Published online Jul 26, 2025. doi: 10.4330/wjc.v17.i7.108901
Revised: May 29, 2025
Accepted: July 4, 2025
Published online: July 26, 2025
Processing time: 88 Days and 13.5 Hours
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide, hosting numerous serious possible complications such as stroke and heart failure. In the past two decades, managing rhythm control was more successful via pulmonary vein isolation (PVI) ablation, generally performed via transfemoral access. Patients with anatomical variations may necessitate a dose of creativity and evidence-based techniques. To our knowledge, we present the first PVI case in a patient with AF via right internal jugular (IJ) vein access using pulse field ablation.
A 76-year-old male with an extensive medical history notable for type 2 diabetes and severe peripheral vascular disease requiring vascular bypass surgery is identified to have paroxysmal AF. Given functional decline and worsening arrhythmia burden refractory to oral antiarrhythmics, an initial PVI ablation was attempted but failed as the catheter could not be advanced secondary to bilateral iliac vein occlusions. This necessitated a novel approach and a subsequent PVI ablation via the right IJ vein was successful without any complications. The success of this case highlights the feasibility of an IJ approach for PVI in patients where traditional access is not possible. This case can be used as a reference for other practitioners who may face similar challenges when attempting to perform PVI for AF or similar procedures requiring access to similar anatomical locations.
The success of this case highlights the feasibility of an IJ approach for PVI when traditional access is impossible.
Core Tip: Atrial fibrillation is typically ablated using pulmonary vein isolation through transfemoral venous access. When this is not possible, alternative entry points are required. The patient showcased in the case report had bilateral femoral vein occlusions due to chronic thrombosis requiring access via the right internal jugular vein. Successful ablation was performed, and the patient was discharged the same day. This case highlights the feasibility of internal jugular vein access for pulmonary vein isolation as well as the importance of physician adaptability in unexpected circumstances.
- Citation: Lester JR, Abolhassani A, Patel H, Hreibe H. Novel approach to pulmonary vein isolation ablation via right internal jugular access: A case report. World J Cardiol 2025; 17(7): 108901
- URL: https://www.wjgnet.com/1949-8462/full/v17/i7/108901.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i7.108901
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the United States, comprising 4.48% of the adult population[1,2]. It carries clinically significant consequences, such as a 2.4-fold increase in the risk of stroke, a 5-fold increase in the risk of heart failure, and a 1.5-fold increase in all-cause mortality[3]. The prevailing theory behind the pathogenesis of AF involves ectopic electrical activity from myocyte sleeves within the pulmonary veins. As the pulmonary veins empty into the posterior left atrium, this aberrant electrical activity leads to irregular atrial beats, with the potential for an irregular ventricular response known as AF with rapid ventricular response, a serious tachyarrhythmia[4,5].
The traditional approach for electrophysiology ablation procedures for AF includes catheter access via the femoral veins to the right atrium and then through a transseptal puncture to access the left atrium where the ablation typically the pulmonary vein isolation (PVI)[1,6]. However, there are instances where femoral access is occluded, or the inferior vena cava (IVC) has interruptions, necessitating a superior approach through the internal jugular (IJ) vein or axillary/subclavian vein[7]. These patients still require interventions; therefore, innovative methods are continuously being developed. Previously, right IJ vein access has been taken due to IVC interruptions with minimal complications[8]. We present the first case where right IJ access was utilized for PVI in AF due to total occlusion of the right iliac and subtotal occlusion of the left iliac veins.
Intermittent palpitations with worsening dyspnea on exertion.
He presented to the cardiology clinic in November 2024 with a several-year history of paroxysmal AF on anticoagulation with apixaban. He reported intermittent palpitations and a steady functional decline over several months with fatigue, increasing shortness of breath, and exercise intolerance. Fourteen-day cardiac monitoring at that time revealed more than six days of AF. Transthoracic echocardiography at that time revealed a preserved left ventricular ejection fraction of 51%-55%, with mild valvular heart disease. At his follow-up appointment a month later, the patient was found to be in persistent and highly symptomatic AF with continued functional decline. He was initiated on oral antiarrhythmic therapy with amiodarone and underwent successful transesophageal echocardiography-direct current cardioversion with plans for PVI at a later date with the restoration of sinus rhythm.
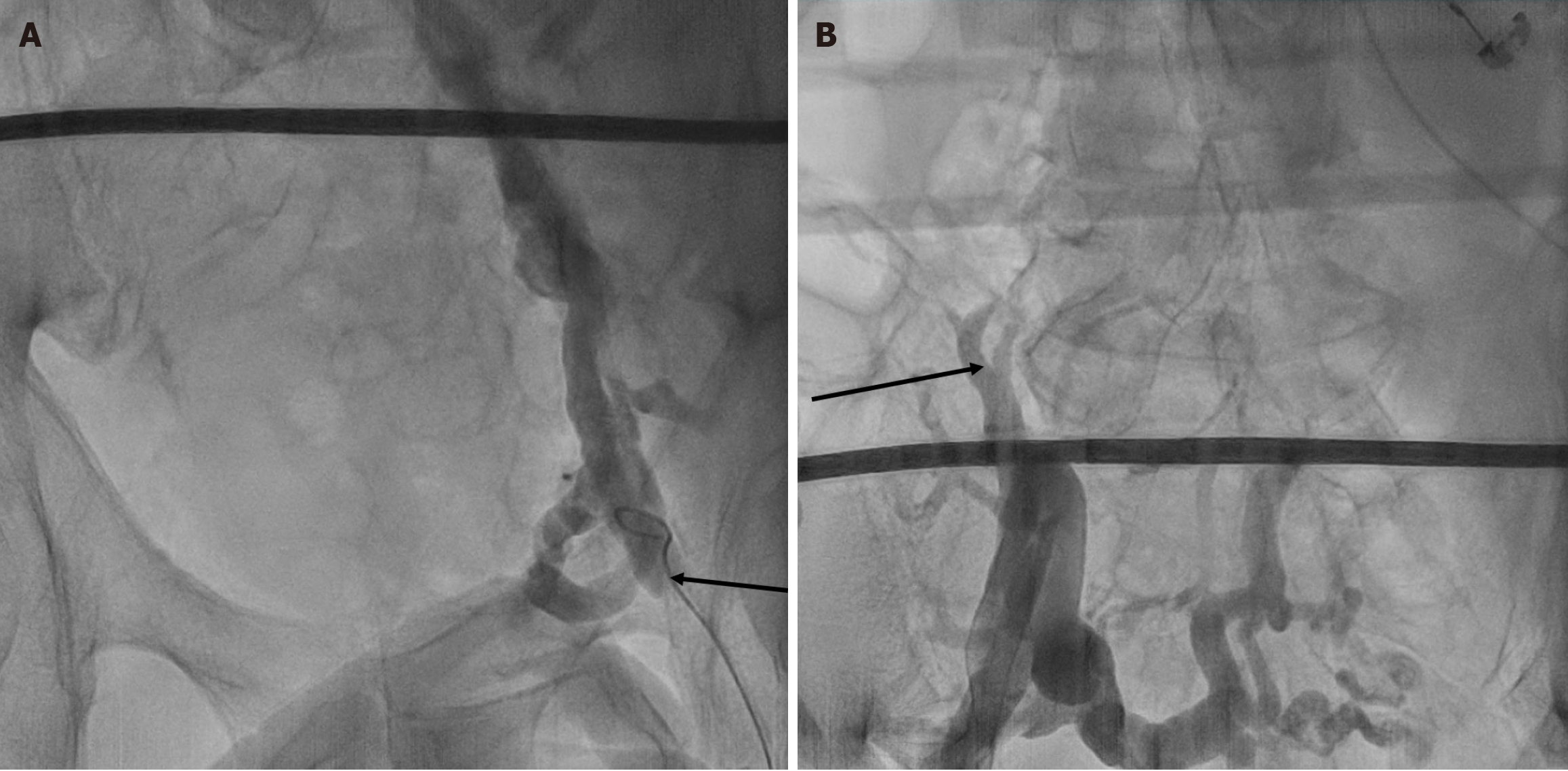
His past medical history is significant for hypertension, type 2 diabetes mellitus, chronic kidney disease, peripheral artery disease, bilateral popliteal artery aneurysm status post-surgical repair, and pulmonary embolism status post IVC filter. In February 2022, bilateral iliac vein occlusions were discovered. The right iliac vein had chronic total occlusions with an extensive network of collateral flow. The left iliac and external iliac veins were heavily diseased with a high thrombotic burden and occlusion. The left common femoral vein was also discovered to be diseased with chronic thrombus development (Figure 1). Recanalization attempts on his femoral and iliac veins have been unsuccessful in the past.
Patient reports no significant family history. He reports drinking two glasses of wine per week, denies tobacco and illicit drug use.
General: Alert, well appearing, in no distress.
Eyes: Pupils are equal and reactive.
Neck: Supple, no jugular venous distention, no carotid bruits, no lymphadenopathy.
Respiratory: Clear to auscultation, no wheezes, rales, or rhonchi; normal respiratory effort.
Cardiovascular: Normal S1 and S2, normal rate and rhythm, no murmur, click, gallop or rub.
Abd: Soft without significant tenderness or guarding, bowel sounds present.
Extremities: No pedal edema, no clubbing or cyanosis, palpable pulses to upper and lower extremities bilaterally.
Skin: Normal coloration, no rashes.
Neuro: Alert, oriented, normal speech, no focal findings.
Psych: Appropriate mood and affect, follows commands.
No laboratory examination data contributed to or was discussed in this case report.
Transthoracic echocardiography revealed a preserved left ventricular ejection fraction of 51%-55%, with mild valvular heart disease. Angiogram revealed bilateral iliac vein occlusions (Figure 1).
Symptomatic AF.
In March 2025, the patient arrived for PVI. During the PVI, accesses were attempted in both femoral veins; however, the J wires could not be advanced past the pelvis. Bilateral angiograms revealed total occlusion of the right iliac vein and subtotal occlusion of the left iliac vein. The procedure was aborted without complication. Two weeks later, he presented again for PVI. Given bilateral iliac occlusions, the decision was made to attempt a right IJ vein approach. His apixaban was continued peri-procedurally without need for heparin bridging, and intraoperatively the activated clotting time goal was > 300 seconds. A 7 French dipolar catheter was placed at the coronary sinus, and a 10 French intracardiac echocardiography catheter was placed in the right atrium both via left subclavian vein access. An SL1 sheath was advanced to the right atrium over a Brockenbrough needle under fluoroscopy via the right IJ vein. The septal puncture was performed, and the SL1 sheath was advanced into the left atrium. A guiding catheter and a pulse-select IV catheter were advanced into the left atrium. The pulmonary veins were isolated with pulse field ablation (PFA), and 8-10 pulse fields were delivered per vein. PVI was confirmed, and the sheaths were removed; a figure of 8 sutures was used to achieve hemostasis in the IJ.
The patient tolerated the procedure well without complications and was discharged the same day. At his 1-month post ablation follow up appointment he reported no recurrence of palpitations, shortness of breath and electrocardiogram revealed normal sinus rhythm. His European Heart Rhythm Association symptom class score improved from III (pre-ablation) to I.
PVI via catheter ablation is an established therapeutic option for symptomatic, drug-refractory AF. Although the standard approach is via transfemoral access, patients carry various individual anatomical differences. Due to these nuances, creativity is needed to provide patients with adequate treatment. In this patient, femoral access was limited due to extensive and chronic occlusions. Despite prior recanalization, obstructions recurred, yielding impossible access to the more traditional transfemoral approach. Therefore, the patient described in this case provides an illustrative example of a challenging venous anatomy and the novel solutions required to navigate the situation.
Although this is the first reported combination of IJ access for AF ablation with PFA; IJ access has been previously described in the literature for other cases. These include implantation of leadless pacemakers and slow pathway ablation for atrioventricular nodal re-entry due to barriers such as IVC interruptions[9,10]. Additionally, there are reports of atypical access sites for atrial ablations in patients lacking an IVC. Singh et al[11] presented two cases of atrial ablations in patients with absent IVCs. Their first patient presented with a congenital absence of the IVC, a single atrium receiving hepatic and pulmonary venous return, and a single ventricle with prior Fontan procedure. A successful flutter ablation was performed with percutaneous transhepatic access achieved via the right hepatic vein with ultrasound and fluoroscopic guidance. The second patient required AF ablation and was found to have a dilated hemiazygos vein, suggestive of an absent IVC. Successful ablation was performed with femoral access, guiding the catheter through the azygous vein, into the left-sided SVC and ultimately past the coronary sinus into the right atrium[11]. These approaches avoid IVC interruptions and offer a reliable route into the targeted location with the standard tools necessary to perform a successful ablation with minimal complications. Therefore, this method can be used in complex patients with unforgiving anatomy, allowing curative options to prevail despite access challenges.
In an analogous situation, transcatheter aortic valve implantations have been studied via subclavian access instead of traditional femoral access, showing that they are safe options for patients with complex or impossible femoral access[12]. De Boulle et al[13] presented a case in which percutaneous coronary intervention access was necessary via the left carotid artery because of total occlusion of bilateral iliac and subclavian arteries. Furthermore, there is a reported case of diagnostic coronary catheterization accessed via the left carotid artery[14]. As with the present report, these cases were successful and without significant complications despite the atypical access sites. These further bolsters the feasibility of this novel IJ approach for AF in patients requiring PVI, which is impossible through traditional methods.
In addition, the decision was made to use PFA over more traditional radiofrequency (RF) or cryoablation techniques. PFA is a relatively new ablative technique that utilizes short burst of electricity to deliver treatment. These bursts are faster and safer to surrounding structures than cryoablation or RF. PFA is also less reliant on contact with the endocardium and can even create lesions without direct tissue contact. Decreased reliance on endocardial contact to create lesions allows for more flexibility, and decreased catheter manipulation and repositioning. The more rapid energy delivery, and decreased catheter manipulation make for reduced procedure times, all desirable benefits given the novelty of this atypical IJ approach[15,16].
This case also highlights the importance of an individualized treatment plan where anatomical variance is critical for successful outcomes. However, IJ access is not without risk. Because of close anatomical proximity there is a risk of arterial injury and pneumothorax. Additionally, as with mechanical intervention in any vasculature, there is a risk of thrombosis[17]. No routine post-procedural duplex ultrasound of the IJ was performed in this case, so asymptomatic jugular thrombosis could not be excluded. A further limitation is the learning curve associated with both PFA energy delivery and superior (IJ) catheter manipulation, especially in those who are unfamiliar with the anatomy[18]. Operators should anticipate altered torque response and fluoroscopic orientation when steering catheters from a superior route, which may render standard maneuvers less intuitive[18]. These techniques require operator experience before outcomes equal those of conventional femoral RF or cryo-ablation.
The IJ offers rapid ultrasound-guided access, lower bleeding risk, and the ability to accommodate large-bore sheaths[19]. Its chief drawbacks are pneumothorax, arterial injury, and potential jugular stenosis[19]. Transhepatic access, while bypassing thoracic complications, carries higher bleeding and hepatic capsular perforation risks, whereas azygos navigation is technically demanding and limited by vessel caliber[20,21]. Although subclavian access has lower risks of bloodstream infections and symptomatic thrombosis, there is a higher risk of pneumothorax compared to IJ access[22]. The risks and benefits of these access sites are further demonstrated in Table 1.
| Access point | Benefits | Risks |
| Internal jugular vein | Rapid ultrasound-guided access | Pneumothorax |
| Lower bleeding risk | Arterial injury | |
| Ability to accommodate large bore sheaths | Potential jugular stenosis | |
| High first-attempt success rate | ||
| Lower rates of arterial puncture and hematoma with ultrasound guidance | ||
| Transhepatic access | Bypass thoracic complications | Bleeding risk |
| Useful in patients with contraindications to thoracic access | Hepatic capsular perforation | |
| Azygous vein | Alternative if all other options limited | Technically demanding with small vessel caliber |
| Subclavian vein | Lower risk of catheter-related bloodstream infection and thrombosis | Higher risk of pneumothorax and subclavian artery cannulization |
This case, however, was completed without these complications, adding to the growing body of evidence suggesting that IJ access is a safe and effective alternative to traditional approaches when necessary. Future research should be conducted to assess further the safety, efficacy, and long-term outcomes of using the right IJ approach in various patient populations. Finally, this case’s success showcases the critical need for physician adaptability and awareness in the field. As the prevalence of AF continues to rise, it is crucial to continue developing and refining techniques to ensure successful ablations in a variety of patients.
We presented a case of PVI using PFA in a patient with bilateral iliac vein occlusion. A novel approach via the right IJ was taken, facilitating a successful ablation of the pulmonary veins and eliminating AF. This broadens curative-ablation eligibility for patients with occluded femoral/IVC pathways who would previously may have been limited to lifelong medical rate control. It also provides a safer alternative to riskier transhepatic, azygous, and subclavian access sites. Finally, IJ access with PFA minimizes collateral injury, may shorten fluoroscopy times, and enable rapid discharge. The patient had minimal complications and was discharged the same day, supporting the feasibility of IJ access for PVI. This case expands the anatomic toolkit for complex AF ablation and may guide centers faced with the need for alternative routes in extraordinary patients.
| 1. | Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger ZD, Gopinathannair R, Gorenek B, Hess PL, Hlatky M, Hogan G, Ibeh C, Indik JH, Kido K, Kusumoto F, Link MS, Linta KT, Marcus GM, McCarthy PM, Patel N, Patton KK, Perez MV, Piccini JP, Russo AM, Sanders P, Streur MM, Thomas KL, Times S, Tisdale JE, Valente AM, Van Wagoner DR; Peer Review Committee Members. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1-e156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1133] [Cited by in RCA: 970] [Article Influence: 970.0] [Reference Citation Analysis (0)] |
| 2. | Noubiap JJ, Tang JJ, Teraoka JT, Dewland TA, Marcus GM. Minimum National Prevalence of Diagnosed Atrial Fibrillation Inferred From California Acute Care Facilities. J Am Coll Cardiol. 2024;84:1501-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 552] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 4. | Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ Res. 2017;120:1501-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 807] [Article Influence: 100.9] [Reference Citation Analysis (2)] |
| 5. | Chard M, Tabrizchi R. The role of pulmonary veins in atrial fibrillation: a complex yet simple story. Pharmacol Ther. 2009;124:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Parameswaran R, Al-Kaisey AM, Kalman JM. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat Rev Cardiol. 2021;18:210-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 7. | Santangeli P, Kodali S, Liang JJ. How to perform left atrial transseptal access and catheter ablation of atrial fibrillation from a superior approach. J Cardiovasc Electrophysiol. 2020;31:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Lim HE, Pak HN, Tse HF, Lau CP, Hwang C, Kim YH. Catheter ablation of atrial fibrillation via superior approach in patients with interruption of the inferior vena cava. Heart Rhythm. 2009;6:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Molitor N, Saleem-Talib S, Ramanna H, Hofer D, Breitenstein A, Steffel J. Leadless pacemaker implantation via the internal jugular vein. Europace. 2024;26:euae199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Salem YS, Burke MC, Kim SS, Morady F, Knight BP. Slow pathway ablation for atrioventricular nodal reentry using a right internal jugular vein approach: a case series. Pacing Clin Electrophysiol. 2006;29:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Singh SM, Neuzil P, Skoka J, Kriz R, Popelova J, Love BA, Mittnacht AJ, Reddy VY. Percutaneous transhepatic venous access for catheter ablation procedures in patients with interruption of the inferior vena cava. Circ Arrhythm Electrophysiol. 2011;4:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Petronio AS, De Carlo M, Bedogni F, Maisano F, Ettori F, Klugmann S, Poli A, Marzocchi A, Santoro G, Napodano M, Ussia GP, Giannini C, Brambilla N, Colombo A. 2-year results of CoreValve implantation through the subclavian access: a propensity-matched comparison with the femoral access. J Am Coll Cardiol. 2012;60:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | De Boulle M, Debing E, Belsack D, Vandeloo B. Carotid access for percutaneous coronary intervention. Clin Case Rep. 2021;9:e04739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Lichtenwalter C, Guleserian KJ, Holper EM. Carotid access for cardiac catheterization: revisiting the past for a complex intra-arterial approach. Cardiovasc Revasc Med. 2011;12:134.e1-134.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Carta-Bergaz A, Ríos-Muñoz GR, Ávila P, Atienza F, González-Torrecilla E, Arenal Á. Pulsed Field Ablation of Atrial Fibrillation: A Novel Technology for Safer and Faster Ablation. Biomedicines. 2024;12:2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Verma A, Haines DE, Boersma LV, Sood N, Natale A, Marchlinski FE, Calkins H, Sanders P, Packer DL, Kuck KH, Hindricks G, Onal B, Cerkvenik J, Tada H, DeLurgio DB; PULSED AF Investigators. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation. 2023;147:1422-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 286] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 17. | Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access--a systematic review. Crit Care Med. 2002;30:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 380] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Bohora S, Tharakan J. Internal jugular/subclavian venous access in electrophysiology study and ablation. Indian Pacing Electrophysiol J. 2009;9:190-194. [PubMed] |
| 19. | Practice Guidelines for Central Venous Access 2020: An Updated Report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2020;132:8-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 20. | Moise MA, Hadro N, El-Arousy H, Alvarez-Tostado JA. The azygos system as a rare alternative for chronic indwelling catheters placement. J Vasc Surg. 2009;50:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Diamanti A, Rollo M, Monti L, Candusso M, de Ville de Goyet J. Surgically assisted trans-hepatic anterior approach for central venous catheter placement: safety and efficacy. J Pediatr Surg. 2012;47:2353-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Parienti JJ, Mongardon N, Mégarbane B, Mira JP, Kalfon P, Gros A, Marqué S, Thuong M, Pottier V, Ramakers M, Savary B, Seguin A, Valette X, Terzi N, Sauneuf B, Cattoir V, Mermel LA, du Cheyron D; 3SITES Study Group. Intravascular Complications of Central Venous Catheterization by Insertion Site. N Engl J Med. 2015;373:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 462] [Article Influence: 46.2] [Reference Citation Analysis (0)] |