INTRODUCTION
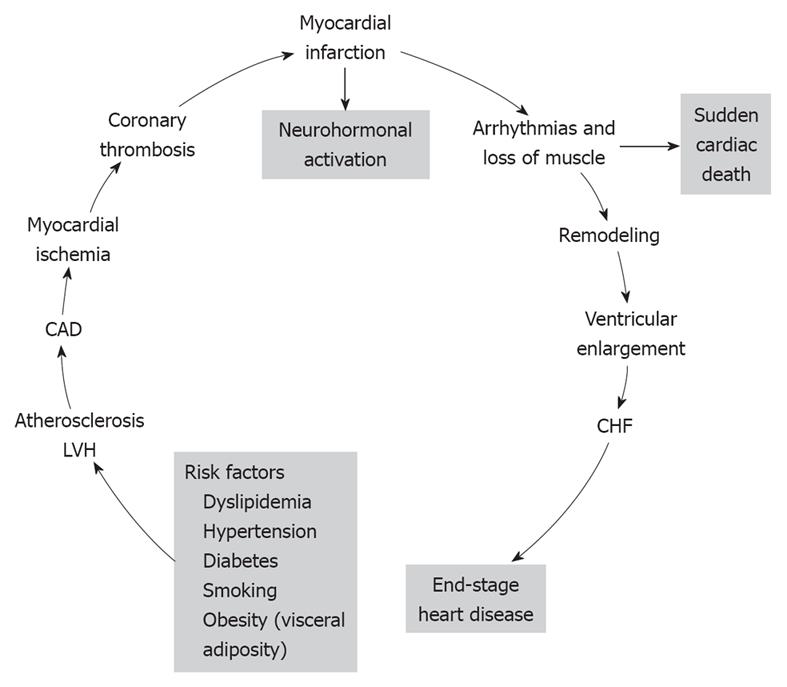
The cardiovascular disease continuum (CVDC) was first conceived by Dzau et al[1] in 1991; it is a chain of events precipitated by several cardiovascular risk factors, which if left untreated, inexorably culminate in end-stage heart failure (HF) and death. The major cardiovascular risk factors that lead to the CVDC are listed at the bottom of Figure 1 and consist of dyslipidemia, hypertension, diabetes, obesity and smoking[2]. All these risk factors, with the exception of smoking, constitute the metabolic syndrome. The metabolic syndrome is defined by the coexistence of any three of the following risk factors: (1) increased waist circumference (≥ 102 cm in men, ≥ 88 cm in women); (2) high triglyceride levels (≥ 150 mg/dL, ≥ 1.68 mmol/L); (3) high-density lipoprotein cholesterol (HDL-C; < 40 mg/dL, < 1.03 mm/L in men, or < 50 mg/dL, < 1.29 mmol/L in women); (4) blood pressure (BP; ≥ 130/85 mmHg); and (5) glucose (≥ 100 mg/dL, ≥ 5.5 mmol/L); and is associated with high incidence of CVD[3]. Since its introduction, the CVDC has been validated by several clinical trials and epidemiological studies, which have provided new insights into its underlying pathophysiology and the possible arrest of its progression by early intervention[4]. Mounting evidence suggests that early intervention in managing the cardiovascular risk factors is more important than treating the CVD itself[4]. CVD complications take years to develop, therefore, this affords ample time for early intervention and treatment of the various cardiovascular risk factors. In this editorial, the main CVD risk factors and their treatment are briefly reviewed.
Figure 1 The various stages of the cardiovascular disease continuum (CVDC) and the different stages of intervention.
CAD: Coronary artery disease; LVH: Left ventricular hypertrophy; CHF: Chronic heart failure. Reprinted with permission[2].
DYSLIPIDEMIA
High cholesterol levels have long been considered an independent risk factor for CVD, and total cholesterol levels of 200 mg/dL (5.17 mmol/L) or higher and low-density lipoprotein cholesterol (LDL-C) levels of 130 mg/dL (3.36 mmol/L) or higher have been found in 50.7% and 45.8% of adult subjects, respectively[5]. In addition, with the increase in obesity, total cholesterol levels > 200 mg/dL (5.17 mmol/L) have been found in 10% of children aged 12-19 years old, and of those screened, only 28.6% knew that high cholesterol is a risk factor for CVD. Also, a recent analysis of data for the United States, Finland and Australia[6] has found that adolescents with dyslipidemia ≥ 95th percentile have a higher incidence of increased carotid intima-media thickness in adulthood, which is a progenitor of coronary artery disease (CAD) in later life[7]. These data suggest that obese adolescents with or without hypertension should be routinely screened for dyslipidemia and treated with lifestyle modification, or more aggressively, with cholesterol-lowering drugs. Adults with dyslipidemia and preexisting CAD should also be aggressively treated according to ATP III guidelines, to LDL-C < 130 mg/dL (3.36 mmol/L) for moderate CVD risk, to < 100 mg/dL (2.59 mmol/L) for high risk, and to < 70 mg/dL (1.81 mmol/L) for very high CVD risk[8]. Several recent outcome trials have shown that aggressive treatment of LDL-C to < 70 mg/dL (1.81 mmol/L) with statins provides protection against recurrent CAD in high risk patients[9-11]. However, despite aggressive LDL-C lowering, CVD continues to increase. According to the American Heart Association statistics, the incidence of CVD increased by 12% from 70.1 million in 2005 to 79.4 million in 2007[12]. Therefore, besides LDL-C, other lipid subclasses have been considered as culprits for this increase in CVD, and recently, high non-HDL-C levels have been the focus for this increase, and have suggested that dyslipidemia is a multifactorial disease and should be treated with a combination of statins and other drugs[13]. Also, the atherogenic phenotype that consists of small dense LDL-C particles, low HDL-C and increased triglyceride levels is also associated with high incidence of coronary heart disease[14].
DIABETES MELLITUS
Diabetes mellitus, especially type 2, accounts for > 97% of the adult diabetic population and its prevalence has increased from 5% in 1988 to 6.5% in 2002, and is in line with the 6% estimate of global prevalence[15]. This rise has been attributed to the increasing incidence of obesity and the aging of the population, which accounts for an annual incidence of 1.3 million Americans with new-onset type 2 diabetes and for the 18.2 million Americans with type 2 diabetes in 2002[5]. The incidence of type 2 diabetes is also rising rapidly in Asian children and adults[16]. Recent reports from China, Japan and the Pacific Islands indicate that > 70% of children are diagnosed with type 2 diabetes. In addition, a recent study from 10 Asian countries has suggested that Asian children are more susceptible in developing diabetes than Caucasian[17]. Overt diabetes mellitus evolves from a pre-diabetic state that is characterized by insulin resistance, impaired glucose tolerance and fasting plasma glucose levels of 100-125 mg/dL (5.55-6.94 mmol/L). Several clinical trials have reported a significant improvement in the metabolic status, a delay in the progression of pre-diabetes to overt diabetes, and a decrease in the incidence of cardiovascular events with a combination of diet, exercise and anti-diabetic drugs, if necessary[18-20]. Overt type 2 diabetes mellitus is a serious CVD risk factor and is presently considered a “cardiovascular risk equivalent”, thus conferring to diabetic patients the same risk for future cardiovascular complications as those who have already sustained a prior myocardial infarction (MI)[21]. It is therefore critical that type 2 diabetes mellitus is treated aggressively with a combination of diet, exercise and anti-diabetic drugs, to a level of hemoglobin A1c ≤ 7% and BP < 130/80 mmHg, in order to prevent cardiovascular and renal complications[22,23]. Three recently published studies have shown mixed results with respect to aggressive control of diabetes (hemoglobin A1c < 7%). One study has shown a significant decrease in renal complications and no effect on cardiovascular and stroke complications by reducing hemoglobin A1c to < 7%[24]. Another has shown an increased incidence in total mortality and no effect on cardiovascular events in the intensively treated compared to the standard treated group[25]. A third study has shown no significant difference between the aggressively treated (hemoglobin A1c < 7%) and the standard treated (hemoglobin A1c > 7%) groups[26]. In that study, LDL-C was decreased to 80 mg/dL (2.1 mmol/L). For the time being, it is prudent not to lower hemoglobin A1c to < 7% in high-risk patients until new information becomes available. Also, it is recommended that LDL-C is lowered to < 100 mg/dL (< 2.59 mmol/L) in diabetic patients because they are considered to be high-risk patients[22].
OVERWEIGHT AND OBESITY
Body overweight and obesity start from an early age. A large epidemiological study of 34 countries involving young persons aged 10-16 years old has shown that overweight and obesity are directly related to television viewing time, lack of exercise, and increased consumption of sweets and soft drinks, and decreased consumption of fruits and vegetables[27]. Countries with the highest obesity rates are the United States, Canada, England and Southwest Europe[27]. Other epidemiological studies have also shown that the percentage of Americans who are either overweight or obese has increased significantly over the past 25 years, and accounts for 64% and 30.5% of subjects age ≥ 20 years who are either overweight or obese, respectively[28]. A rapid rise in obesity and the metabolic syndrome has also been noted in many Asian countries as a result of changes in nutrition and physical activity[29]. This rise has resulted in increased incidence of diabetes and CVD[29]. Excess body weight, besides being an independent risk factor for CVD, also contributes to other risk factors, such as type 2 diabetes mellitus, hypertension and dyslipidemia, which further increase the prevalence and severity of CVD[28,30]. Central obesity is associated with insulin resistance and has been shown to be an independent risk factor for ischemic stroke, even after adjustments for body mass index and other risk factors[31]. Because of these alarming trends in obesity increase, major medical societies have issued recent guidelines instructing healthcare professionals about how to stem the rise in this epidemic, by advising their patients about weight loss, diet, exercise, and pharmacological treatment, if necessary[27,28,32,33].
SMOKING
Cigarette smoking is a well-established risk factor for the CVDC[34,35], and is listed in the Framingham CVD risk factors[36]. Long-term prospective studies have clearly demonstrated the considerable mortality risk reduction associated with smoking cessation[35,37,38]. A 50-year follow-up of 34 439 male British physicians has shown that quitting cigarette smoking at any age is associated with prolongation of life expectancy[35]. In that study, physicians who stopped cigarette smoking at age 60, 50, 40 or 30 years, gained about 3, 6, 9, or 10 years in life expectancy, respectively[35]. A recent study has also shown that subjects with CVD improve their survival[39]. In 1521 patients aged ≤ 65 years with a first MI, who were followed for a mean 13.2 years, the odds of dying were 0.57 for never smokers, and 0.50 for pre-MI quitters compared to persistent smokers. In addition, among the persistent smokers, in those who reduced the number of cigarettes smoked, there was an 18% decline in mortality for every five-cigarette decrease in smoking[39]. The mechanisms by which cigarette smoking exerts its cardiovascular damaging effects is not clearly delineated. The most plausible mechanisms include, lipid oxidation, inflammation and thrombosis, with lipid oxidation being the most dominant[40]. Additional factors include vasospasm from nicotine and decreased oxygen delivery due to formation of carboxyhemoglobin. Quitting cigarette smoking is very difficult and recidivism is very high. According to a current American report, approximately 44% of smokers attempt to quit annually, but only 4%-7% succeed[41]. The smoking cessation rate is a little higher in persons who suffer from CAD. It has been estimated that between 28% and 74% quit after an acute MI[42,43]. However, about 40% of the quitters relapse and the major reason is post-MI depression[42]. Medical intervention has resulted in a higher percentage of patients who quit smoking: 61% vs 42% of controls[43]. Smoking cessation or abstinence altogether has been a major undertaking by many countries, by forbidding cigarette smoking in closed places or increasing the price of cigarettes. Family guidance and student education in schools about the health hazards of cigarette smoking will help stop young people from taking up cigarette smoking.
HYPERTENSION
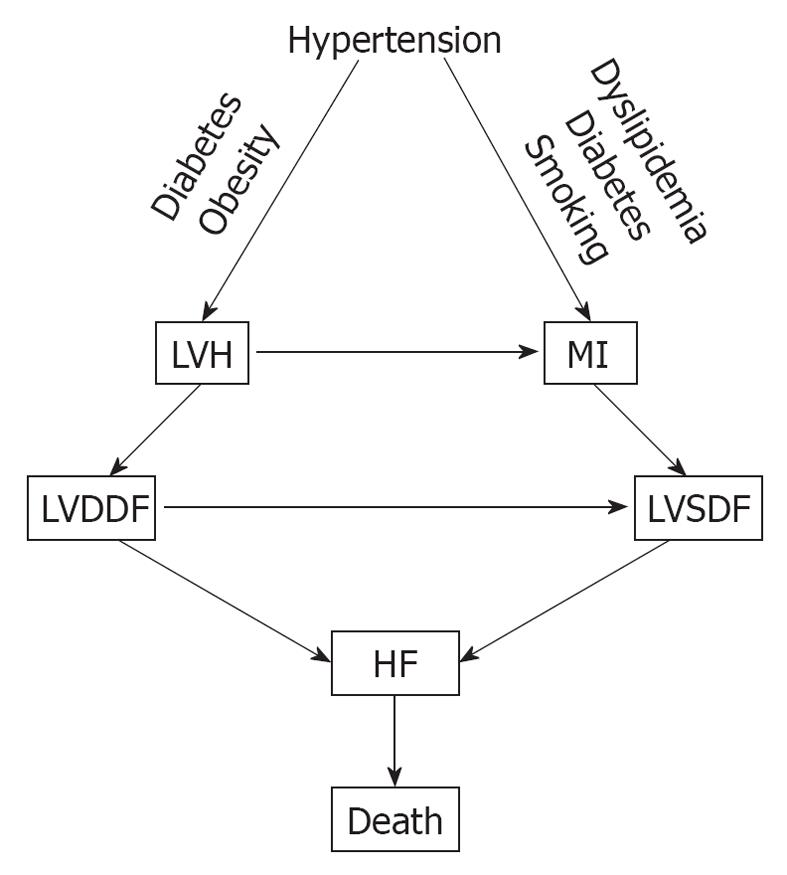
Hypertension is one of the major cardiovascular risk factors for the CVDC and its incidence continues to rise. It increased by 10% between 2005 and 2007, from 65 million to 72 million[12]. Hypertension evolves from a pre-hypertensive state, and this evolution can be delayed or prevented by treating pre-hypertension with diet, salt restriction or drugs[44-48]. There is a linear and continuous relationship between BP level and cardiovascular morbidity and mortality, regardless of age or sex[49], and its reduction is also directly related to the decreased incidence of cardiovascular and cerebrovascular complications[50,51]. In addition, hypertension is one of the most common conditions that predispose to HF. A recent meta-analysis of clinical trials of 193 424 patients has found that 24 837 patients suffered major cardiovascular events[52]. Of these, 7171 (28.9%) were cases of HF, 10 223 (41.1%) were cases of CAD, and 7443 (30.0%) were stroke cases. The incidence of HF was similar to that of stroke and was more prevalent in older persons (> 65 years), in blacks, and in diabetics. Other investigators have also reported that hypertension is a major risk factor for HF[53,54]. In a meta-analysis by Moser et al[53], of 13 342 subjects with hypertension, 1493 (11.2%) from the control groups progressed from less severe to severe hypertension, compared with only 95 of 13 389 (0.17%) from the treated groups. The incidence of left ventricular hypertrophy (LVH) and HF was higher in the control (placebo) than in the treated groups[53]. In the Framingham Heart Study, the association of baseline systolic, diastolic and pulse pressures was examined in 2040 subjects aged 50-79 years old who were free from HF at the baseline examination, and HF developed in 11.8% after 24 years of observation[54]. All these studies point to a common pathophysiological mechanism for the development of HF. Untreated or poorly treated hypertension, alone or in combination with obesity and diabetes mellitus, eventually leads to cardiac remodeling, LVH, left ventricular diastolic dysfunction (LVDDF), HF and death. In addition, LVDDF may also lead to left ventricular systolic dysfunction, which also can arise from MI as a result of hypertension, dyslipidemia and diabetes. This eventually leads to HF and death as depicted in Figure 2. It is, therefore, prudent that instead of focusing on treating the end-stage disease, our attention should be directed to the early diagnosis and treatment of hypertension and other comorbid conditions. There are several options for the treatment of hypertension, including diet[48], salt restriction[47], or drug therapy with any class of antihypertensive drugs, but preferably with drugs that block the renin angiotensin system (RAS), such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and direct renin inhibitors (DRIs). These drugs are more effective in preventing or regressing cardiac remodeling and LVH[55-60].
Figure 2 Pathophysiological mechanisms that lead to heart failure (HF) from hypertension.
MI: Myocardial infarction; LVDDF: Left ventricular diastolic dysfunction; LVSDF: Left ventricular systolic dysfunction.
CONCLUSION
From the evidence presented, it appears that early detection and treatment of the risk factors that initiate the CVDC could stop or greatly delay its further progression. The emphasis, therefore, should be based on preventing the disease, instead of waiting for it to develop and then treat it. The new treatment paradigm is a shift to the left on the events that comprise the CVDC (Figure 1). On this theme, there have been several recent calls to practicing physicians by national scientific committees for proactive treatment of cardiovascular risk factors[27,30,32]. There is an urgent need to stem the rising tide of obesity and the metabolic syndrome and their consequences by stressing weight loss through diet and exercise, starting from childhood and continuing through adult life. Pre-diabetes should be recognized and treated early to prevent its progression to overt diabetes. Overt diabetes should be treated aggressively to hemoglobin A1c < 7%, with a combination of diet, exercise and anti-diabetic drugs. Caution should be exercised in older, high-risk patients to avoid serious hypoglycemia. High cholesterol level should also be recognized and treated early, and cigarette smoking should be discouraged through parental guidance and school education about the serious health problems that it can cause later in life. Above all, hypertension should be diagnosed and brought under control early, before it causes target organ damage that is difficult to repair. Whether to treat pre-hypertension pharmacologically, on a large scale, is a question that needs to be addressed soon. Preliminary studies have shown that treatment of pre-hypertension can delay or stop its progression to overt hypertension. Non pharmacological means such as weight loss, salt restriction and exercise should be tried first because they are known to work. Recent reports that aggressive treatment of hypertension is associated with higher cardiovascular complications concerns older, high-risk subjects with preexisting CAD[61,62]. Aggressive control of uncomplicated hypertension is very important because it prevents target organ damage and the incidence of stroke, HF and renal failure. There are several classes of antihypertensive drugs to choose from because they are all effective in lowering BP[63], but individualization of treatment may be necessary. Diuretics, although effective in lowering BP, may not be a good choice for hypertensive subjects with diabetes or the metabolic syndrome, because they increase blood glucose, which is associated with high incidence of cardiovascular morbidity and mortality[64-66]. Drugs that block the RAS such as ACEIs, ARBs, and DRIs are preferable in such cases, because they interfere with the action of angiotensin II, which is responsible for cardiovascular remodeling, new-onset diabetes mellitus, and HF (Figure 2). In the Acute Decompensated Heart Failure National Registry (ADHERE), 91% of patients with HF and preserved ejection fraction had hypertension, CAD and diabetes[67]. In the Antihypertensive and Lipid-Lowering Treatment to prevent Heart Attack Trial (ALLHAT), control of BP was the most important factor in the reduction of hospitalization for HF[68]. Since these comorbidities that are associated with hypertension greatly influence the patient’s outcome, clinicians should try to identify and treat aggressively all these conditions associated with hypertension.
Peer reviewers: Paul Erne, MD, Professor, Head, Department of Cardiology, Luzerner Kantonsspital, CH-6000 Luzern 16, Switzerland; Carmine Gazzaruso, MD, PhD, Section of Cardiovascular & Metabolic Diseases, Center for Applied Clinical Research (Ce.R.C.A.), Clinical Institute, "Beato Matteo" ICBM, Corso Pavia, 84, 27029 Vigevano, Italy; Filippo Cademartiri, MD, PhD, Department of Radiology - c/o Piastra Tecnica - Piano 0, Azienda Ospedaliero-Universitaria di Parma, Via Gramsci, 14 - 43100 Parma, Italy
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM