INTRODUCTION
Systemic autoimmune diseases represent a family of different pathologies with common pathogenetic mechanisms and occur as a consequence of the loss of physiological tolerance to self antigens. The targets of the autoantibodies are ubiquitous antigens, so that tissue damage is generalized, resulting in multiple organ involvement, including the heart. Circulating antibodies do not always play a pathogenetic role but they represent specific markers of ongoing tissue damage[1].
The most frequent systemic autoimmune diseases are rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), primary antiphospholipid syndrome, systemic sclerosis and systemic vasculitis. Patients affected by these diseases show an increased cardiovascular (CV) morbidity and mortality, only partially related to traditional CV risk factors and mainly due to enhanced atherosclerosis[2,3]. In particular, CV disease occurs at a younger age than in the general population and often remains asymptomatic, at least in the early stages[4].
The excess of CV morbidity and mortality could be explained by specific risk factors strictly related to autoimmune diseases, such as chronic inflammation, disease duration and activity and immunosuppressive therapy [glucocorticoids, methotrexate or anti-tumor necrosis factor α (TNFα)][5]. All components of the heart can be potentially affected by several pathogenetic mechanisms involving valves, coronary arteries, conduction system, myocardium, endocardium and pericardium such that a wide spectrum of clinical manifestations can occur; e.g. pericarditis, myocarditis and myocardial fibrosis, rhythm and conduction disturbances, coronaritis with ischemic heart disease, valvular diseases, pulmonary hypertension, syncope, diastolic or systolic heart failure[6].
Several studies have shown that chronic inflammation plays an important role in the development of atherosclerotic plaque[7] and endothelial dysfunction; in particular, a reduced bioavailability of nitric oxide (NO) seems to be the primum movens in this process[8]. Asymmetric dimethylarginine (ADMA) is widely recognized as the major endogenous inhibitor of NO-synthase and is considered an emerging CV risk factor. Elevated plasma ADMA levels have been found in patients affected by systemic autoimmune diseases, for example, in RA patients[9,10].
Since CV damage in autoimmune diseases is characterized by adverse outcomes, an early identification of patients at higher risk is very important to improve long term prognosis. CV imaging techniques provide a reliable approach to CV involvement in systemic autoimmune diseases, both for screening, diagnosis and follow up. In this report, we analyze the different characteristics and applications of various imaging modalities, pointing out advantages and disadvantages.
ULTRASOUND APPLICATIONS
Ultrasound techniques are easy and useful diagnostic tools that enable detection of cardiac morphological and functional damage. Transthoracic echocardiography is a reliable, inexpensive and non-invasive technique that allows an accurate evaluation of valvular abnormalities, pericardial diseases and ventricular wall motion defects, while Doppler analysis is useful in studying left ventricular diastolic filling, valvular functioning and pulmonary pressures. Rexhepaj et al[11] found significant differences in early diastolic flow velocity (E), atrial flow velocity (A) and E/A ratios in RA patients compared to a control group, suggesting that a subclinical impairment of left and right ventricular function is present in RA patients, when left ventricular thickness, dimensions and myocardial performance indexes were still normal.
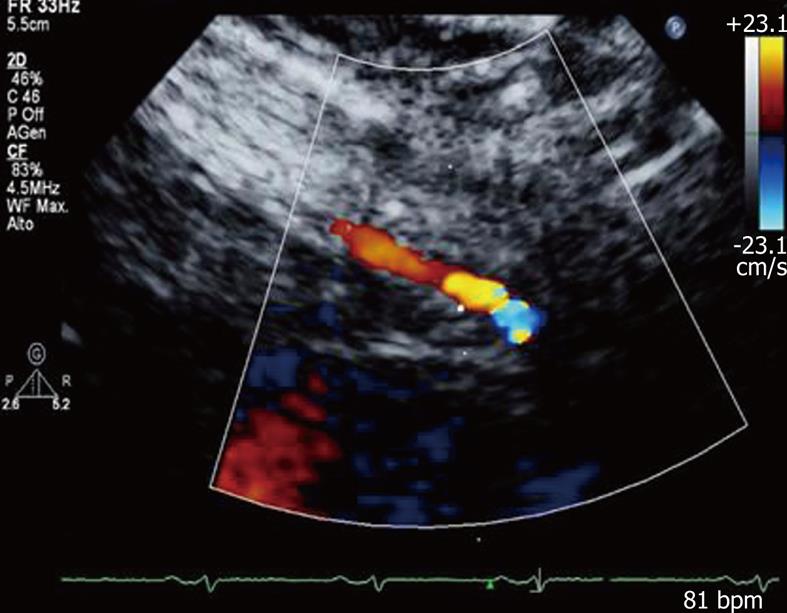
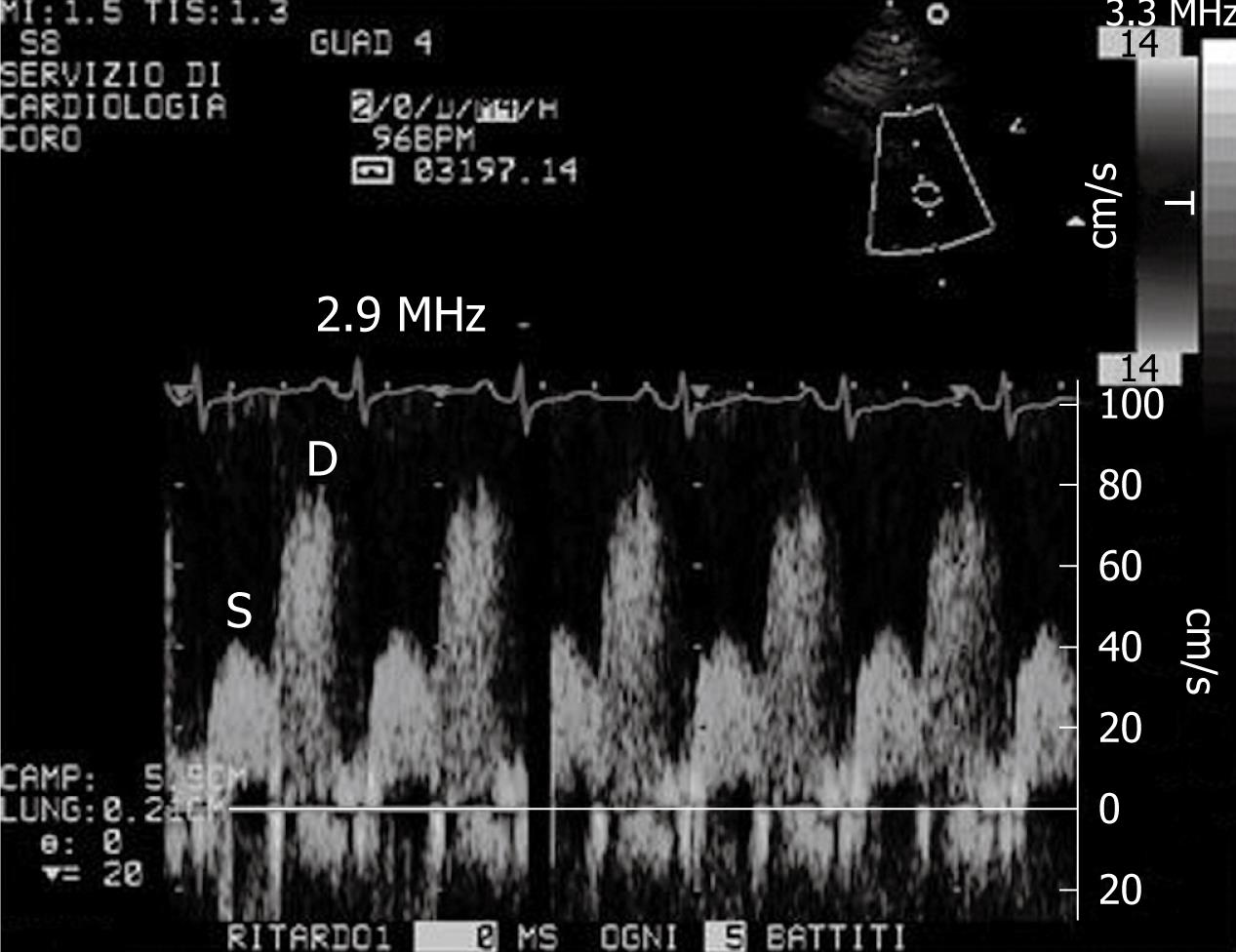
A new clinical application of ultrasound imaging is transthoracic dipyridamole stress echocardiography with coronary flow reserve (CFR) evaluation (Figure 1). CFR is assessed in the distal left anterior descending coronary artery defined by the ratio between peak diastolic velocity during stress and at baseline (Figure 2). It is a highly sensitive (> 90%) diagnostic marker for coronary artery disease (CAD)[12,13] and, when associated with evaluation of the regional wall motion analysis, it becomes also highly specific[14]. In literature reports, a value of CFR < 2 has been shown to accurately predict the presence of coronary stenosis[13]. In the absence of epicardial coronary stenosis, an abnormal CFR may reflect an impaired coronary microcirculation in patients with reperfused myocardial infarct, arterial hypertension with or without left ventricular hypertrophy, diabetes mellitus, hypercholesterolemia, syndrome X, hypertrophic cardiomyopathy and other diseases[15]. The assessment of CFR also has a prognostic value, in that a reduced CFR correlates with a negative prognosis[16]. Recently, new evidence underlined that, not only the binary (normal-abnormal) response in CFR, but the continuous spectrum of CFR values is a strong independent prognostic predictor in patients with known or suspected CAD[17].
Figure 1 Color-Doppler signal in the distal left anterior descending coronary artery.
Figure 2 Example of coronary flow Doppler signal during dypiridamole-induced hyperaemia.
S: Systolic flow; D: Diastolic flow.
Hirata et al[18] found a significant reduction of CFR in premenopausal women with SLE compared with age- and sex-matched controls. They concluded that microvascular impairment in SLE could be explained by functional alteration of the endothelium, which is responsible for the decrease in vasodilation in response to pharmacological stress. Turiel et al[10] detected a significant impairment of CFR in 25 early RA patients, with disease duration less than 1 year and without any anti-rheumatic therapy. The reduced CFR in the absence of wall motion abnormalities at rest and during pharmacological stress showed a coronary microcirculation involvement present in early RA that was associated with endothelial dysfunction.
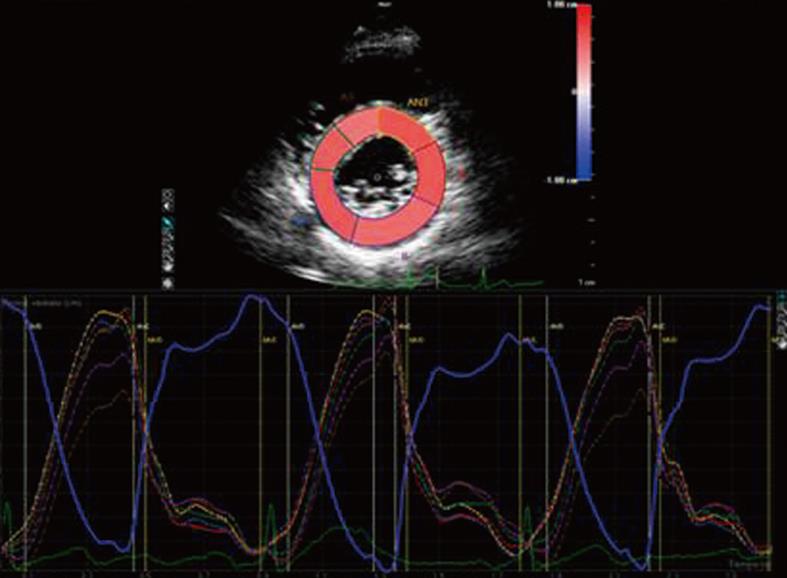
Tissue doppler imaging (TDI) is a new imaging modality that allows the measurement of myocardial velocities. Until now, TDI has been considered a reliable tool for the assessment of myocardial deformation, but this method is limited by angle-dependency and only deformation along the ultrasound beam can be derived from velocities, while myocardium deforms simultaneously in 3 dimensions[19]. Recently, Birdane et al[20] demonstrated that RA patients had a significant impairment of TDI biventricular diastolic functional parameters compared to healthy controls, depending on age and use of steroids. To overcome TDI limitations, speckle tracking analysis has been introduced to evaluate myocardial strain along the longitudinal, circumferential and radial axes (Figure 3)[21].
Figure 3 Speckle tracking analysis of radial strain in a left ventricle short axis view.
Another very useful application of echocardiography in systemic autoimmune diseases is the echo transesophageal approach, which is widely recognized as more sensitive than a transthoracic evaluation for the detection of valvular lesions[22] and identification of intracardiac masses. In particular, Turiel et al[23] observed a large prevalence (61%) of valvular thickening or vegetations and/or potential embolic sources by a transesophageal echocardiographic approach in 56 patients with primary antiphospholipid syndrome followed up for 5 years. Recently, the development of 3-dimensional (3D) transesophageal echocardiography makes it possible to obtain cross-sectional visualization of the mitral, aortic and tricuspid valves, improving the diagnostic sensitivity compared to traditional 2D imaging[24]. The main advantages over conventional 2D echocardiography and the clinical applications of 3D echocardiography include more accurate and reproducible assessment of LV volumes, mass and ejection fraction, more accurate identification of wall motion abnormalities, study of the right ventricle and better understanding of valve and subvalvular apparatus abnormalities[25].
COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE APPLICATIONS
Coronary artery calcification (CAC) has long been known to occur as a part of the atherosclerotic process and there is good evidence that the extent of CAC reflects the total coronary atherosclerotic burden[26]. The Agatston coronary calcium score assesses the extent and the density of calcification in the coronary tree[27]. Electron-beam computed tomography (EBCT) has been recognized as a highly sensitive tool that is able to detect small amounts of calcium in the coronary arteries. Radiation doses received during an EBCT study are much lower than invasive coronary angiography[28]. Recent studies, using multislice computed tomography (CT) with administration of iodinate contrast medium to visualize the coronary artery lumen, demonstrated accuracy in the detection of CAD[29]. This technique plays a diagnostic role not only for the detection of significant coronary artery luminal narrowing but also for the study of the atherosclerotic plaque texture. Moreover, it allows coronary calcium scanning along the coronary tree[30].
Kiani et al[9] evaluated coronary calcium by means of helical CT in 200 asymptomatic SLE patients and they found increased coronary calcium significantly related with plasma ADMA levels. However, while echocardiography and, mainly, stress echocardiography provide functional evaluations of the heart and the coronary tree, EBCT is limited to an anatomical and morphological description, without any functional information.
As reported above, CT requires the administration of iodinated contrast medium, which could induce intolerance symptoms or renal impairment and, moreover, patients undergo ionizing radiation exposure, which is much higher than in invasive coronary angiography. To overcome these limitations, coronary magnetic resonance (MR) angiography has been introduced in clinical practice with non-invasive visualization of the epicardial coronary arteries in the majority of subjects. It has high sensitivity, negative predictive value and overall accuracy for detecting CAD, but it is not an exercise-dependent exam[31]. Moreover, coronary MR angiography has an unsuccessful rate of 13%-14% depending on patient’s features and coronary arteries with diameters less than 1.5 mm are not well visualized, so that the diagnostic accuracy of distal coronary artery lesions is inferior to multislice CT[27]. A meta-analysis of 48 studies showed that multislice CT has higher sensitivity and specificity than MR for non invasive detection of coronary artery stenosis[32]. Moreover, MR imaging protocols are variable and the imaging procedure is time-consuming[28].
Because of its non-invasiveness, MR angiography might be the most feasible imaging modality for the detection of CAD in patients with chronic kidney disease, as well as in young and asymptomatic patients. Panting et al[33] demonstrated the high sensitivity of myocardial perfusion MR in the detection of subendocardial hypoperfusion in patients with syndrome X, characterized by chest pain with normal coronary arteries, but these results were not supported by Vermeltfoort et al[34]. Pilz et al[35] confirmed the ability of MR to address the status of coronary microvascular impairment in the presence of normal epicardial vessels. In particular, coronary MR has been shown to be effective in detecting congenital coronary artery abnormalities[36]. Moreover, MR plays an important role in the diagnosis of myocardial inflammation that often coexists with different systemic autoimmune diseases[37,38]. Edwards et al[39] detected a high prevalence of late gadolinium enhancement in the left ventricular myocardium, not related to coronary artery territories in patients with SLE and Wagner granulomatosis, raising the possibility that myocardial damage is due to a combination of subclinical inflammatory and immunological processes.
Finally, coronary angiography remains the gold standard for the diagnosis and therapy of coronary epicardial stenosis and the assessment of the presence, extent and site of atheromatous lesions, but because of its invasiveness and potential high risk, should not be used as a screening tool[40].
NONINVASIVE IMAGING OF MYOCARDIAL PERFUSION
Single-photon emission CT (SPECT) is the most widely available nuclear technique to assess myocardial perfusion at rest and during stress (maximal exercise or pharmacological stress) using diffusible radiotracers[41]. While under normal conditions, myocardial blood flow during stress increases about 3 to 5 fold compared to during rest. In the presence of significant coronary stenosis, myocardial perfusion will not increase appropriately in the territory supplied by the stenotic artery, creating heterogeneous uptake. The available SPECT radiotracers are characterized by a rapid myocardial extraction and by a cardiac uptake proportional to blood flow[42]. Although SPECT is very sensitive, specificity is relatively lower[43], mainly due to the occurrence of artifacts due to soft-tissue attenuation. Disadvantages of SPECT are represented by the need to use radioactive materials. However, the diagnostic applications are based on the ability to detect a hemodynamically significant flow-limiting stenosis[44].
Positron emission tomography (PET) has higher spatial resolution than SPECT and provides absolute quantitative measurements of physiologic parameters; moreover, it has high sensitivity and specificity for detection of myocardial ischemia. Myocardial perfusion by PET is particularly useful in reducing the number of false-positive SPECT studies because of attenuation artifacts and allows a quantitative evaluation of myocardial blood flow[36].
The noninvasive study of myocardial perfusion by nuclear imaging could be a useful tool for the detection of subtle CV involvement in systemic autoimmune diseases, such as LES or RA[45], however, more study is required.
USEFULNESS OF BIOMARKERS OF ENDOTHELIAL DYSFUNCTION
In the field of systemic autoimmune disease, the new challenge for cardiology is the early diagnosis of subtle cardiac abnormalities in a preclinical stage. In addition to instrumental diagnostic tools, there is increasing evidence for a strict association between plasma levels of ADMA and CV disease in autoimmune diseases. Increased ADMA plasma levels have been demonstrated in different pathological conditions characterized by high CV risk, such as hypercholesterolemia[46], hypertrigliceridemia[47], peripheral arterial disease[48], hypertension[49], type 2 diabetes mellitus[50], acute coronary syndrome[51] and end-stage renal disease[52]. Recently, Kiani et al[9] described higher ADMA levels among SLE patients. In this group ADMA levels appeared to be associated with coronary calcium and poor prognosis. Recently, Turiel et al[10] found that increased plasma ADMA levels in early RA patients who were free of anti-rheumatic therapy were associated with a subclinical impairment of coronary microcirculation. Interestingly, the same authors[53] observed that after 18 mo of treatment with methotrexate or anti-TNFα agents, the improvement in inflammatory status and better control of disease activity were able to induce a significant amelioration of CFR.
Evaluation of the arterial distensibility and stiffness represents a good index of endothelial dysfunction and preclinical atherosclerosis. A reduced arterial distensibility disturbs coronary perfusion and has been related to increased CV risk[54]. Yildiz[55] reported a subclinical impairment of the aortic pulse wave velocity in chronic inflammatory rheumatic disorders, such as SLE, RA, psoriasis and systemic sclerosis, mainly due to the chronic inflammatory status.
CONCLUSION
CV involvement represents the most likely cause of morbidity and mortality in systemic autoimmune disease, with a large spectrum of clinical manifestations. However, the cardiologist should be able to make an early diagnosis of cardiac disease when it is still clinically silent. Echocardiographic techniques, with several applications, and in particular stress echocardiography with CFR evaluation, represent a first line approach for the assessment of endothelial dysfunction, such as ADMA, in addition to biomarkers. Nuclear medicine can provide a functional evaluation of myocardial perfusion, while CT and cardiac MR can be used to evaluate the morphological and anatomical integrity of coronary vessels. A careful study of CV function should be done in patients affected by systemic autoimmune diseases, early after diagnosis, to detect preclinical involvement and improve long-term prognosis.
Peer reviewers: Guenter Pilz, MD, Assistant Professor, FESC, Department of Cardiology, Clinic Agatharied, Academic Teaching Hospital, University of Munich, Norbert-Kerkel-Platz, D-83734 Hausham, Germany; Mustafa Yildiz, MD, PhD, Associate Professor, EC, Cardiologist, Internal Medicine Specialist and Physiologist, Department of Cardiology, Kartal Kosuyolu Yuksek Ihtisas Educational and Research Hospital, Istanbul 81410, Turkey
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM