children: a meta-analysis. Arch Pediatr Adolesc Med. 2007;161:172-178. |
| 26. | Amin RS, Carroll JL, Jeffries JL, Grone C, Bean JA, Chini B, Bokulic R, Daniels SR. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169:950-956. |
| 27. | Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J, Staessen JA. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension. 2005;45:493-498. |
| 28. | Stabouli S, Kotsis V, Toumanidis S, Papamichael C, Constantopoulos A, Zakopoulos N. White-coat and masked hypertension in children: association with target-organ damage. Pediatr Nephrol. 2005;20:1151-1155. |
| 29. | Björklund K, Lind L, Zethelius B, Andrén B, Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;107:1297-1302. |
| 30. | Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:406-411. |
| 31. | Parati G, Faini A, Valentini M. Blood pressure variability: its measurement and significance in hypertension. Curr Hypertens Rep. 2006;8:199-204. |