ATHEROGENESIS: LIPID PEROXIDATION MEETS INFLAMMATION
Much of the recent research on the origin of atherosclerosis has concentrated on the interplay between lipid metabolism, cytokines and cellular activity within arterial wall. Oxidative modification of low density lipoprotein (LDL) and ‘response to injury’ are two fundamental mechanisms proposed to be involved in the pathogenesis of atherosclerosis. Oxidized LDL has been shown to bypass the negative feedback mechanism in macrophages that inhibits the excessive uptake of native LDL[5]. Additionally, a vast number of oxidation products of lipids and proteins have been demonstrated in atherosclerotic lesions and in the plasma of patients with atherosclerosis[6-8]. On the other hand according to the ‘response to injury hypothesis[9], endothelial nudation is the initial step in atherosclerosis, however recent research has focused on endothelial dysfunction rather than endothelial nudation as being the first pathognomonic feature of atherosclerosis[10]. The risk factors influencing the development of atherosclerosis mainly involve hypercholesterolemia, altered LDL, hypertension, elevated levels of homocystine, genetic alteration, smoking, diabetes mellitus and infectious microorganisms such as Chlamydia pneumoniae[11].
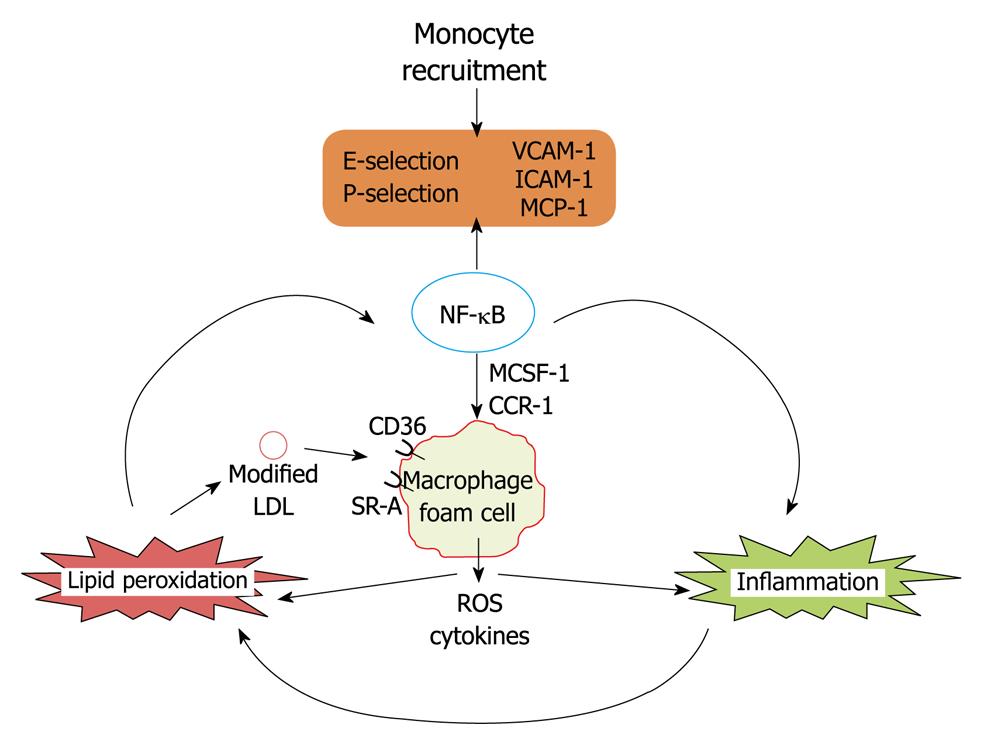
The first step in the pathogenesis of initial lesion development is lipid deposition followed by monocyte recruitment to the sub-endothelial space which is regulated by nuclear factor κB (NF-κB) induced gene expression[1]. At first LDL shuttling in and out of the vessel wall are trapped as a result of oxidative modifications by myeloperoxidase and 12/15 lipoxygenase enzymes[12,13]. Trapped LDL aggregates undergo further oxidation and oxidized LDL induces expression of adhesion factors in an NF-κB dependent manner[14]. Further monocyte attach to vascular endothelial cells which is mediated by selectins[15], vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1[16]. Monocyte transmigration into the sub-endothelial space is mediated by endothelium monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR-2[17,18]. In the subendothelial space monocytes differentiate into macrophages under the influence of monocyte colony stimulating factor and express CD36, scavenger receptor-A which specifically recognizes oxidized LDL. Uptake of oxidized LDL by macrophages leads to foam cell formation, which is a crucial landmark for the pathogenesis of atherosclerosis. Lipid loaded macrophages in the subendothelial space release reactive oxygen species and cytokines which leads to further expression of adhesion factors and subsequent monocyte migration and exacerbation of vascular inflammation. Macrophages may also be an active player in both intracellular and extracellular lipid peroxidation. Foam cells also mediate the release of reactive oxygen species and inflammatory cytokines and oxidation of lipoprotein particles in the subendothelial space[19-23]. Thus co-operativity between lipid peroxidation and inflammation is a hallmark for the development of atherosclerosis (Figure 1).
Figure 1 Lipid peroxidation vs inflammation in the development of coronary heart disease.
VCAM-1: Vascular cell adhesion molecule-1; ICAM-1: Intracellular cell adhesion molecule-1; MCP-1: Monocyte chemoattractant protein-1; MCSF-1: Monocyte colony stimulating factor; LDL: Low density lipoprotein; NF-κB: Nuclear factor κB; ROS: Reactive oxygen species.
In summary, endothelial dysfunction followed by cooperative action of lipid peroxidation and inflammation on vascular wall leads to the initial lesion development, mediated by the NF-κB dependent expression of various cell adhesion molecules and mediators which leads to the recruitment of monocytes and furthers their differentiation into macrophage foam cells is the hallmark feature for the development of CHD.
LXR-α EPIGENOMICS FROM BASIC BIOLOGY TO CLINIC
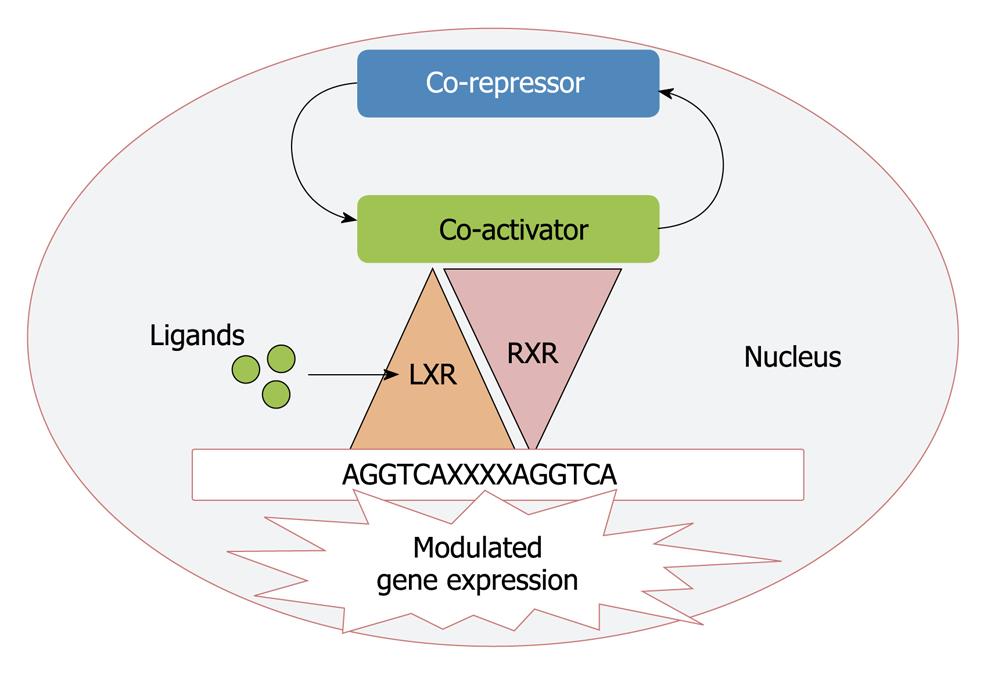
Based upon the sequence homology with other nuclear receptors LXRs (LXR-α, NR1H3 and LXR-β, NR1H2) were cloned a decade ago and considered originally as “orphan” nuclear receptors as their natural ligands were unknown[24,25]. LXR-α is highly expressed in liver, adipose tissue and macrophages whereas the β isoform is ubiquitously expressed in all tissues[26]. The LXRs are ligand activated transcription factors that form permissive heterodimers with retinoid X receptor (RXR). This heterodimer binds to LXR response element in the DNA consisting of direct repeats of the core sequence AGGTCA separated by four nucleotides[27]. In the absence of ligand, LXRs recruit complexes of co-repressors that are substituted by co-activators upon ligand binding[28] and thus regulate the gene expression (Figure 2).
Figure 2 Mechanism for the transcriptional regulation by liver X receptor (LXR).
RXR: Retinoid X receptor.
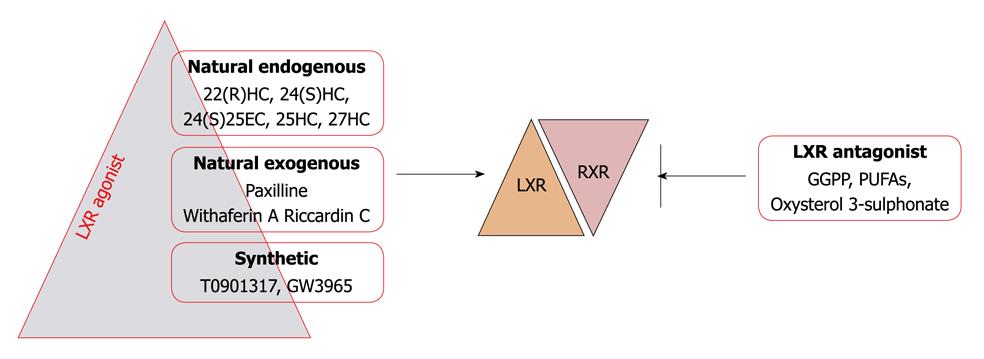
Mono-oxygenated derivatives of cholesterol or oxysterols are the major physiological LXR ligands. Intermediates between steroid hormone synthesis, 22(R) hydroxy cholesterol and 20(S) hydroxy cholesterol, have been shown to bind and stimulate LXR-α transcriptional activity in the physiological range. In the brain 24(S) hydroxy cholesterol and in the liver 24(S),25-epoxycholesterol is the abundant LXR agonist[29-32]. Apart from these, studies have demonstrated that D-glucose and D-glucose 6 phosphate are endogenous LXR agonists with equal efficacy to that of oxysterols[33], however recent findings have questioned this fact on the basis of inability of glucose and its metabolites to influence the interaction of cofactors with LXR and the lack of involvement of LXRs in the regulation of glucose sensitive genes in the liver[34]. Based upon molecular docking and in vitro studies, our recent finding have revealed withaferin A as a novel LXR-α agonist, which interacts in a similar fashion within the ligand binding domain, as its natural physiological ligands and these have potential to activate LXR-α[35]. An indol alkaloid, paxilline, produced by a fungus Penicillium paxilli was the first natural non oxysterol LXR agonist[36] but due to its toxicity in in vivo studies it is unsuitable. Riccardin C is also a natural nonsteroidal compound isolated from liverworts, which acts as an LXR-α agonist and LXR-β antagonist[37]. Synthetic LXR agonists T0901317 and GW3965 are commonly used in experimental studies. In addition to LXR agonists geranyl-geranyl pyrophosphate has been found to inhibit LXR activity by antagonizing their interaction with coactivators[38,39]. Further transcriptional activity of LXR has also been shown to be inhibited by oxidized cholesterol 3 sulphates (normally found in human plasma) and polyunsaturated fatty acids in various cell lines[40,41] (Figure 3).
Figure 3 Classification of LXR modulators.
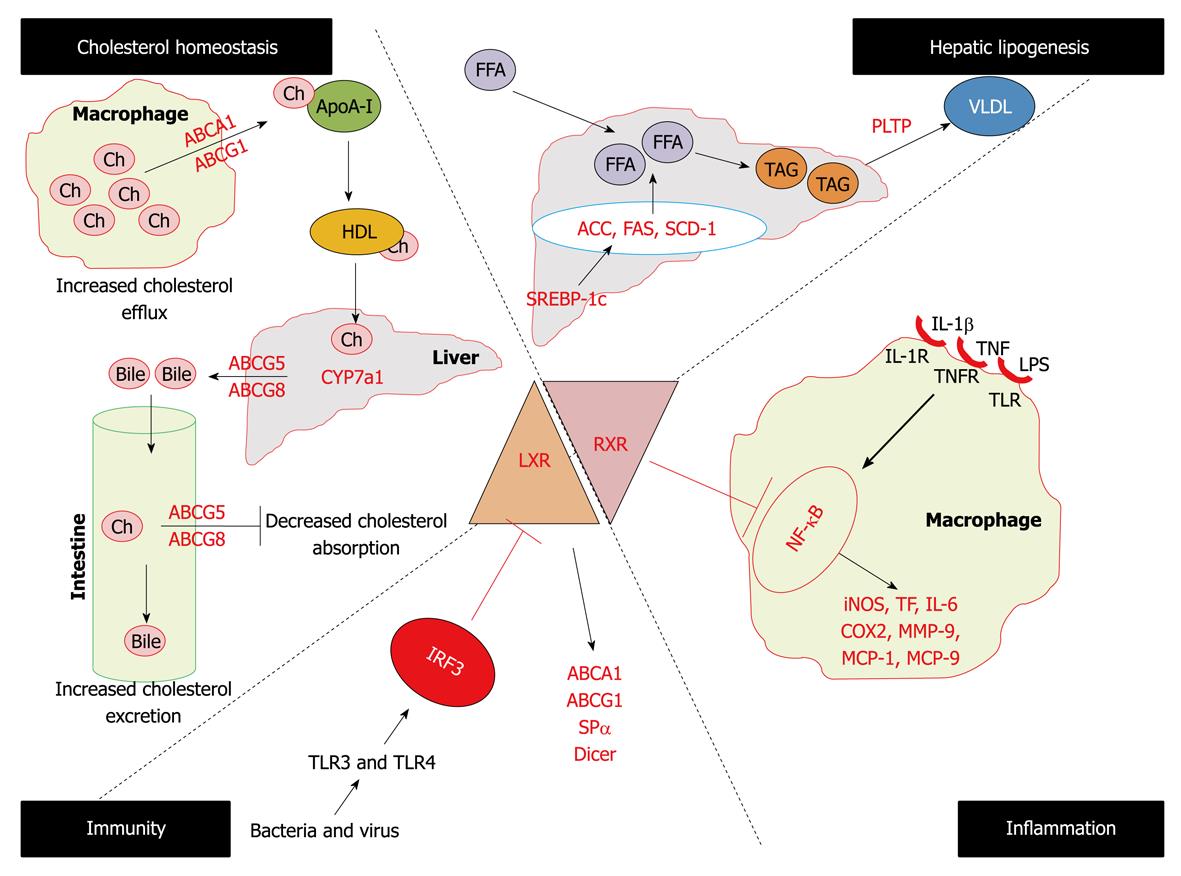
Studies on LXR-α-/- mice, but not LXR-β-/- mice, showed a marked cholesterol ester accumulation in the liver when fed with diets containing cholesterol[42]. This led to the identification of the first known LXR-α direct target CYP7A1 (the rate limiting enzyme in bile acid synthesis). The different phenotypes of the two knockout mice strains indicates that despite the considerable sequence homology between two LXR isoforms they have different distinct biological functions. With the identification of several LXR-α target genes, this molecule became a fascinating player for understanding its role in the mechanism of regulation of macrophage cholesterol metabolism, hepatic lipogenesis, and enterohepatic circulation by inhibiting cholesterol absorption. Apart from its role in atherosclerosis, recent data have uncovered the role of LXR-α in inflammation and immunity also leading to the integration of three fundamental cellular processes i.e. lipid metabolism, inflammation and immunity (Figure 4).
Figure 4 Diverse biological functions of LXRs.
ACC: Acyl coenzyme A carboxylase; FAS: Fatty acid synthase; SCD1: Sterol coenzyme A desaturase 1; PLTP: Phospholipids transfer protein; iNOS: Inducible nitric oxide synthase; COX2: Cyclo-oxygenase 2; IL: Interleukin; MMP-9: Matrix mettaloproteinase-9; TNF: Tumor necrosis factor; TF: Tissue factor.
LXR-α in the trafficking of cholesterol
A primary function of LXR-α is to maintain cellular cholesterol homeostasis by participating in the process of reverse cholesterol transport[43]. In vivo activation of LXR-α with synthetic high affinity ligand increases HDL levels and net cholesterol secretion[44]. These activities are mediated by LXR-α by upregulating the expression of the ABC superfamily of membrane transporters including ABCA1, ABCG5, ABCG8 and ABCG1[45-51]. Mutations in the ABCA1 gene are the cause of Tangier disease, characterized by the complete absence of HDL in plasma of afflicted patients, resulting in the accumulation of cholesterol in tissue macrophages and an increased incidence of cardiovascular diseases[52]. In addition to ABC transporters, LXR-α driven reverse cholesterol is promoted by the induction of a subset of apolipoproteins that serves as cholesterol acceptors. It is now well recognized that in macrophages, which play a central role in the pathogenesis of atherosclerosis[53], ABCA1 facilitates the efflux of cholesterol and phospholipids to the lipid poor lipoproteins (apoA-I) and its induction may contribute to the increases in the plasma HDL level seen with LXR-α ligand treatment[54,55]. In addition LXR-α also induces the expression of apoE in macrophages and adipose tissues but not in liver[56], further LXR-α also induces apoC gene clusters in macrophages and apoD in adipose tissues[57,58]. The importance of the activation of apoC and apoD by LXR-α in lipoprotein metabolism are unknown but the protective role of apoE in atherogenesis has been uncovered. Loss of macrophage apoE leads to the increased lesion, whereas overexpression of apoE by LXR agonists leads to the reversed phenotype[59]. Further LXR-α have been shown to modulate the expression of various enzymes that act on lipoproteins including lipoprotein lipase, cholesterol ester transfer protein and phospholipids transfer protein (PLTP)[60-63]. Thus under increased intracellular cholesterol levels, these pathways would be expected to impact the progression of cardiovascular diseases and LXR-α agonists can be exploited for the therapeutic interventions.
LXRs in hepatic lipogenesis
In addition to their ability to modulate cholesterol metabolism, LXR-α also plays a regulatory role in hepatic lipogenesis. Findings that treatment of mice with LXR agonist elevates triglyceride levels in liver and plasma have raised an obstacle to the development of these compounds as human therapeutics[64,65]. The primary mechanism by which LXR-α agonists stimulate lipogenesis appears to be through direct activation of the SREBP-1c promoter[66,67]. Further LXR-α have direct actions on certain lipogenic genes such as fatty acid synthase, PLTP, sterol coenzyme A desaturase 1 and acyl coenzyme A carboxylase[68].
LXR-α in inflammation
Several lines of evidence show that excessive inflammation within the arterial wall is a risk factor for cardiovascular disease and promotes atherogenesis[53,69]. A growing body of data has indicated that apart from the reverse cholesterol transport, LXR-α reciprocally regulates a set of inflammatory genes after bacterial, LPS, tumor necrosis factor (TNF) or interleukin (IL)-1β stimulation, such as inducible nitric oxide synthase (iNOS), cyclo-oxygenase 2, IL-6, MCP-1, MCP-9, and matrix mettaloproteinase-9 (MMP-9)[4,70]. Further in two mouse models of chronic atherogenic inflammation, Apoe-/- and ldlr-/- mice, it has been reported that administration of LXR-α ligands repressed the aortic expression of MMP-9 and tissue factor (TF) while inducing the expression of ABCA1[4,71]. The mechanisms for the repression of inflammatory genes are not well understood but evidence supports the involvement of the NF-κB pathway[72]. Thus all the observations confirm the anti-atherogenic effects of LXR-α agonists not only by promoting cholesterol efflux but also by repressing the inflammatory mediators.
LXR-α in immunity
Recent studies have revealed a common mechanism by which different microbial pathogens might contribute to foam cell formation and accelerated lesion development by interfering with LXR dependent cholesterol metabolism[73]. Activation of TLR3 and TLR4 during bacterial or viral infection of macrophages severely compromises the expression of ABCA1, ABCG1 and ApoE and other LXR target genes. TLR3/4 dependent inhibition of LXR is accomplished through activation of viral response transcription factor IFN regulatory response factor-3, however the mechanism by which this factor blocks LXR activation remains to be determined. Further macrophages from Spα-/- (antiapoptotic gene) mice are highly susceptible to oxidized LDL loading induced apoptosis in vitro and undergo massive apoptosis within atherosclerotic lesions in vivo[74]. A study from our laboratory has revealed for the first time that the LXR-α knock down cellular model has lower expression of the dicer gene, which shows the involvement of LXR-α in RNAi mediated innate immune responses[75].
LXRs in glucose homeostasis
Identification of LXRs as a mediator of insulin action in the liver, have pointed the role of these receptors in glucose homeostasis. Several studies have demonstrated potent glucose lowering and insulin sensitizing effects of synthetic LXR agonists in various rodent models of diabetes and insulin resistance[76-78]. It has been demonstrated that LXR activation leads to the suppression of various genes involved in gluconeogenesis (phosphoenolpyruvate carboxykinase, fructose-1,6 biphosphatase, and glucose 6 phosphatase) in the liver of wild type but not in LXRα/β deficient mice[79]. A further LXR response element was identified in the promoter region of glucose transporter 4 (GLUT4) gene in mice and humans[78,80] and synthetic LXR agonists were shown to increase GLUT4 expression in white adipose tissues of mice and rats as well as in cultured murine and human adipocytes[78,80,81-83]. In addition to suppression of gluconeogenesis and increased uptake of peripheral glucose uptake by LXR activators, it was shown that prolonged exposure of rat pancreatic islet insulinoma cell lines to T0901317 increases insulin secretion by glucose and glucagon-like peptide[84,85]. Thus potent glucose lowering properties of LXR agonists (T0901317 and GW3965) demonstrated in the rodent studies suggest a potential clinical use of LXR agonists as antidiabetic drugs.
In summary, ligand activated nuclear receptor LXR-α maintains cellular cholesterol homeostasis by regulating the genes involved in reverse cholesterol transport as well as hepatic lipogenesis. Further LXR activators have also been found to regulate glucose homeostasis by inhibiting gluconeogenesis and promoting peripheral glucose uptake as well insulin secretion. Apart from the fundamental cellular processes, LXRs reciprocally regulate the genes involved in inflammation.
REGULATION OF LXR-α EXPRESSION AND ACTIVITY
Despite extensive research in the field of LXR biology very little is known about the regulation of expression and activity of these receptors. LXR-β is constitutively expressed while LXR-α expression can be modulated. There are three LXR-α isoforms. All are derived from the same gene via alternative splicing[86] although relevance of the various isoforms has not yet been characterized. The LXR-α2 isoform lacks the first 45 amino acids of LXR-α1 and LXR-α3 lacks 50 amino acids within the ligand binding domain. LXR-α2 and LXR-α3 are expressed at lower levels, except in the testis where LXR-α2 is predominant. LXR-α2 shows reduced transcriptional activity and LXR-α3 is unable to bind ligand and is transcriptionally inactive[86]. Further in human LXR-α expression can be regulated by the auto regulatory loop mechanism as the LXR-α promoter itself contains its response element[87]. In addition to responding with their agonist or antagonist or by co-activator and co-repressor, the phosphorylation status of LXR-α also affects its activity. Under basal conditions, LXR-α is phosphorylated at S198 a general target for the mitogen-activated protein kinase (MAPK) family. A phosphorylation site mutant LXR-α remains nuclear and responds to ligands like the wild-type protein and the biological significance of phosphorylation remained to be elucidated. Phosphorylation is enhanced by LXR ligands. Expression of some but not all established LXR target genes is increased in macrophages expressing mutant LXR-α[88].
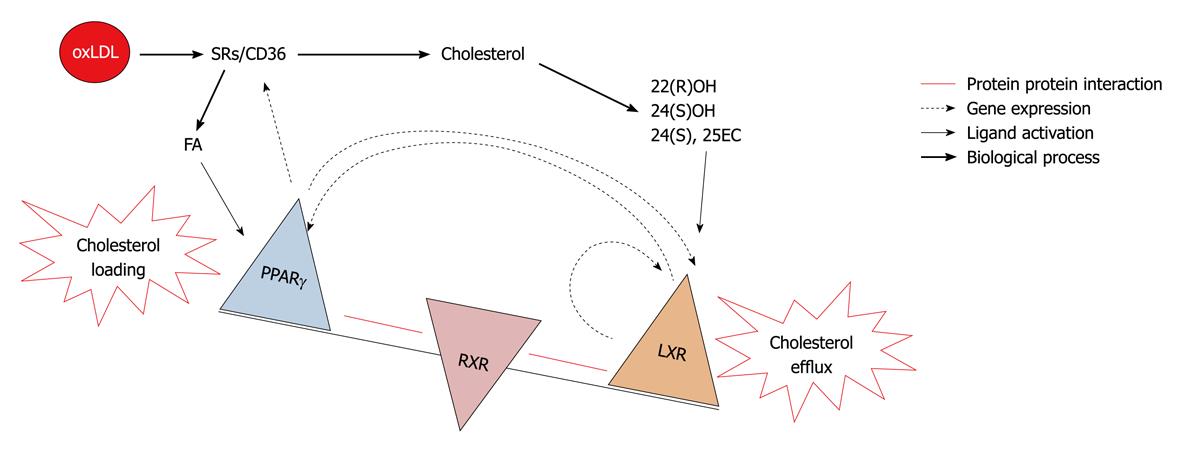
PPARγ is the closely related nuclear receptor to LXR and shares a common role in macrophage cholesterol turn over. Both receptors shares some common features, such as they form heterodimers with RXR and their endogenous activators are oxidized lipid molecules i.e. oxidized fatty acids for PPARγ and oxidized sterols for the LXR. Both are involved in the regulation of lipid metabolism in adipose tissue, macrophage and liver. Transplantation of PPARγ null bone marrow into LDLR-/- mice resulted in a significant increase in atherosclerosis[89]. Further it was reported that TZDs inhibited the development of atherosclerosis in LDLR deficient male mice[90], similar results were reported in the atherosclerotic model of apoE-/- mice[91]. All the above reported observations consider PPARγ as an antiatherogenic molecule. PPARγ expression has also been found in foam cells of atherosclerotic lesions and its expression could be increased with the oxidized LDL. PPARγ enhances uptake of oxidized LDL but not native LDL[92] by inducing the scavenger receptor CD36 leading to a vicious cycle of cholesterol loading and foam cell formation. In concordance with others, our studies suggest that PPARγ and LXR-α regulate each other. PPARγ activators have been found to enhance cholesterol efflux via the LXR-ABCA1 dependent pathway[93]. In two independent studies from our laboratory with PPARγ and LXR-α knock down cellular models using an siRNA approach it was found that both regulate expression of each other[75,94]. So the existence of this transcriptional cascade predicts that alterations in one of the elements in the cascade will affect all others and the net effect on cholesterol levels in the cell depends on how the balance between influx and efflux changes. Thus central activity of LXR-α determines if lipids are eliminated through cholesterol efflux towards HDL or accumulate and form foam cells from macrophages to induce lesion formation. This model highlights LXR-α molecule as a decision maker for atherosclerosis (Figure 5).
Figure 5 Decision maker: LXR vs PPARγ.
LXR-α AND CHD
From in vitro to in vivo model systems, it is now clear that LXR-α have the ability to regulate key processes i.e. lipid metabolism and inflammation, which are associated with the pathogenesis of atherosclerosis, making it a candidate molecule for the therapeutic intervention of the world’s
highest death causing disease. It has been shown that treatment with a synthetic LXR agonist GW3965 can reduce atherosclerotic lesion development in two mouse models (i.e. LDLR-/- and apoE-/- mice)[95]. In addition to this, effectiveness of another synthetic agonist T-0901317 has also been reported[96]. It is evident that anti-atherosclerotic actions of synthetic LXR agonists in murine models is to a large extent independent from changes in the plasma lipid profile which indicates that this effect is predominantly a consequence of direct action of LXR activators on the vascular wall. Consistent with this notion, synthetic LXR agonists were shown to stimulate ABCA1 and ABCG1 expression in the atherosclerotic lesions of both LDL receptor- and apoE-deficient mice[95,96]. Subsequent experiments using bone marrow transplantation approaches provided direct evidence for a protective role of macrophage LXRs in atherosclerosis development. Tangirala et al[97] demonstrated that hematopoietic stem cell-specific LXRα/β deficiency aggravates atherosclerosis in both apoE and LDL receptor null mice. Another mechanism that could potentially contribute to the antiatherosclerotic action of LXR activators is their suppressing effect on macrophage inflammatory mediators production. Joseph et al[4] demonstrated that GW3965 and T0901317 inhibit expression of iNOS, cyclooxygenase-2 and interleukin-6 in macrophages subjected to bacterial infection or lipopolysaccharide stimulation. This inhibition depends on both LXRα and LXRβ and is mediated through suppression of the NF-κB signaling. Anti-inflammatory action of LXR agonists has been confirmed in vivo in a model of contact dermatitis and in the aortas of atherosclerotic mice[4].
In addition to stimulating reverse cholesterol transport and repressing inflammatory responses, LXR-α agonists may inhibit atherogenesis in several ways. For example T0901317 and GW3965 suppress platelet derived growth factor or insulin induced proliferation of vascular smooth muscle cells by inhibiting cell cycle progression from G1 to S phase[98]. The proliferation of smooth muscle cell plays an important role in growth of atherosclerotic plaques. It has been found that LXR agonist reduces the expression of cyclin D1 and cyclin A, which stimulates cyclin dependent kinases. Further TF is abundant in the lipid rich core of atherosclerotic plaques, and plaque rupture induces coagulation by exposing TF to circulating blood. It has been reported that synthetic LXR agonist attenuates LPS, TNF-α, and IL-1β induced TF expression in murine and human macrophages[71]. GW3965 has also been found to reduce cytokine induced synthesis and secretion of MMP-9, which is responsible for degrading the fibrous cap of atherosclerotic plaques and contributes to plaque rupturing[70]. A recent report shows that[99] LXR agonist attenuates the stimulatory effect of homocysteine (Hcy) on immunoglobin production by B-lymphocyte, by attenuating reactive oxygen species (ROS) and NF-κB activity. Although this study relates to a specific function of immune cells, these results are of great interest, taking into consideration the important role of the immune response in atherogenesis as well as an involvement of ROS and Hcy in cardiovascular pathology.
The immunohistochemical study indicated that LXR-α is highly expressed in macrophages present in human atherosclerotic lesions[100]. Our previous study also demonstrated that blood cellular LXR-α gene expression was higher in normolipidemic and hyperlipidemic CHD groups as compared to their corresponding groups[101], suggesting nature’s protection against the development of CHD. In concordance with others[102] findings from our laboratory also stated that the statins, which are the best drug of choice for the treatment of CHD, exert their effect via the upregulation of LXR-α gene expression[103]. Vitamin C also shares a common pathway for the upregulation of LXR-α[103]. The naturally occurring polyphenol resveratrol has been associated with the beneficial effects of red wine consumption on cardiovascular disease and has been shown to inhibit atherosclerosis in animal models. Resveratrol was shown to regulate the expression of LXR-α in human macrophages, which could be a possible molecular explanation for the beneficial effects of polyphenols[104]. However our recent study revealed the existence of deregulated LXR-α transcriptome and a paradoxical relationship between blood cellular LXR-α mRNA expression and the severity of coronary occlusion, which was explained by the presence of three critical mutations in the ligand binding domain comprising Asp324, Pro327 and Arg328, responsible for the inability of this domain to interact with its natural ligands leading thereby to a deregulated LXR-α transcriptome[35]. Keeping in view the importance of LXR-α signaling in CHD our study has raised the thrust for the search for alternative ligands for the restoration of deregulated LXR-α genomics in subjects suffering from CHD.
In summary, from cellular to animal model systems LXR activators have been found to be atheroprotective in nature by promoting reverse cholesterol transport, suppressing inflammatory processes and inhibiting vascular smooth muscle cell proliferation. Further higher expression of LXRs in the atherosclerotic lesions as well as in the peripheral blood mononuclear cells of CHD patients shows nature’s protection against the development of CHD.