Published online Nov 26, 2010. doi: 10.4330/wjc.v2.i11.391
Revised: October 7, 2010
Accepted: October 14, 2010
Published online: November 26, 2010
In spite of advances in the management of mediastinitis following sternotomy, mediastinitis is still associated with significant morbidity. The prognosis is much better in pediatric surgery compared to adult surgery, but the prolonged hospital stays with intravenous therapy and frequent required dressing changes that occur with several therapeutic approaches are poorly tolerated. Prevention includes nasal decontamination, skin preparation, antibioprophylaxis and air filtration in the operating theater. The expertise of the surgical team is an additional factor that is difficult to assess precisely. Diagnosis is often very simple, being made on the basis of a septic state with wound modification, while retrosternal puncture and CT scan are rarely useful. Treatment of mediastinitis following sternotomy is always a combination of surgical debridement and antibiotic therapy. Continued use of numerous surgical techniques demonstrates that there is no consensus and the best treatment has yet to be determined. However, we suggest that a primary sternal closure is the best surgical option for pediatric patients. We propose a simple technique with high-vacuum Redon’s catheter drainage that allows early mobilization and short term antibiotherapy, which thus decreases physiological and psychological trauma for patients and families. We have demonstrated the efficiency of this technique, which is also cost-effective by decreasing intensive care and hospital stay durations, in a large group of patients.
- Citation: Durandy Y. Mediastinitis in pediatric cardiac surgery: Prevention, diagnosis and treatment. World J Cardiol 2010; 2(11): 391-398
- URL: https://www.wjgnet.com/1949-8462/full/v2/i11/391.htm
- DOI: https://dx.doi.org/10.4330/wjc.v2.i11.391
Mediastinitis is a complication of pediatric cardiac surgery, with an incidence of 0.2% to 5%[1-3]. It is a retrosternal wound infection frequently associated with a macroscopically sternal osteomyelitis. The prognosis of mediastinitis is better for pediatric patients than for adult patients but this complication is still serious, particularly in the rare instances where mediastinitis is associated with endocarditis. Mediastinitis is uncomfortable for patients, is poorly accepted by parents, leads to a prolonged hospital stay and, thus, leads to an increase in health care costs.
There is considerable lack of consensus on the prevention, diagnosis and optimal treatment of mediastinitis. The goal of this manuscript is to describe the different suggestions that have been offered: (1) to prevent mediastinitis; (2) to confirm the diagnosis of mediastinitis; and (3) to treat poststernotomy deep wound infection. We will especially focus on an original and simple closed chest technique that we described in 1989[4] and reevaluated in 2007[5].
Mediastinitis is a post-operative infection that is mainly due to intra-operative contamination. The origin of the germ is the patient, the surgical team or the operating room air[6-8]. Among the factors involved in germ pathogenesis in mediastinitis are three highly relevant factors: (1) the quality of the pre- and intra-operative patient disinfection; (2) the expertise of the surgical team; and (3) the quality of the air in the operating theater. Airborne particles with germs can reach the wound or enter into the bypass circuit through the suckers used during the cardiopulmonary bypass.
Nasal carriers of staphylococcus aureus are at increased risk for post-operative mediastinitis[9,10], but there is no consensus about the efficiency of mupirocin prophylaxis in decreasing post-operative wound infection. Some works have demonstrated a significant reduction in surgical-site infection in patients treated with mupirocin[11-13], while others failed to show any difference between patients treated with mupirocin nasal ointment and patients treated with placebo[14,15]. One of the most recent studies on mupirocin pre-operative nasal decontamination concluded that decolonization of nasal and extra nasal sites decreased the risk of surgical site infection in adults. However, this work did not analyze mupirocin alone; the treated group received nasal mupirocin twice daily for 5 d and also had a total-body wash with chlorhexidine soap every day, while the control group had only placebo[16].
Before mupirocin ointment nasal therapy, nasal carriage must be identified either by microbiological culture techniques or with real-time polymerase chain reaction (which allows detection of staphylococcus within a few hours instead of days when using a classical microbiological approach). The treatment is prescribed for 5 d (delay consistent with the manufacturer’s recommendations) so that emergency patients and neonates, who are usually considered at higher risk for mediastinitis, are not included in many studies. Staphylococcus resistance to mupirocin is rare and mupirocin is a safe and inexpensive product, however, the emergence of mupirocin high-level resistance has been described in many countries[17]. Prolonged or widespread use of mupirocin is likely to be a significant predisposing factor for acquired resistance[14-17]. In many studies, lack of information regarding skin disinfection and the type of antibiotic prophylaxis that was used limits the pertinence of the results. As a final remark, nasal decontamination with mupirocin has been tested for over 20 years without clearly demonstrating an advantage.
There is less debate about skin preparation. Skin disinfection is considered as a major factor in the reduction of post-operative infection. There are two major products used as antiseptic solutions: povidone-iodine and chlorhexidine. Because of the permeable nature of the skin in small infants, significant iodine absorption is possible and likely to occur in pre-term infants. However, even in neonates, TSH levels may be significantly affected by povidone-iodine and transient thyroid dysfunction may result from topical exposure to iodine-containing antiseptic solutions[18-20]. This is known as the acute Wolff-Chaikoff effect, described in 1948, and is the reason why iodine containing solution should be avoided in neonates. Furthermore, chlorhexidine-based antiseptic solution is often considered as the best therapy for skin disinfection[21,22]. Compared to iodine solution, it has a more prolonged action and no known sensitivity[23]. Intra-operative skin disinfection is of the utmost importance, however, it seems that showers or baths with skin disinfectant before surgery are not required[24,25].
The rationale for systematic antibioprophylaxis in cardiac surgery is questionable; however, it is accepted worldwide and classically performed with a second generation cephalosporin, in the absence of a patient sensitivity. The vast majority of mediastinitis cases are due to staphylococcus and the incidence of resistance to methicillin (varying from one country to another and from one surgical unit to another, with an increase in incidence for US hospitals from 35.9% in 1992 to 64.4% in 2003[26]). Antibiotic prophylaxis induces a change in staphylococcal flora. There is some relation between the efficiency in eradication of carriage of staphylococcus aureus and the emergence of resistant strains of coagulase-negative staphylococci[27]. Staphylococci cultured from the skin of cardiac surgery patients are more resistant after surgery than before surgery and, furthermore, staphylococci causing post-operative infections have the same antimicrobial resistant phenotypes as do colonizing isolates[28]. This supports a modification of patient skin flora induced by antibiotics and is most unlikely due to in-hospital acquired germs. Pefloxacin was compared to cefamandole in perioperative cardiac surgery prophylaxis. Eradication of germs was better achieved with pefloxacin, however, while pefloxacin and oxacillin resistant strains were 0% before prophylaxis in the perianal area, 70% of the patients had resistant strains after pefloxacin prophylaxis. Such an emergence is not seen with cefamandole prophylaxis[29]. Antimicrobial agents given as prophylaxis may select resistant organisms. In patients who do not receive antibiotic prophylaxis, the staphylococcal flora remains unaffected. In patients receiving cephalosporin prophylaxis, 61% of the sites colonized with a low-level of methicillin-resistant strains before surgery were colonized with high levels of methicillin-resistant staphylococci on the third post-operative day[30]. In this study, the authors also demonstrated that the plasmid profile patterns were identical between pre- and post-operative methicillin-resistant staphylococci. This is in accordance with previous works suggesting that the resistant pathogen is an alteration of patient skin flora rather than a contamination from in-hospital flora. The benefit of a prophylactic antibiotic must be balanced with the possible selection of resistant strains that are likely to make up a nosocomial reservoir for new patients and for the hospital staff.
Obviously many risk factors of mediastinitis could be decreased with a high-quality level of care. Immunomodulation and an increase in gut mucosal permeability, induced by cardiopulmonary bypass and increased by hypothermia or deep hypothermic circulatory arrest, are also considered as predisposing factors[31,32]. They can be minimized with a more physiological cardiopulmonary bypass and warm surgery. In pediatric patients younger than 1-year-old, length of surgery, redo for bleeding, post-operative open-chest, ECMO and blood transfusion are also classical risk factors for deep wound infection[33-35].
Optimal thoracic blood drainage is important in the prevention of post-operative infection. Stagnant blood in the mediastinum is a perfect growth medium for microorganisms, which are protected from immunologic host defense. A hematoma collected in the supra-sternal space is clearly, in some patients, the origin of an abscess that may diffuse in the retrosternal space.
In an adult retrospective study of 18 532 patients who underwent on-pump coronary artery bypass grafting, blood transfusion was considered as the major preventable risk factor of post-operative mediastinitis[36]. The risk associated with blood transfusion also exists after off-pump coronary artery bypass[37]. It is noteworthy that the association of red blood cell transfusion with infection is dose-dependent[38]. In neonates and young infants, blood-free surgery is unrealistic but efforts have to be taken to decrease blood use. Blood conservation with a miniaturized bypass circuit, vacuum-assisted venous drainage and microplegia is effective[39-41] and, thus, is likely to decrease the risk of pediatric post-operative mediastinitis.
Skin closure with cyanoacrylate glue, initially used for treatment of sternal instability, was also described as a protective factor against deep wound infection[42].
Finally, the “human factor” is probably the most difficult to assess and the most difficult to control but not the least important. An optimal protocol is the first step of an efficient prophylaxis and its practical application is crucial, which depends on the motivation and quality of the medical staff.
One particularity of open heart surgery is the need for cardiopulmonary bypass. In this technique, the sucker system drains blood into the cardiotomy from the mediastinum or from cardiac cavities to the venous reservoir of the bypass circuit. In the sucker system tubing, room air is mixed with blood and thus potentially contaminated air enters into the bypass circuit. There is some evidence of transmission of fungal infections through contaminated air-handling systems[43,44], and air filtration as well as radiation were considered efficient ways to protect from mediastinitis[45-47]. Gram negative bacilli may also be transmitted from the environmental flora[48] but contamination with coagulase negative staphylococci is probably only due to patient or surgical team flora[49].
The delay between surgery and diagnosis of mediastinitis varies from a few days to a few weeks. When a patient does not receive any antibiotic therapy, mediastinitis is usually an early complication and simple to diagnose. Babies are often grouchy and tired, and fever is constant. The incision is erythematous and painful, and wound dehiscence and purulent drainage from the incision are frequent as well as sternal instability. Biological signs of infection are also constant (i.e. leukocytosis and C reactive protein elevation) and blood cultures are often positive. Bacterial examination of the purulent drainage confirms the presence of altered leukocytes and germs. Antibiotic treatment based on empirical probability should be immediately initiated, followed within a few hours by surgical drainage, which may confirm the retrosternal infection.
The diagnosis may be more difficult when the patient has received antibiotic therapy. Epicardial pacing wire cultures are not satisfactory for the diagnosis of mediastinitis[50], while bacteriological samples from the sternal or retrosternal puncture are considered safe and powerful[51]. CT scans have been considered of great value to localize infected tissues[52], however, there is also some doubt about the validity of a CT scan in performing an early diagnosis[53,54]. Furthermore, erroneous diagnoses due to Surgicel packing[55], to Surgicel body foreign reaction[56] or to iodine accumulation following irrigation[57] have been reported.
Treatment of mediastinitis is based on surgery and antibiotic therapy, but there is still controversy about the best strategy to help speed-up wound healing.
There are at least some consensual points: (1) Surgical revision with careful debridement of the infected areas, removal of foreign material and curettage of sternal edges until normal bleeding are essential; (2) Samples of purulent fluids must be collected to confirm the diagnosis and to perform germ identification and antibiotic susceptibility testing; and (3) Intravenous antibiotics, based on empiric probability, need to be injected before surgery and modified according to germ susceptibility.
Following surgery, several therapeutic approaches have been successively described. Open dressing or closed irrigation are two conventional treatments.
Open dressing has disadvantages, namely, thoracic instability requiring mechanical ventilation and prolonged immobilization, increasing risk of muscular weakening and patient discomfort. Multiple open dressing changes require heavy sedation and are time consuming for the medical staff. Cytotoxicity of classical antiseptics has been demonstrated[58] and even lethal iodine toxicity following povidone-iodine irrigation in a 34-mo-old patient has been described[59]. To overcome this problem, topical treatment with granulated sugar was also proposed and was considered by several authors as a simple, efficient and inexpensive alternative to irrigation during open chest management[60,61]. However, the numerous drawbacks of open chest management have stimulated the emergence of new therapies, such as primary closed sternum with continuous irrigation and drainage. This approach was described in 1963 and had theoretical advantages[62,63]. Mechanical ventilation was not required and the overall length of treatment was shorter than with the open chest technique. However, the results were disappointing and far from the expected progress, with a high rate of failure[64,65] and mortality. Cardiac tamponade induced by imbalance between irrigation and drainage, or even cardiac rupture, were reported[66,67]. A combination of two techniques, primary open chest management followed by delayed closed chest irrigation, was also proposed but was also less than satisfactory. However, irrigation is still used and is considered a cost-effective therapy[68].
Procedures using reconstructive plastic surgery with vascularized soft tissue flaps were described in 1980. The pectoral muscle flap was proposed for patients who fail conventional closed irrigation techniques. The obliteration of all of the dead space with well-vascularized tissue is probably the major positive aspect of this approach[69]. By eliminating any residual cavity and by filling all of the space with healthy tissue, from an infectious point of view, this treatment represents real progress. The mediastinal cavity is anfractuous, thus failure of irrigation and drainage is probably due to incomplete irrigation and drainage with persistence of one or several septic residual cavities. Unfortunately, drawbacks of plastic procedures are also numerous. Morbidity associated with the muscle flap technique includes pain, weakness, hernia and an esthetic prejudice, which is of great significance in pediatric patients and more specifically in young girls[70,71]. Chest wall instability, bleeding, recurrence of infection were noticed and omental transfer was proposed as an alternative to the pectoral flap[72]. On the other hand, the omental flap has been credited to alter respiratory function by decreasing the percent vital capacity and oxygen consumption at the anaerobic threshold[73].
When compared to closed mediastinal irrigation, the benefit of muscle flap reconstruction is not obvious[70]. Plastic procedures may have short-term and long-term results equivalent to irrigation, but the length of stay in intensive care is longer for patients treated with muscle flap closure[74].
One of the latest developments in mediastinitis surgical treatment is the introduction of vacuum-assisted closure therapy. The application of negative pressure has several advantages: (1) Wound drainage is enhanced by negative pressure; (2) The negative pressure avoids any residual mediastinal cavity so that the mediastinum is fill with healthy tissue; (3) The negative pressure helps approximate the wound edges and favors stabilization of the chest; and (4) Some benefits in local microcirculation have been described.
This newly emerging technique was used with success in small groups of pediatric patients[75-78]. The mechanism by which the vacuum technique improves wound healing is still unclear. A reduction in germ burden was suspected but not demonstrated[79].
Vacuum-assisted closure was also used following continuous irrigation, when irrigation was ineffective or in patients with low cardiac output syndrome after a successful treatment of their hemodynamic instability[80].
The benefit obtained with vacuum-assisted wound closure over classical management is unquestionably accepted by the medical community. However, there are constraints for the patient: the vacuum-assisted closure system must be changed every 2 or 3 d, positive results are seen after a long time and confinement in bed is necessary for days or weeks; following vacuum device therapy, a delayed closure is necessary and, thus, the intensive care stay is prolonged. Cardiac ruptures were described in adults during topical negative pressure[81,82] and the best negative pressure in pediatric patients is still to be determined. There is also a real concern about the risk of hemodynamic instability during negative pressure application in patients with Fontan- or Rastelli-type procedures; experimental data remains conflicting, and the precise location of the foam placement is important[83]. Magnetic resonance imaging has confirmed a reduction in cardiac output and stroke volume after initiation of vacuum therapy at the levels currently used in clinical human applications. This hemodynamic effect can be minimized by interposition of paraffin gauze dressing over the heart during application of negative pressure[84].
More interesting is a recent approach with an early primary closure over a single chest tube that serves as a routine mediastinal drain (usually removed in 1 d or 2 d). This simple technique is more comfortable for the patient and achieves a high rate of success[85]. However, in 3 of 42 patients, this approach failed and they all required reoperation for continuing sepsis with suspicion of ongoing mediastinitis. Furthermore, the medical therapy is a lengthy course of intravenous antibiotics. The 6-wk intravenous antibiotic treatment has a significant disadvantage, especially when a central venous line is necessary (because of small-sized patients or the poor peripheral venous tolerance to antibiotics).
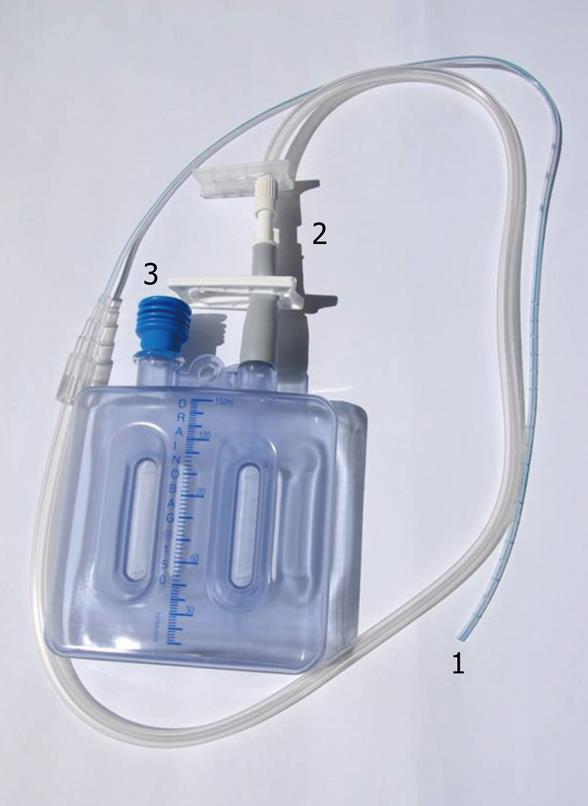
In 1989, we described a simple closed technique with primary closure and high-vacuum drainage[4]. The technique was progressively modified to decrease the length of hospital stay and to decrease patient physical and psychological trauma[5]. The surgical technique is classic with debridement of all infected or necrotic tissue and sternal edge revision until normal bleeding. Drainage is achieved through high-vacuum Redon’s catheters connected to lightweight plastic bottles. The catheter is 2.7 mm in diameter with multiperforated polyvinyl tubing, and the plastic graduated bottle is manufactured with a negative pressure of 90 kPa and has a vacuum indicator that inflates in case of loss of negative pressure (Drainobag® Lock 150, Braun, Melsungen, Germany, Figure 1). The antibiotic therapy is composed of a synergistic association of two molecules. We usually have a bacteriological monitoring of the effluent fluids every day. Sterility of the effluent fluids is obtained in 4 to 5 d for staphylococcus aureus or epidermitis, however methicillin sensitive or resistance they might be. Sterility is delayed 8 d for gram-negative bacilli. When the effluent is sterile, the catheter is progressively withdrawn (2 cm every day). This technique avoids any recurrence of sepsis. In the rare cases of unusual delay in mediastinal sterilization, antibiotic dosage in the blood and in the effluent fluid allows an assessment of antibiotic diffusion, and increased antibiotic dosage or prescription of a third antibiotic is always efficient in achieving sterility. In the group of 64 patients studied, 40 cases had isolated mediastinitis (nine in neonates, 20 in infants and 11 in children), seven patients had mediastinitis associated with endocarditis who needed a longer antibiotic therapy course, and 17 had mediastinitis associated with organ failure. The mortality rate was 4%; one patient had pneumococcal endocarditis and mediastinitis following a pulmonary atresia with a ventricular septal defect cure. One patient had a redo operation for coronaroplasty following an arterial switch operation with mediastinitis and acute respiratory distress syndrome, and the third patient had an arterial switch and Senning procedure. The three patients died from associated complications after sterilization of the mediastinal effluent and withdrawal of Redon’s catheters.
The major advantages of this technique are: (1) The primary sternal closure allows for a short mechanical ventilation time and short intensive care stay; (2) The lightweight plastic bottles allow patient mobilization; they are in fact less inconvenient than the intravenous perfusion needed for antibiotics therapy; (3) The high-vacuum is well-tolerated. This drainage is used in pediatric or adult cardiac surgery (instead of conventional underwater-seal drains) without hemodynamic drawback[86] (except for Norwood stage 1) and without damage to surrounding tissue; (4) The high-vacuum avoids any residual cavity and helps filling all of the mediastinum with healthy well-vascularized tissue; (5) Duration of antibiotic therapy is short: 11 d in cases without organ failure and 15 d in patients with organ failure; namely, respiratory failure, renal failure or ECMO; (6) A short antibiotic course is probably the best way to avoid fungal superinfection and to prevent catheter-related complications; (7) The efficiency of the short antibiotic course is due to the efficiency of the mediastinal drainage; (8) The overall infection eradication rate is 100% on a group of 64 patients treated during a 10-year period[5]; (9) There is no need for sophisticated or expensive material and the technique is not time consuming for the medical staff; and (10) As a result of these advantages, the technique is cost effective.
This therapy was adopted by several centers in Europe[87-90] and via humanitarian activities in Asia, South America and Africa.
The literature about mediastinitis is prolific for adult cardiac surgery, but there is much less data for pediatric cardiac surgery. Pediatric patients are very different from adult patients; many factors make the prognosis of mediastinitis worse including diabetes, chronic lung disease, and unilateral or bilateral use of internal mammary artery for coronary artery bypass. All of the different techniques were first used in adults and subsequently applied in pediatric cases. The lack of consensus about surgical management is illustrated by the numerous strategies proposed. None of the techniques are totally satisfactory, but we must keep in mind the differences between the pediatric and adult populations. We have demonstrated, on a large group of patients, that primary closure, allowing quick mobilization with or without minimal discomfort, is a valid alternative to all other techniques. This technique is, to our knowledge, the shortest, simplest and most efficient method for mediastinitis that has been published.
Prevention of mediastinitis is still a difficult challenge. The prognosis of mediastinitis is very different in adult compared to pediatric patients, with mortality being very rarely related to mediastinal infection in pediatric cardiac surgery. A primary sternal closure is the technique of choice in pediatric patients. Short-term therapy is the main goal to decrease the physical and psychological trauma induced by this complication.
Peer reviewers: Luiz César Guarita-Souza, MD, PhD, Adjunct Professor, Cardiovascular Surgery, Adjuncto Professor PUCPR, Head of Cardiovascular Surgery Department of Costantini Hospital, Inácio Wichnewski, 1249 CEP: 82310-420, 55 41 30771700-84177188, Curitiba Pr, Brazil; Jack CJ Sun, MD, BSc, MSc, Division of Cardiac Surgery, Hamilton General Hospital, McMaster University, 237 Barton St. E., Hamilton, Ontario, L8L 2X2, Canada
S- Editor Cheng JX L- Editor Luze M E- Editor Zheng XM
| 1. | Erez E, Katz M, Sharoni E, Katz Y, Leviav A, Vidne BA, Dagan O. Pectoralis major muscle flap for deep sternal wound infection in neonates. Ann Thorac Surg. 2000;69:572-577. |
| 2. | Tortoriello TA, Friedman JD, McKenzie ED, Fraser CD, Feltes TF, Randall J, Mott AR. Mediastinitis after pediatric cardiac surgery: a 15-year experience at a single institution. Ann Thorac Surg. 2003;76:1655-1660. |
| 3. | Mehta PA, Cunningham CK, Colella CB, Alferis G, Weiner LB. Risk factors for sternal wound and other infections in pediatric cardiac surgery patients. Pediatr Infect Dis J. 2000;19:1000-1004. |
| 4. | Durandy Y, Batisse A, Bourel P, Dibie A, Lemoine G, Lecompte Y. Mediastinal infection after cardiac operation. A simple closed technique. J Thorac Cardiovasc Surg. 1989;97:282-285. |
| 5. | Anslot C, Hulin S, Durandy Y. Postoperative mediastinitis in children: improvement of simple primary closed drainage. Ann Thorac Surg. 2007;84:423-428. |
| 6. | Jakob HG, Borneff-Lipp M, Bach A, von Pückler S, Windeler J, Sonntag H, Hagl S. The endogenous pathway is a major route for deep sternal wound infection. Eur J Cardiothorac Surg. 2000;17:154-160. |
| 7. | von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11-16. |
| 8. | San Juan R, Chaves F, López Gude MJ, Díaz-Pedroche C, Otero J, Cortina Romero JM, Rufilanchas JJ, Aguado JM. Staphylococcus aureus poststernotomy mediastinitis: description of two distinct acquisition pathways with different potential preventive approaches. J Thorac Cardiovasc Surg. 2007;134:670-676. |
| 9. | Cimochowski GE, Harostock MD, Brown R, Bernardi M, Alonzo N, Coyle K. Intranasal mupirocin reduces sternal wound infection after open heart surgery in diabetics and nondiabetics. Ann Thorac Surg. 2001;71:1572-1578; discussion 1578-1579. |
| 10. | Muñoz P, Hortal J, Giannella M, Barrio JM, Rodríguez-Créixems M, Pérez MJ, Rincón C, Bouza E. Nasal carriage of S. aureus increases the risk of surgical site infection after major heart surgery. J Hosp Infect. 2008;68:25-31. |
| 11. | Carrier M, Marchand R, Auger P, Hébert Y, Pellerin M, Perrault LP, Cartier R, Bouchard D, Poirier N, Pagé P. Methicillin-resistant Staphylococcus aureus infection in a cardiac surgical unit. J Thorac Cardiovasc Surg. 2002;123:40-44. |
| 12. | van Rijen MM, Bonten M, Wenzel RP, Kluytmans JA. Intranasal mupirocin for reduction of Staphylococcus aureus infections in surgical patients with nasal carriage: a systematic review. J Antimicrob Chemother. 2008;61:254-261. |
| 13. | Reagan DR, Doebbeling BN, Pfaller MA, Sheetz CT, Houston AK, Hollis RJ, Wenzel RP. Elimination of coincident Staphylococcus aureus nasal and hand carriage with intranasal application of mupirocin calcium ointment. Ann Intern Med. 1991;114:101-106. |
| 14. | Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871-1877. |
| 15. | Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, Bogaers-Hofman D, de Baere GA, Stuurman A, van Belkum A, Kluytmans JA. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35:353-358. |
| 16. | Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R, Troelstra A, Box AT, Voss A, van der Tweel I. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9-17. |
| 17. | Cookson BD. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother. 1998;41:11-18. |
| 18. | Linder N, Sela B, German B, Davidovitch N, Kuint J, Hegesh J, Lubin D, Sack J. Iodine and hypothyroidism in neonates with congenital heart disease. Arch Dis Child Fetal Neonatal Ed. 1997;77:F239-F240. |
| 19. | Mitchell IM, Pollock JC, Jamieson MP, Fitzpatrick KC, Logan RW. Transcutaneous iodine absorption in infants undergoing cardiac operation. Ann Thorac Surg. 1991;52:1138-1140. |
| 20. | Aliefendioğlu D, Sanli C, Cakmak M, Ağar A, Albayrak M, Evliyaoğlu O. Wolff-Chaikoff effect in a newborn: is it an overlooked problem? J Pediatr Surg. 2006;41:e1-e3. |
| 21. | Mimoz O, Villeminey S, Ragot S, Dahyot-Fizelier C, Laksiri L, Petitpas F, Debaene B. Chlorhexidine-based antiseptic solution vs alcohol-based povidone-iodine for central venous catheter care. Arch Intern Med. 2007;167:2066-2072. |
| 22. | Darouiche RO, Wall MJ Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, Miller HJ, Awad SS, Crosby CT, Mosier MC. Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. N Engl J Med. 2010;362:18-26. |
| 23. | McGrath DR, McCrory D. An audit of pre-operative skin preparative methods. Ann R Coll Surg Engl. 2005;87:366-368. |
| 24. | Webster J, Osborne S. Meta-analysis of preoperative antiseptic bathing in the prevention of surgical site infection. Br J Surg. 2006;93:1335-1341. |
| 25. | Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2007;CD004985. |
| 26. | Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin Infect Dis. 2006;42:389-391. |
| 27. | Archer GL, Armstrong BC. Alteration of staphylococcal flora in cardiac surgery patients receiving antibiotic prophylaxis. J Infect Dis. 1983;147:642-649. |
| 28. | Archer GL. Alteration of cutaneous staphylococcal flora as a consequence of antimicrobial prophylaxis. Rev Infect Dis. 1991;13 Suppl 10:S805-S809. |
| 29. | Baiocchi P, Venditti M, Santini C, Santoro R, Tarasi A, Gelfusa V, Brandimarte C, Serra P. Alterations of coagulase-negative staphylococcal flora in cardiac surgery patients: comparative study between cefamandole and pefloxacin perioperative prophylaxis. J Chemother. 1989;1:305-309. |
| 30. | Kernodle DS, Barg NL, Kaiser AB. Low-level colonization of hospitalized patients with methicillin-resistant coagulase-negative staphylococci and emergence of the organisms during surgical antimicrobial prophylaxis. Antimicrob Agents Chemother. 1988;32:202-208. |
| 31. | Markewitz A, Faist E, Lang S, Hültner L, Weinhold C, Reichart B. An imbalance in T-helper cell subsets alters immune response after cardiac surgery. Eur J Cardiothorac Surg. 1996;10:61-67. |
| 32. | Malagon I, Onkenhout W, Klok G, van der Poel PF, Bovill JG, Hazekamp MG. Gut permeability in paediatric cardiac surgery. Br J Anaesth. 2005;94:181-185. |
| 33. | Costello JM, Graham DA, Morrow DF, Morrow J, Potter-Bynoe G, Sandora TJ, Pigula FA, Laussen PC. Risk factors for surgical site infection after cardiac surgery in children. Ann Thorac Surg. 2010;89:1833-1841; discussion 1841-1842. |
| 34. | Long CB, Shah SS, Lautenbach E, Coffin SE, Tabbutt S, Gaynor JW, Bell LM. Postoperative mediastinitis in children: epidemiology, microbiology and risk factors for Gram-negative pathogens. Pediatr Infect Dis J. 2005;24:315-319. |
| 35. | Al-Sehly AA, Robinson JL, Lee BE, Taylor G, Ross DB, Robertson M, Rebeyka IM. Pediatric poststernotomy mediastinitis. Ann Thorac Surg. 2005;80:2314-2320. |
| 36. | Risnes I, Abdelnoor M, Almdahl SM, Svennevig JL. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg. 2010;89:1502-1509. |
| 37. | Rosmarakis ES, Prapas SN, Rellos K, Michalopoulos A, Samonis G, Falagas ME. Nosocomial infections after off-pump coronary artery bypass surgery: frequency, characteristics, and risk factors. Interact Cardiovasc Thorac Surg. 2007;6:759-767. |
| 38. | Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, Herruzo-Avilés A, Camacho-Laraña P, Garnacho-Montero J, Amaya-Villar R. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119:1461-1468. |
| 39. | Durandy Y. The impact of vacuum-assisted venous drainage and miniaturized bypass circuits on blood transfusion in pediatric cardiac surgery. ASAIO J. 2009;55:117-120. |
| 40. | Charette K, Hirata Y, Bograd A, Mongero L, Chen J, Quaegebeur J, Mosca R. 180 ml and less: cardiopulmonary bypass techniques to minimize hemodilution for neonates and small infants. Perfusion. 2007;22:327-331. |
| 41. | Golab HD, Takkenberg JJ, van Gerner-Weelink GL, Wijers MJ, Scohy TV, de Jong PL, Bogers AJ. Effects of cardiopulmonary bypass circuit reduction and residual volume salvage on allogeneic transfusion requirements in infants undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2007;6:335-339. |
| 42. | Souza EC, Fitaroni RB, Januzelli DM, Macruz HM, Camacho JC, Souza MR. Use of 2-octyl cyanoacrylate for skin closure of sternal incisions in cardiac surgery: observations of microbial barrier effects. Curr Med Res Opin. 2008;24:151-155. |
| 43. | Lutz BD, Jin J, Rinaldi MG, Wickes BL, Huycke MM. Outbreak of invasive Aspergillus infection in surgical patients, associated with a contaminated air-handling system. Clin Infect Dis. 2003;37:786-793. |
| 44. | Fox BC, Chamberlin L, Kulich P, Rae EJ, Webster LR. Heavy contamination of operating room air by Penicillium species: identification of the source and attempts at decontamination. Am J Infect Control. 1990;18:300-306. |
| 45. | Soots G, Leclerc H, Pol A, Savage C, Fieve R. Air-borne contamination hazard in open heart surgery. Efficiency of HEPA air filtration and laminar flow. J Cardiovasc Surg (Torino). 1982;23:155-162. |
| 46. | Hart D, Postlethwait RW, Brown IW Jr, Smith WW, Johnson PA. Postoperative wound infections: a further report on ultraviolet irradiation with comments on the recent (1964) national research council cooperative study report. Ann Surg. 1968;167:728-743. |
| 47. | Brown IW Jr, Moor GF, Hummel BW, Marshall WG Jr, Collins JP. Toward further reducing wound infections in cardiac operations. Ann Thorac Surg. 1996;62:1783-1789. |
| 48. | Andersen BM, Sørlie D, Hotvedt R, Almdahl SM, Olafsen K, George R, Gilfillian A. Multiply beta-lactam resistant Enterobacter cloacae infections linked to the environmental flora in a unit for cardiothoracic and vascular surgery. Scand J Infect Dis. 1989;21:181-191. |
| 49. | Bitkover CY, Marcusson E, Ransjö U. Spread of coagulase-negative staphylococci during cardiac operations in a modern operating room. Ann Thorac Surg. 2000;69:1110-1115. |
| 50. | Maroto LC, Aguado JM, Carrascal Y, Pérez A, Pérez-de-la-Sota E, Cortina JM, Delgado R, Rodriguez E, Molina L, Rufilanchas JJ. Role of epicardial pacing wire cultures in the diagnosis of poststernotomy mediastinitis. Clin Infect Dis. 1997;24:419-421. |
| 51. | Benlolo S, Matéo J, Raskine L, Tibourtine O, Bel A, Payen D, Mebazaa A. Sternal puncture allows an early diagnosis of poststernotomy mediastinitis. J Thorac Cardiovasc Surg. 2003;125:611-617. |
| 52. | Goodman LR, Kay HR, Teplick SK, Mundth ED. Complications of median sternotomy: computed tomographic evaluation. AJR Am J Roentgenol. 1983;141:225-230. |
| 53. | Misawa Y, Fuse K, Hasegawa T. Infectious mediastinitis after cardiac operations: computed tomographic findings. Ann Thorac Surg. 1998;65:622-624. |
| 54. | Bitkover CY, Cederlund K, Aberg B, Vaage J. Computed tomography of the sternum and mediastinum after median sternotomy. Ann Thorac Surg. 1999;68:858-863. |
| 55. | Alves Júnior L, Vicente WV, Ferreira CA, Manso PH, Arantes LR, Pinheiro KS, Luciano PM, Rodrigues AJ, Evora PR. Surgicel packing and an erroneous diagnosis of mediastinitis in a neonate. Tex Heart Inst J. 2010;37:116-118. |
| 56. | Ibrahim MF, Aps C, Young CP. A foreign body reaction to Surgicel mimicking an abscess following cardiac surgery. Eur J Cardiothorac Surg. 2002;22:489-490; author reply 490. |
| 57. | Bauernschmitt R, Martinoff S, Poerner M, Lange R. Pitfall in the computed-tomography-diagnosis of postcardiotomy infection: iodine accumulation after irrigation mimicking retrosternal abscess. Eur J Cardiothorac Surg. 2005;27:707; discussion 707-707; discussion 708. |
| 58. | Lineaweaver W, Howard R, Soucy D, McMorris S, Freeman J, Crain C, Robertson J, Rumley T. Topical antimicrobial toxicity. Arch Surg. 1985;120:267-270. |
| 59. | Glick PL, Guglielmo BJ, Tranbaugh RF, Turley K. Iodine toxicity in a patient treated by continuous povidone-iodine mediastinal irrigation. Ann Thorac Surg. 1985;39:478-480. |
| 60. | Trouillet JL, Chastre J, Fagon JY, Pierre J, Domart Y, Gibert C. Use of granulated sugar in treatment of open mediastinitis after cardiac surgery. Lancet. 1985;2:180-184. |
| 61. | Szerafin T, Vaszily M, Péterffy A. Granulated sugar treatment of severe mediastinitis after open-heart surgery. Scand J Thorac Cardiovasc Surg. 1991;25:77-80. |
| 62. | Schumacker HB Jr, Mandelbaum I. Continuous antibiotic irrigation in the treatment of infection. Arch Surg. 1963;86:384-387. |
| 63. | Bryant LR, Spencer FC, Trinkle JK. Treatment of median sternotomy infection by mediastinal irrigation with an antibiotic solution. Ann Surg. 1969;169:914-920. |
| 64. | Rand RP, Cochran RP, Aziz S, Hofer BO, Allen MD, Verrier ED, Kunzelman KS. Prospective trial of catheter irrigation and muscle flaps for sternal wound infection. Ann Thorac Surg. 1998;65:1046-1049. |
| 65. | Molina JE. Primary closure for infected dehiscence of the sternum. Ann Thorac Surg. 1993;55:459-463. |
| 66. | Piwnica A, Abdelmeguid I, Mesnildrey P, Laborde F, Menasche P, Romano M, Guedon C. Rupture of the right ventricular free wall. An unusual complication of mediastinitis after cardiac surgery. Eur J Cardiothorac Surg. 1988;2:172-175. |
| 67. | Niclauss L, Delay D, Stumpe F. Right ventricular rupture due to recurrent mediastinal infection with a closed chest. Interact Cardiovasc Thorac Surg. 2010;10:470-472. |
| 68. | Vida VL, Leon-Wyss J, Larrazabal A, Cruz S, Castaneda AR. Mediastinitis in pediatric cardiac surgery: treatment and cost-effectiveness in a low-income country. Pediatr Cardiol. 2007;28:163-166. |
| 69. | Jurkiewicz MJ, Bostwick J 3rd, Hester TR, Bishop JB, Craver J. Infected median sternotomy wound. Successful treatment by muscle flaps. Ann Surg. 1980;191:738-744. |
| 70. | Ringelman PR, Vander Kolk CA, Cameron D, Baumgartner WA, Manson PN. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg. 1994;93:1208-1214; discussion 1215-1216. |
| 71. | Yuen JC, Zhou AT, Serafin D, Georgiade GS. Long-term sequelae following median sternotomy wound infection and flap reconstruction. Ann Plast Surg. 1995;35:585-589. |
| 72. | Milano CA, Georgiade G, Muhlbaier LH, Smith PK, Wolfe WG. Comparison of omental and pectoralis flaps for poststernotomy mediastinitis. Ann Thorac Surg. 1999;67:377-380; discussion 380-381. |
| 73. | Morotomi N, Saitoh M, Takanashi S, Nagayama M, Mizuma M. After omental flap transposition, respiratory function and exercise capacity decrease. Ann Thorac Cardiovasc Surg. 2010;16:9-15. |
| 74. | Scully HE, Leclerc Y, Martin RD, Tong CP, Goldman BS, Weisel RD, Mickleborough LL, Baird RJ. Comparison between antibiotic irrigation and mobilization of pectoral muscle flaps in treatment of deep sternal infections. J Thorac Cardiovasc Surg. 1985;90:523-531. |
| 75. | Hiramatsu T, Okamura Y, Komori S, Nishimura Y, Suzuki H, Takeuchi T. Vacuum-assisted closure for mediastinitis after pediatric cardiac surgery. Asian Cardiovasc Thorac Ann. 2008;16:e45-e46. |
| 76. | Kadohama T, Akasaka N, Nagamine A, Nakanishi K, Kiyokawa K, Goh K, Sasajima T. Vacuum-assisted closure for pediatric post-sternotomy mediastinitis: are low negative pressures sufficient? Ann Thorac Surg. 2008;85:1094-1096. |
| 77. | Salazard B, Niddam J, Ghez O, Metras D, Magalon G. Vacuum-assisted closure in the treatment of poststernotomy mediastinitis in the paediatric patient. J Plast Reconstr Aesthet Surg. 2008;61:302-305. |
| 78. | Fleck T, Simon P, Burda G, Wolner E, Wollenek G. Vacuum assisted closure therapy for the treatment of sternal wound infections in neonates and small infants. Interact Cardiovasc Thorac Surg. 2006;5:285-288. |
| 79. | Mouës CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11-17. |
| 80. | Ugaki S, Kasahara S, Arai S, Takagaki M, Sano S. Combination of continuous irrigation and vacuum-assisted closure is effective for mediastinitis after cardiac surgery in small children. Interact Cardiovasc Thorac Surg. 2010;11:247-251. |
| 81. | Sartipy U, Lockowandt U, Gäbel J, Jidéus L, Dellgren G. Cardiac rupture during vacuum-assisted closure therapy. Ann Thorac Surg. 2006;82:1110-1111. |
| 82. | Abu-Omar Y, Naik MJ, Catarino PA, Ratnatunga C. Right ventricular rupture during use of high-pressure suction drainage in the management of poststernotomy mediastinitis. Ann Thorac Surg. 2003;76:974; author reply 974-975. |
| 83. | Steigelman MB, Norbury KC, Kilpadi DV, McNeil JD. Cardiopulmonary effects of continuous negative pressure wound therapy in swine. Ann Thorac Surg. 2009;88:1277-1283. |
| 84. | Petzina R, Ugander M, Gustafsson L, Engblom H, Sjögren J, Hetzer R, Ingemansson R, Arheden H, Malmsjö M. Hemodynamic effects of vacuum-assisted closure therapy in cardiac surgery: assessment using magnetic resonance imaging. J Thorac Cardiovasc Surg. 2007;133:1154-1162. |
| 85. | Ohye RG, Maniker RB, Graves HL, Devaney EJ, Bove EL. Primary closure for postoperative mediastinitis in children. J Thorac Cardiovasc Surg. 2004;128:480-486. |
| 86. | Newcomb AE, Alphonso N, Nørgaard MA, Cochrane AD, Karl TR, Brizard CP. High-vacuum drains rival conventional underwater-seal drains after pediatric heart surgery. Eur J Cardiothorac Surg. 2005;27:395-399; discussion 399-400. |
| 87. | Calvat S, Trouillet JL, Nataf P, Vuagnat A, Chastre J, Gibert C. Closed drainage using Redon catheters for local treatment of poststernotomy mediastinitis. Ann Thorac Surg. 1996;61:195-201. |
| 88. | Kirsch M, Mekontso-Dessap A, Houël R, Giroud E, Hillion ML, Loisance DY. Closed drainage using redon catheters for poststernotomy mediastinitis: results and risk factors for adverse outcome. Ann Thorac Surg. 2001;71:1580-1586. |
| 89. | Mekontso-Dessap A, Kirsch M, Brun-Buisson C, Loisance D. Poststernotomy mediastinitis due to Staphylococcus aureus: comparison of methicillin-resistant and methicillin-susceptible cases. Clin Infect Dis. 2001;32:877-883. |
| 90. | Berg HF, Brands WG, van Geldorp TR, Kluytmans-VandenBergh FQ, Kluytmans JA. Comparison between closed drainage techniques for the treatment of postoperative mediastinitis. Ann Thorac Surg. 2000;70:924-929. |