Published online Jul 26, 2025. doi: 10.4330/wjc.v17.i7.108363
Revised: April 26, 2025
Accepted: July 2, 2025
Published online: July 26, 2025
Processing time: 101 Days and 18.7 Hours
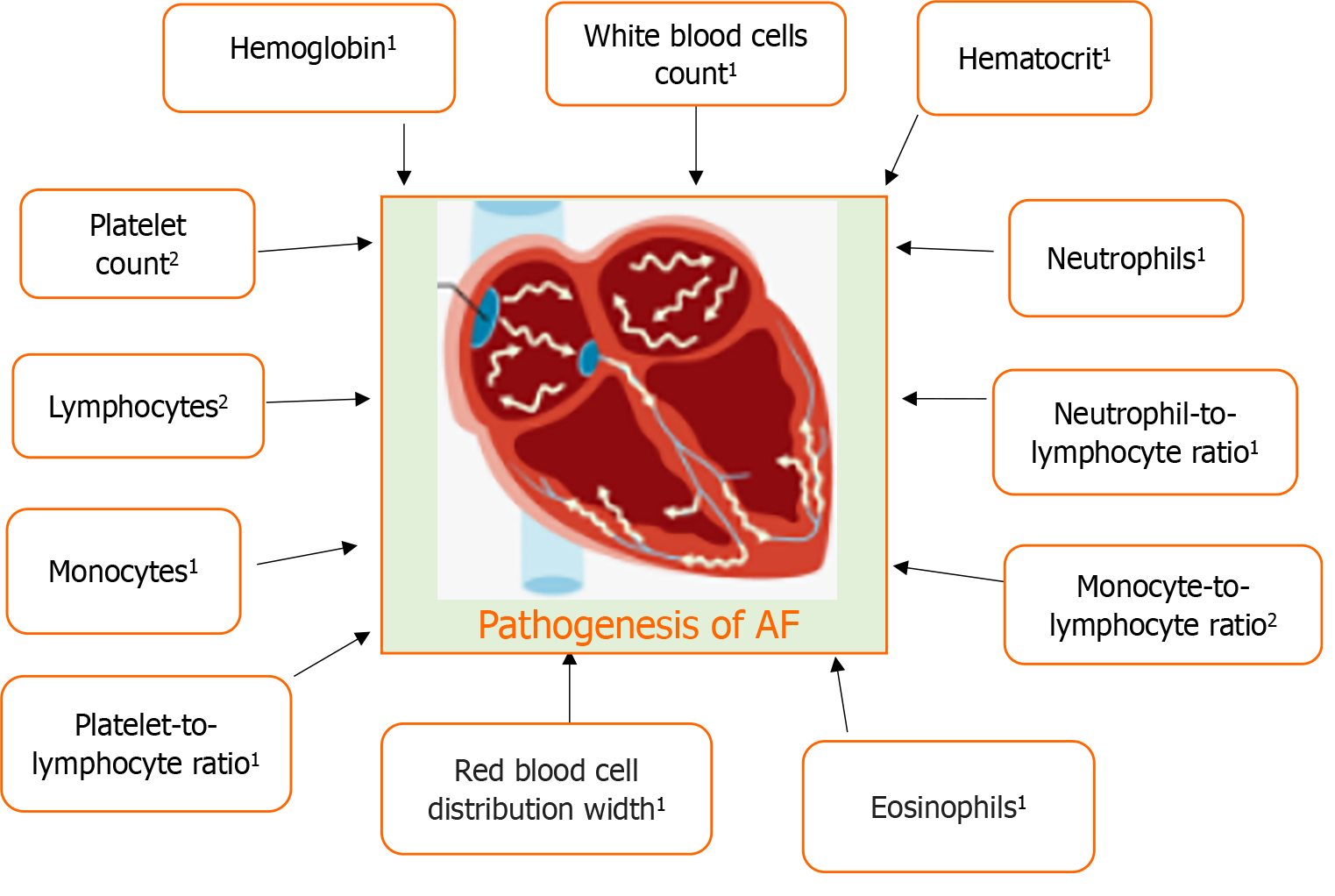
Atrial fibrillation (AF) is a frequent cardiac arrhythmia in the general population, which is associated with an increased risk of several health issues. It has been demonstrated that hematological variables predict the occurrence and recurrence of AF. This review article specifically only focuses on haemoglobin, hematocrit, platelet count, white blood cells (WBCs), lymphocytes, neutrophils, monocytes, neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR) and red blood cells in the pathophysiology of AF. It emphasizes that there is a higher risk of new-onset AF linked with both low and high haemoglobin levels. A quantitative investigation showed that hematocrit is not linked to the development of AF. The predictive significance of platelet count was reported in nonvalvular AF patients. WBCs are consistent inflammatory markers that are associated with postoperative new-onset AF. Inflammation and in particular, leukocyte activation predisposes to AF. Enhanced migratory activity in circulating and local monocytes may play a pivotal role in the pathogenesis of progression in atrial remodeling in AF patients. In particular, the peripheral eosinophil and left atrial diameter may be important in mediating inflammation and atrial remodeling in AF. In nonvalvular AF patients, PLR may be an independent risk factor for left atrial appendage thrombogenic milieu. NLR and MLR changes are associated with early recurrence of AF, and NLR change is related to late recurrence of AF after pulmonary vein isolation. Red blood cell distribution width and left atrial dimension were the only independent risk factors associated with AF.
Core Tip: Hematological variables predict the occurrence and recurrence of atrial fibrillation (AF). This review article specifically only focuses on haemoglobin, hematocrit, platelet count, white blood cells, lymphocytes, neutrophils, monocytes, neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio and red blood cells in the pathophysiology of AF.
- Citation: Rafaqat S, Hassan A, Usman A, Hussain I, Hussain Rathore AW, Tariq MF, Naseem H, Khan S, Zaidi M. Hematological parameters in atrial fibrillation: A literature review. World J Cardiol 2025; 17(7): 108363
- URL: https://www.wjgnet.com/1949-8462/full/v17/i7/108363.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i7.108363
Atrial fibrillation (AF), a frequent cardiac arrhythmia in the general population, is associated with an increased risk of several health issues. These problems include major and minor organ malfunction or failure, thromboembolism, stroke, neurological injury, and hospital readmissions, which result in a considerable rise in medical costs. In 2017, the database had information on 3.046 million new instances of AF globally. With 403/million people, the predicted incidence rate for 2017 was 31% higher than the similar incidence for 1997. The number of instances of AF globally is 37574 million, or 0.51% of the global population. Over the past 20 years, this number has mounted by 33%. High sociodemographic index nations bear the burden of the cost, whereas moderate sociodemographic index countries have witnessed the most recent growth. The absolute burden of AF may rise by more than 60% in 2050, according to predictions for the future[1].
A blood cell, also known as a hematopoietic cell, hemocyte, or hematocyte, is a kind of cell that is mostly found in blood and is created during hematopoiesis. Red blood cells (erythrocytes), white blood cells (leukocytes), and platelets (thrombocytes) are the three main kinds of blood cells. It has been demonstrated that hematological variables predict the occurrence and recurrence of AF. The complete blood count, which counts many blood components such as platelets, red blood cells, and white blood cells, is one of the blood tests that physicians recommend the most frequently. The complete blood count is one of the most important blood tests in clinical practice and is typically part of the diagnostic procedure for cardiovascular disease. The clinical practice recommends a complete blood count as part of the diagnostic process for AF due to the potential predictive relevance of haematological markers for both new-onset and recurrent AF[2].
Another study reported that the AF group had elevated levels of mean corpuscular volume, red blood cells, and white blood cells as compared to the control group. In contrast, the AF group showed reduced levels of platelets, hematocrit, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, and haemoglobin when compared to the control group. Finally, it demonstrated a strong correlation between AF and haematological markers[3].
An increased risk of morbidity and death is AF, a frequent consequence following acute myocardial infarction (AMI). High white and red blood cell counts were found to be possible risk factors for newly diagnosed AF in earlier research. The incidence of AF following AMI was significantly correlated with serum haemoglobin, haemoglobin concentration, and erythrocyte count. More research is necessary to fully understand the pathophysiologic mechanism behind these relationships and if it has any therapeutic implications[4].
This review article specifically only focuses on haemoglobin, hematocrit, platelet count, white blood cells, lymphocytes, neutrophils, monocytes, neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR) and red blood cells in the pathophysiology of AF.
To conduct this literature review, multiple databases such as Google Scholar, PubMed, and Science Direct were searched. The search process was concluded on 5 April 2025. Various keywords, including ‘atrial fibrillation’, ‘hematological parameters’, ‘blood cells’, ‘red blood cells’, ‘white blood cells’, and ‘platelets’ were employed. It should be noted that the clinical investigations were limited to articles published in English. While the focus was on more recent studies, no specific time limit was set. Furthermore, the reference lists of relevant articles were examined, leading to the discovery of additional related articles for comparison.
Circulating levels of hematological parameters in AF are elaborated in Figure 1. Additionally, Table 1 illustrates the pathophysiological aspects of hematological parameters in AF.
| Hematological parameters | Pathophysiological aspects |
| Haemoglobin | Undetermined |
| Hematocrit | Undetermined |
| Platelet | (1) A specific reduction in platelet aggregation in response to thrombin receptor activating peptide, which activates the thrombin receptor protease-activated receptor-1, was observed in all AF patients; (2) Acute episodes of AF results in a decrease in MPs-associated tissue factor activity, possibly corresponding to consumption, which in turn favors coagulation and the local production of thrombin; and (3) A decreased platelet basal aggregation to thrombin receptor activating peptide may result from protease-activated receptor-1 desensitization, whereas the improved response after an induced episode of AF suggests activation of coagulation and protease-activated receptor-1 re-sensitization |
| WBC count | Only consistent inflammatory marker associated with postoperative new-onset AF |
| Lymphocytes | (1) CD4+ CD28null T lymphocytes play a crucial role in the development and progression of AF; and (2) Notably, these cells are believed to be a key player in a T-cell-mediated autoimmune reaction against myocardial tissue |
| Neutrophils | (1) Inflammation and in particular leukocyte activation predisposes to AF; (2) CD11b-integrin mediated atrial polymorphonuclear neutrophils infiltration to the formation of fibrosis, which promotes the initiation and propagation of AF; (3) Neutrophil extracellular traps were increased significantly in AF patients and positively correlated with Spontaneous echo contrast grades; and (4) Neutrophil extracellular trap levels increased significantly in the left atrial appendage and promoted thrombosis |
| Monocytes | (1) High monocyte counts independently predict the occurrence of MACE, major bleeding and mortality, but not SSE; (2) A higher number of CCR2-positive monocytes/macrophages in the left atrial appendages in the enlarged left atrium group; (3) Enhanced migratory activity in circulating and local monocytes may play a pivotal role in the pathogenesis of progression in atrial remodeling in AF patients; (4) An elevated preablation monocyte/high-density lipoprotein ratio was associated with an increased risk of the postoperative recurrence of AF; (5) Intermediate CD14++ CD16+ monocytes are associated with incident atrial fibrillation independently of other common risk factors in the general population, supporting the role of inflammatory cells in AF; (6) Elevated pre-ablation monocyte-to-high-density lipoprotein high-density lipoprotein ratio was associated with an increased recurrence of AF after cryoballoon-based catheter ablation; (7) The monocyte-to-high-density lipoprotein ratio was found to be associated with the occurrence of atrial high-rate episodes in patients with cardiac implantable electronic devices; (8) Increased monocyte count in subjects with heart failure is related to the development of AF; (9) The chemokine receptor CXCR-2 is a critical regulator of monocyte mobilization in hypertension and cardiac remodeling; (10) CXCR-2 in driving monocyte infiltration of the atria, which accelerates atrial remodeling and AF after hypertension; and (11) The intermediate monocytes and toll-like receptor 4 expressions positively correlated with the expansion of low-voltage zones in AF patients |
| Lymphocyte-to-monocyte ratio | Undetermined |
| Eosinophils | Especially, the peripheral eosinophil and left atrial diameter may play important roles in mediating inflammation and atrial remodeling in AF |
| Neutrophil to lymphocyte ratio | (1) Independent predictors of AF recurrence; (2) In PAF patients, high NLR indicates thrombogenesis with a high degree of certainty and is associated with reduced left atrial appendage contraction rather than with left atrial body function; (3) NLR increases in diabetic patients with AF when compared to diabetic patients without atrial fibrillation; and (4) NLR might be associated with thrombosis and bleeding risk scores and might predict cardio-embolic risk in nonvalvular atrial fibrillation patients within the therapeutic international normalized ratio |
| Platelet to lymphocyte ratio | (1) Elevated PLR is a marker of increased inflammation and may serve as a practical and inexpensive predictor for recurrence during 6 months of follow-up in patients with non-valvular persistent AF who had restoration of the sinus rhythm after successful ECV; (2) Patients with an elevated preoperative PLR were at higher risk of AF after coronary artery bypass graft surgery; (3) The PLR was lower in patients with nonvalvular atrial fibrillation and with a decreased left atrial appendage flow velocity; (4) Its correlation with left atrial strain might indicate the role of inflammation in the progression of atrial remodeling and the prothrombotic state; and (5) PLR may be an independent risk factor for left atrial appendage thrombogenic milieu in nonvalvular AF patients |
| Monocyte to lymphocyte ratio | (1) Indicators of systemic inflammatory response because systemic inflammation is associated with the alternation in peripheral blood leukocytes; and (2) NLR and MLR changes are associated with early recurrence of atrial fibrillation, and NLR change is related to late recurrence of AF after pulmonary vein isolation |
| Red blood cell distribution width | (1) Reflects the heterogeneity of the volume and size of red blood cells; (2) RDW and left atrial dimension were the only independent risk factors associated with AF; (3) Red blood cell distribution width was associated with the incidence of AF independently of several cardiovascular, nutritional and hematological factors; (4) Elevated RDW levels may be an independent risk marker for nonvalvular AF, affected by the type of AF and altitude; and (5) Red cell distribution width is directly associated with the risk of stroke regardless of anemia status and improves the predictive accuracy for stroke in patients with AF |
The protein in red blood cells called hemoglobin transports oxygen from the lungs to the body’s other organs. Additionally, it returns carbon dioxide to the lungs. Your blood’s hemoglobin content is determined via a hemoglobin test. In the general Korean population’s risk of developing new-onset AF was examined concerning haemoglobin levels and their variations. One major risk factor for the development of AF has been identified as the presence of anemia, which is defined as a haemoglobin level of < 13 g/dL in males and < 12 g/dL in women. Even after accounting for cardiovascular risk factors, haemoglobin levels continued to show a U-shaped connection with the incidence of AF. With haemoglobin values of 14-14.9 g/dL in men and 12-12.9 g/dL in women, the lowest incidence of AF was noted[5].
Both a drop and a rise in haemoglobin levels outside these normal limits at the second assessment were linked to an increased risk of AF in those whose haemoglobin levels were within normal ranges. In particular, compared to individuals with normal haemoglobin levels, a drop in haemoglobin levels resulted in an 11% and 21% increase in AF risk for males and a 3% and 36% increase for women, respectively. It emphasizes that there is a higher risk of new-onset AF linked with both low and high haemoglobin levels. Furthermore, it has been hypothesized that keeping haemoglobin levels within the normal range reduces the likelihood of developing AF. According to Lim et al[5], study, there is little chance of developing AF if haemoglobin levels are kept within normal ranges.
An investigation of the possible correlation between haemoglobin levels and rhythm outcomes following AF catheter ablation (AFCA) was the focus of another study. Warfarin usage, body mass index, baseline red cell distribution width (RDW), and paroxysmal AF were found to be independent risk factors for anemia in AF patients. Compared to patients without anemia, those with pre-AFCA anemia had a much greater clinical recurrence rate of AF[6].
Those who were male and had paroxysmal AF showed the greatest difference in this regard. The following factors are independent predictors of post-AFCA clinical recurrence: Anemia, female sex, left atrial diameter (LAD), and persistent AF. An important direct causative association between anemia and AF recurrence at the genetic level was not found by a mendelian randomization investigation incorporating genetic variants related to haemoglobin levels. An independent predictor of clinical recurrence after AFCA was shown to be pre-AFCA anemia. On the other hand, the mendelian randomization study did not show a genetically based causal link between anemia and AF recurrence. These results highlight the therapeutic significance of taking haemoglobin levels into account when forecasting outcomes following AFCA, particularly in particular patient subgroups[6].
Qin et al[7] investigated the relationship between all-cause mortality and the hemoglobin-to-RDW ratio (HRR) in ischemic stroke patients with AF. It showed that serum HRR levels were negatively linked with 180-day all-cause mortality. In individuals with ischemic stroke with AF, the correlation between HRR levels and all-cause mortality was non-linear. There was a negative correlation between HRR levels and all-cause mortality when the HRR value was ≤ 9.74.
Postoperative anemia affects the majority of individuals who undergo heart surgery. Both AF and delirium are frequent and reliable indicators of morbidity and death. They are rarely examined for postoperative anemia. The purpose of another study was to measure the relationship between anemia and these outcomes in patients after heart surgery. In the postoperative phase, the majority of patients receiving major heart surgery were anemic. There was no significant correlation between postoperative hemoglobin and delirium or AF, which occurred in 34% and 12% of patients, respectively[8].
The risk of worsening anemia in individuals following AF ablation was examined in this three-centre cardiovascular investigation. The study investigated whether individuals with anemia using oral anticoagulant treatment were at a higher risk of hemorrhage following AF ablation. To compare the decline in haemoglobin levels 24 hours after the treatment and assess bleeding consequences across patients with and without preexisting anemia, the research included AF patients who had missed a single dosage of a direct oral anticoagulant before the ablation[9].
Hemoglobin levels were reduced in anemic individuals, but not in non-anemic patients. The presence of preexisting anemia, female gender, and chronic or long-standing persistent AF vs paroxysmal AF were independent predictors linked to a considerable hemoglobin reduction. Between anemic and non-anemic individuals, there was no discernible difference in the risk of serious bleeding problems. Contrary to expectations, individuals who already had anemia seemed to have a lower risk of blood loss after AF ablation[9].
The impact of platelet counts and haemoglobin concentration on opposed outcomes in Japanese patients with non-valvular AF (NVAF) was examined in a post hoc analysis of the J-RHYTHM Registry. Important conclusions from the research showed that as haemoglobin levels dropped, there was an increase in major bleeding, all-cause mortality, and composite events[10].
Platelet counts declined with an increase in composite events. Haemoglobin levels < 12.0 g/dL were associated with a greater risk of all-cause mortality and composite events after controlling for numerous covariates than hemoglobin levels ≥ 14.0 g/dL. Even in multivariate analysis with the stepwise forward approach, platelet count was not related to any events. In conclusion, in Japanese patients with NVAF, a low hemoglobin level was found to be an independent risk factor for all-cause mortality and composite events. However, in this population, platelet count did not affect the results[10].
Wang et al[11], study demonstrates how the HRR and all-cause mortality among AF patients who are septic are related. A lower HRR (< 5.877) was connected with an increased risk of all-cause mortality, which made it relevant as a predictive predictor of clinical outcomes for those septic patients with AF. AF and its consequences are linked to elevated risks for both low and high hemoglobin levels. For patients who have AF or are at risk for it, routine haemoglobin level monitoring and control should be essential parts of their overall therapy.
A simple blood test called a hematocrit test calculates the proportion of red blood cells in your blood. The oxygen that red blood cells deliver throughout your body makes them vital. Low or high hematocrit levels in test results might indicate blood problems or other illnesses. The most common arrhythmia seen after heart surgery is postoperative AF (POAF) and there has been a connection shown between AF and preoperative anemia and higher rates of postoperative morbidity and death. The question of whether preoperative haemoglobin or hematocrit levels are helpful indicators for the development of POAF following heart surgery was examined in another research. A connection between preoperative haemoglobin/hematocrit levels and AMI was one of the outcome measures[12].
The effect size was not diverse for studies including haemoglobin [Q(df) = 11.10, P = 0.26, I2 = 18.96%], whereas it was heterogeneous for studies involving hematocrit [Q(df) = 30.76, P < 0.001, I2 = 73.99%]. Based on a random-effect model, the analysis findings for hematocrit studies showed a standardized mean difference of 0.013, 95% confidence interval (CI): -0.21 to 0.18, and P = 0.89 (P > 0.05). A fixed-effect model analysis of the studies utilizing haemoglobin revealed a standardized mean difference of 0.172, 95%CI: -0.23 to -0.11, and P < 0.001. Preoperative hemoglobin is linked to the development of AF, although hematocrit is not, according to the quantitative analysis’s findings. To definitively demonstrate this link, more research using both hemoglobin and hematocrit in the same trials could be necessary[12]. AF and hematocrit levels have a complicated connection that affects both the risk of thromboembolic events and overall mortality. Lower hematocrit levels have been associated with higher mortality in AF patients, although rising hematocrit during AF episodes may aid in thrombus development. These results highlight how crucial customized evaluation and hematocrit level monitoring are to the treatment of AF.
The smallest component of your blood is called platelets, which are fragments of cells. Their main responsibility is to prevent the bleeding if you are hurt. When a blood vessel is broken, platelets group to stop the blood loss by first forming a plug and subsequently a clot. Platelet-related disorders such as thrombocytopenia and thrombocytosis are common. The test that determines how many platelets are in your blood is called a platelet count. Another study investigated new facets of platelet biology in AF, primarily concentrating on platelet activation and the effects of co-administration of antiplatelet and anticoagulant medication. Two hundred and thirty-eight patients were enrolled in the trial, divided into three groups: Ninety-three patients were not receiving antithrombotic medication; Sixty patients were taking 75-325 mg of aspirin daily; And eighty-five patients were on dose-adjusted warfarin (international normalized ratio range: 2.0-3.0)[13].
The investigation focused on platelet markers, coagulation indicators, and platelet aggregation in response to conventional platelet agonists. Platelet markers included plasma beta-thromboglobulin and soluble glycoprotein V. There was no difference in plasma fibrinogen or platelet aggregation between patients with AF and healthy controls. Those using aspirin showed a decreased platelet aggregation response to epinephrine, whilst those on warfarin showed a markedly decreased plasma fibrin D-dimer level[13].
No appreciable deviations in platelet aggregation were found, even though AF patients showed alterations in plasma indicators of platelet function. Aspirin or warfarin treatment did not significantly improve platelet activation, while warfarin usage was linked to a decrease in thrombogenesis (fibrin D-dimer). According to the study, platelet activation may not be a major factor in thromboembolism pathogenesis in patients with AF[13].
The predictive significance of platelet count in nonvalvular AF patients was revealed by Park et al[14]. A decreased platelet was linked to a higher incidence of bleeding episodes and a reduced risk of stroke. Platelet significantly improved stroke prediction when paired with traditional risk variables[14]. Another study suggested that the duration of AF affects platelet aggregation and coagulation, which are enhanced by AF itself. After AF occurred, platelet activity and coagulability increased 12 hours later[15].
A major danger associated with AF is thrombotic consequences, and little is known about the functions of platelets and microparticles (MPs) in this setting. Pourtau et al[16] sought to evaluate the impact of acute AF episodes on platelet function as well as the procoagulant and fibrinolytic activities of MPs in AF patients. In all individuals with AF, there was a particular decrease in platelet aggregation in response to thrombin receptor activating peptide (TRAP), which activates the thrombin receptor protease-activated receptor-1 (PAR-1)[16].
The expression of platelet receptors, however, did not alter. Remarkably, platelet responsiveness improved with acute AF induction. The left atrium (LA)’s tissue factor-dependent procoagulant activity of MPs also showed a significant decline. The results imply that MPs-associated tissue factor activity is decreased during acute bouts of AF, which may indicate consumption and promote local coagulation and thrombin generation. While the enhanced response following provoked AF supports coagulation activation and PAR-1 re-sensitization, the lower baseline platelet aggregation to TRAP may be linked to PAR-1 desensitization[16].
When AF patients are receiving percutaneous coronary intervention (PCI), platelet function testing (PFT) may prove to be an invaluable therapeutic tool for customizing antithrombotic medication. Another research used real-world data from a multicenter, countrywide observational study of AF patients receiving PCI while taking oral anticoagulants to examine platelet reactivity (PR). Significant bleeding episodes during follow-up were not predicted by PFT findings, nor did they affect the aspirin prescription at discharge. Comparing ticagrelor to clopidogrel, the former produced decreased PR and a decreased incidence of high on-treatment PR. The different ways that clinicians responded to patients’ PR highlighted the need for more studies to determine how PFT influences the individualization of antithrombotic therapy[17].
The transcriptome of platelets is altered in many illnesses. Platelets are recognized for their ability to produce proteins and for maintaining cytoplasmic messenger RNA. Nevertheless, little is known about how NVAF affects the transcript of platelet RNA. Wysokinski et al[18] examined platelet gene expression in consecutive NVAF patients before and 3-4 months following pulmonary vein isolation (PVI), comparing the results to normal sinus rhythm controls (NSR), to assess the impact of NVAF on platelet RNA transcript[18]. Notably, genes encoding for prostacyclin receptor and von Willebrand factor had fourfold lower expression compared to NSR controls, and the insulin-like growth factor binding protein acid labile subunit gene (IGFALS) showed more than a 16-fold decrease[18].
Gender, AF type, heart failure, hypertension, previous stroke, diabetes mellitus, and atherosclerosis have all been linked to different gene expressions. The expression of four genes rose dramatically after PVI, with the IGFALS gene increasing 256-fold and the ADAMT gene increasing 16-fold. Three genes showed a substantial drop in expression, whilst eight genes showed no change in expression. These results imply that platelets can change their transcript in response to the NVAF circulatory environment, perhaps going through molecular “reprogramming” brought on by the NVAF-related flow disruptions[18].
In individuals with AF, platelet vesiculation is frequently a contributing factor to coagulation and thrombosis. Numerous physiological processes, such as coagulation, thromboembolism, microvascular inflammation, arterial stiffness, vascular calcification, formation and rupture of atherosclerotic plaque, endothelial dysfunction, cardiac remodeling, and kidney dysfunction are all significantly impacted by platelet-derived vesicles[19].
Increased levels of platelet-derived vesicles have been seen in the peripheral blood of individuals with both active and past AF. It is proposed that circulating levels of platelet-derived vesicles can be measured on a serial basis to help stratify individuals with AF who are at risk of thromboembolic consequences. It is crucial to remember that there is currently little data supporting their predictive value. To prove their validity and usefulness in foretelling opposed consequences related to AF, more research in sizable clinical trials is required[19]. Assessing platelet counts as part of AF treatment procedures can help identify individuals who are more likely to hemorrhage and die. Clinicians may need to modify anticoagulant doses or take into account alternate therapy for AF patients with thrombocytopenia to balance the risks of bleeding and stroke. Throughout therapy, regular platelet count monitoring can aid in the early identification of thrombocytopenia and prompt action to reduce related risks.
White blood cells perform specialized and unique roles that together constitute the immune system’s core. Together, these cells can build a quick, effective, targeted, and long-lasting defense against invasive infections. In addition to controlling infections, the immune system is essential for wound healing, homeostasis preservation, and cancer prevention. white blood cells must work together harmoniously since any disruption might trigger immunological responses that are not suitable, which can cause immunopathology, autoimmunity, allergies, and even cancer. Numerous of these activities take place in lymphoid organs as well as in the original site of activity[20].
Numerous investigations have demonstrated the connection between AF and inflammatory markers. The relationship between incident AF and the white blood cell count, a commonly accessible indicator of systemic inflammation, was examined, with an emphasis on determining if this correlation was mediated by variables including smoking, myocardial infarction, and heart failure[21].
In addition to smoking, previous myocardial infarction, intermediate myocardial infarction, and heart failure before the event AF, adjustments were made for conventional AF risk variables. The median white blood cell count was 6.4 ×
The inflammatory response has been investigated as a possible underlying mechanism for postoperative new-onset AF (PNAF), a frequent complication following heart surgery. According to earlier research, the only reliable inflammatory measure connected to post-neutrophil autoimmunization is the white blood cell count. A bigger cohort was used in a different study to examine the relationship between perioperative white blood cell response and PNAF. In summary, a larger cohort in this investigation did not show a significant correlation between the development of PNAF and the perioperative white blood cell response. Other variables that were shown to be connected with the incidence of PNAF were age and the kind of heart surgery[22]. Elevated white blood cell counts are increasingly recognized as a significant marker for AF risk, underscoring the importance of systemic inflammation in AF pathophysiology. Incorporating white blood cell count monitoring into clinical practice could enhance early detection and prevention strategies for AF.
White blood cells with a consistent appearance but a variety of functions are known as lymphocytes. They consist of natural killer (NK) cells, T cells, and B cells, each of which has a specific function in the immune system. T cells have a role in the generation of antibodies, the control of the immune response, and the direct cell-mediated destruction of tumor and virus-infected cells. Antibodies are proteins that detect and neutralize infections, and they are produced by B cells. NK cells aid in the direct destruction of aberrant or infected cells. The discovery and characterization of novel effector populations inside lymphocytes have been made possible by recent developments in immunology. T cells that produce interleukin-17, T cells that have regulatory roles, and NK T cells are a few examples. These findings have aided in the reworking of immunology’s conventional concepts[23].
Previous research has established a strong connection between inflammatory processes and the multifactorial pathogenesis of AF, with a particular focus on auto-reactive cluster of differentiation (CD) 4+ CD28null T cells. This article outlines a potential pathophysiological pathway highlighting the impact of CD4+ CD28null T lymphocytes on the development and progression of AF[24].
Based on existing literature, it is suggested that CD4+ CD28null T lymphocytes play a crucial role in the development and progression of AF. Notably, these cells are believed to be a key player in a T-cell-mediated autoimmune reaction against myocardial tissue. However, the mechanisms responsible for recruiting CD4+ CD28null cells to cardiac tissue remain unclear and necessitate further investigation. Understanding these mechanisms could provide valuable insights into the autoimmune aspects of AF pathogenesis[24]. Lymphocytes, particularly T and B cells, play critical roles in the immunopathogenesis of AF. Their involvement in inflammation, structural remodeling, and autoimmunity highlights the importance of immune mechanisms in AF. Ongoing research into lymphocyte function and regulation may pave the way for innovative diagnostic tools and targeted therapies in AF management.
White blood cells called neutrophils, or granulocytes, are an essential component of the immune system and are mostly in charge of warding off wounds and infections. Their proportion of the total white blood cell count is substantial, making them the most prevalent form of white blood cell in the body. Polymorphonuclear neutrophils (PMNs), another name for neutrophils, were formerly thought to be transient, indiscriminate white blood cells that formed pus and, coincidentally, fought against incoming microorganisms[25].
Neutrophils only play a supporting role in immunological responses. Beyond their basic job of eliminating germs and fungus, neutrophils are now understood to be essential for regulating the host’s response to infection and preserving immune system homeostasis. This change in understanding of neutrophils’ function in the immune system is mostly the consequence of in vitro research, which has been made possible by the advancement of fresh and more effective techniques and methodologies for studying these cells[25].
Recent observational clinical studies and ex-vivo experiments have suggested that inflammation, particularly leukocyte activation, may contribute to the predisposition to AF. However, it has remained unclear whether the local binding and extravasation of leukocytes into the atrial myocardium are essential prerequisites for initiating and propagating AF. The researchers investigated the role of atrial CD11b/CD18-mediated infiltration of PMNs in the susceptibility to AF. The study involved C57bl/6J wildtype (WT) mice and CD11b/CD18 knock-out (CD11b-/-) mice treated with angiotensin II (Ang II) for 14 days, a known stimulus for PMN activation. Atria of Ang II-treated WT mice showed increased PMN infiltration, as assessed in immune-histochemically stained sections[26].
In contrast, atrial sections of CD11b-/- mice did not exhibit a significant increase in PMN infiltration upon Ang II infusion. PMN infiltration was accompanied by markedly enhanced atrial fibrosis in Ang II-treated WT mice compared to CD11b-/- mice. Upon in-vivo electrophysiological investigation, Ang II treatment significantly increased susceptibility to AF in WT mice, characterized by an increased number and duration of AF episodes. In contrast, CD11b/CD18-deficient mice were entirely protected from AF induction. Epicardial activation mapping revealed decreased electrical conduction velocity in the atria of Ang II-treated WT mice, which was preserved in CD11b-/- mice. Additionally, atrial PMN infiltration was enhanced in atrial appendage sections of patients with persistent AF compared to patients without AF. These findings suggest a critical link between CD11b-integrin-mediated atrial PMN infiltration, fibrosis formation, and the initiation and propagation of AF. The study not only provides mechanistic insights into the role of leukocytes in AF but also suggests a potential novel avenue for treating AF[26].
Another research examined the possible correlation between thrombogenesis and neutrophil extracellular traps (NETs) in individuals with AF. Furthermore, NETs’ impact on thrombogenesis was investigated using rat models. Transesophageal echocardiography was used to evaluate spontaneous echo contrast (SEC) to determine the prothrombotic condition. It was discovered that NET levels were positively connected with SEC grades and that there was a substantial rise in NETs in AF patients. The insertion of NET levels improved the congestive heart failure, hypertension, age ≥ 75 (doubled), diabetes mellitus, prior stroke or transient ischemic attack (doubled), vascular disease, age 65-74, female (CHA2DS2-VASc) scores’ prediction power for the prothrombotic condition of AF substantially. The left atrial appendage (LAA) of receptor activating peptide (RAP) models showed a considerable rise in NET levels, which encouraged thrombosis[27].
The NET formation inhibitor may reduce these alterations. According to a transcriptomic investigation of LAA tissue from RAP rats, RAP may promote the expression of adhesion genes and inflammatory cytokines, which in turn may drive the creation of NETs. According to the research, NETs may be helpful thrombogenesis risk indicators in patients with AF and offer a viable treatment approach for managing AF[27].
Meulendijks et al[28] evaluated the relationship between epicardial adipose tissue (EAT) features and AF in individuals receiving thoracoscopic AF ablation. The study specifically examined whether patients with paroxysmal and persistent AF, as well as those with and without AF recurrence following thoracoscopic ablation, differed in terms of computed tomography (CT) scan attenuation and volume of EAT, as well as histological parameters like adipocyte size and immune cell infiltration[28].
The CT scan parameters were evaluated and adjusted for body surface area and beam intensity, including EAT attenuation and volume. The findings showed that compared to paroxysmal AF, chronic AF had higher CT-EAT attenuation. CT-EAT attenuation was correlated with neutrophil count and adipocyte size, while persistent AF was linked to a larger neutrophil count. Patients with AF recurrence had bigger adipocytes, but there was no difference in immune cell counts, EAT volume, or CT-EAT attenuation between those with and without AF recurrence. In summary, CT-EAT attenuation, which represents the histological infiltration of neutrophils, may be used as a non-invasive diagnostic to distinguish between paroxysmal and chronic AF. Nevertheless, following thoracoscopic ablation, CT-EAT attenuation did not indicate the return of AF[28].
A correlation was evaluated between the levels of NLR and the recurrence of lone AF following catheter ablation. NLR levels were measured before and following catheter ablation, and the relationship between NLR and recurrence of AF was examined using Cox regression analysis. Compared to patients without recurrence, those with AF showed a greater post-ablation NLR (post-NLR)[29].
Post-NLR, LAD, and body mass index were found to be independent predictors of AF recurrence by multivariate Cox regression analysis. Post-NLR predicted AF recurrence with a sensitivity of 73% and specificity of 67% at a cut-off level of 5.15. Finally, it implies that following catheter ablation, a greater incidence of lone AF recurrence is linked to an increased post-NLR. A straightforward and perhaps helpful measure to identify high-risk individuals who can benefit from pharmacologic intervention to prevent AF recurrence is monitoring NLR levels[29].
White blood cells called monocytes are produced in the bone marrow and are essential to the innate immune response. They are mostly in charge of maintaining cellular homeostasis, but they also have a major part in fighting inflammation and infections. Monocytes, which typically make up 5% of nucleated cells in normal adult blood, only last one to three days in the bloodstream. In myelodysplastic syndromes, a decrease in circulating monocytes, or monocytopenia, is commonly seen. On the other hand, a rise in monocyte counts, or monocytosis, is frequently observed in several ailments, including infections, trauma, drug use, autoimmune diseases, and some types of cancer[30].
Increased monocyte counts have been associated with worse outcomes in cardiovascular illness, although it is unknown how important they are for prognosis in AF. The highest quartile of monocyte counts (≥ 580 μL) was linked to a higher risk of death and major adverse cardiovascular events (MACE), according to Cox regression analysis after adjusting for the CHA2DS2-VASc score[31].
Moreover, a further increase in the risk of mortality and MACE was associated with persisting monocyte levels ≥ 580 per μL during follow-up. Interestingly, even after controlling for the HAS-BLED score, persistently elevated monocyte levels were linked to a substantial increase in major bleeding episodes. In summary, elevated monocyte counts in AF patients are predictive of MACE, severe hemorrhage, and death in an independent manner, but not of other systemic embolisms. The comprehension of the connections between monocytes and unfavorable thrombotic and bleeding outcomes in patients with AF may be improved by more investigation of the underlying pathophysiological processes[31].
It is yet unknown what role monocytes play pathophysiological in AF, namely in the development of structural remodeling of the LA. The purpose of the investigation was to determine if the properties of local and circulating monocytes and the degree of structural remodeling of the lung, as measured by lung size, were related in patients with AF[32]. To assess the RNAs and protein levels of the CC chemokine receptor 2 (CCR2) as well as the monocytes’ migratory behaviour toward the monocyte chemoattractant protein 1, monocytes were also separated from the blood. There was no significant difference seen in the proportions of monocyte subsets based on CD14 and CD16 expressions between the normal and enlarged LA groups. It is yet unknown what role monocytes play pathophysiological in AF, namely in the development of structural remodeling of the LA. The purpose of the investigation was to determine if the properties of local and circulating monocytes and the degree of structural remodeling of the lung, as measured by lung size, were related in patients with AF[32].
To assess the RNAs and protein levels of the CCR2 as well as the monocytes’ migratory behavior toward the monocyte chemoattractant protein 1, monocytes were also separated from the blood. There was no discernible difference in the proportions of monocyte subsets between the normal and enlarged LA groups based on CD14 and CD16 expressions. The expanded LA group’s monocytes had greater amounts of CCR2 transcripts and total protein than the regular LA group’s. Monocytes showed greater elevated migratory activity in the expanded LA group compared to the standard LA group. Additionally, in the larger LA group, we discovered a noticeably greater quantity of CCR2-positive monocytes/macrophages in the LAA. The pathophysiology of the course of atrial remodeling in individuals with AF may be significantly influenced by increased migratory activity in local and circulating monocytes[32].
Recently, there has been increased interest in the monocyte/high-density lipoprotein ratio (MHR) as a possible biomarker in AF and cardiovascular disease. A different research sought to evaluate MHR’s prognostic value for late-stage AF recurrence after radiofrequency ablation. As a result, a higher preablation MHR was linked to a higher likelihood of POAF recurrence, and MHR showed comparable predictive power to LAD in its ability to independently predict the late recurrence of paroxysmal AF following radiofrequency ablation[33].
Increased inflammation is known to be associated with AF, and new research points to a possible relationship between inflammation and the pathophysiology of AF. Studies on circulating immune cells and their function in AF are, nevertheless, few. Specifically, there are few prospective analyses examining monocyte subsets and their relationship to AF risk. These kinds of research are essential for determining who is at a high risk of developing AF and for learning more about the pathophysiology of the illness[34].
In a sizable community-based cohort, variations in circulating monocyte subsets and the incidence of AF were evaluated. Monocyte subsets, including classical CD14++ CD16-, intermediate CD14++ CD16+, and non-classical CD14+ CD16++ monocytes, were enumerated in frozen mononuclear leukocytes using flow cytometry. The study found no sig
Moreover, both the number and percentage of intermediate CD14++ CD16+ monocytes and classical CD14++ CD16- monocytes were higher in subjects with incident AF. After adjusting for common risk factors, both percentages and numbers of intermediate monocytes were independently associated with an increased risk of developing AF during follow-up. The intermediate CD14++ CD16+ monocytes are linked to incident AF independently of other common risk factors in the general population, providing evidence supporting the involvement of inflammatory cells in AF[34].
Because of the pro-inflammatory and pro-oxidant properties of monocytes, previous research has linked lower levels of high-density lipoprotein cholesterol to an increased prevalence of AF. Similarly, increasing monocyte count or activity has been linked to these levels. The predictive significance of the monocyte-to-high-density lipoprotein ratio (M/H ratio), a newly identified marker of oxidative stress and inflammation mostly observed in patients with chronic renal disease, was examined about the recurrence of AF following cryoballoon-based catheter ablation[35].
From the lowest to the highest M/H ratio quartiles, AF recurrence rates increase. The pre-ablation M/H ratio, LAD, length of AF history, and early AF recurrence were found to be independent predictors of AF recurrence by multivariate Cox regression analysis. The pre-ablation M/H ratio had an 85% sensitivity and 74% specificity in predicting the recurrence of AF during follow-up, with a cut-off level of 11.48. In summary, following cryoballoon-based catheter ablation, a greater pre-ablation M/H ratio was linked to a higher risk of recurrence of AF. Although the study indicates that other variables may also be involved, these results support the involvement of a pro-inflammatory and pro-oxidant milieu before ablation therapy in AF recurrence[35].
Technological developments have made it possible for cardiac implanted electronic devices, or cardiac implantable electronic devices (CIEDs), to recognize and record atrial high-rate episodes (AHREs), which serve as a stand-in marker for silent AF. The correlation between AHREs and unfavourable clinical outcomes has been shown in several recent research, indicating that early identification and treatment can greatly reduce morbidity and death. The aetiology of AF is influenced by mechanisms like inflammation and oxidative stress. One new biomarker of both inflammation and oxidative stress is the M/H ratio[36].
Different research sought to evaluate the M/H ratio’s predictive usefulness in identifying AHREs found by CIEDs. During pacemaker interrogation, patients were split into two groups: Group 1 (AHRE present) and group 2 (AHRE missing). Group 1 had a significantly higher M/H ratio than group 2. The M/H ratio was shown to be substantially correlated with the frequency of AHREs in patients with CIEDs in multivariate Cox regression analysis. This implies that the M/H ratio has the potential for use as a predictive marker for AHREs in patients with CIEDs, emphasizing its ability to detect silent AF and direct prompt therapies[36].
Given that AF is linked to higher rates of morbidity and death, it is imperative to identify patients with heart failure who are at a high risk of acquiring the condition. In individuals with heart failure, the development of AF is known to be significantly influenced by the inflammatory response. In individuals with heart failure, the association between the development of AF and monocyte count-a crucial element of the inflammatory response was examined[37].
After the follow-up period, the subjects were split into two groups: Group 1 (kept sinus rhythm) and group 2 (developed AF). There were no statistically significant differences seen between the groups about smoking status, hypertension, diabetes mellitus, age, or sex. However, there were notable variations between the two groups when it came to the existence of monocyte count and moderate-to-severe mitral regurgitation (MR). After adjusting for variables that were statistically significant in the univariate analysis and related to monocyte count, the association between monocyte count and moderate-to-severe MR and the development of AF remained in the multivariate Cox regression model. In heart failure patients, the onset of AF was linked to an elevated monocyte count. However, a more extensive study is required to validate these results[37].
An increased inflammatory response that is typified by monocyte/macrophage infiltration is frequently associated with AF. When it comes to controlling monocyte mobilization in diseases like hypertension and cardiac remodeling, the chemokine receptor CXCR-2 is essential. Nevertheless, it was previously unclear if CXCR-2 had a role in the development of hypertensive AF. Male C57BL/6 WT mice, CXCR-2 knockout mice, bone marrow-reconstituted chimera mice, and mice treated with the CXCR-2 inhibitor SB225002 were used to induce AF in these experiments. The infusion of Ang II was done for three weeks[38].
Four CXCR-2 chemokine ligands were significantly upregulated in the atria throughout the three-week Ang II infusion, according to microarray analysis. The number of CXCR-2+ immune cells and CXCR-2 expression in the atria of mice undergoing Ang II infusion both gradually rose with time. Furthermore, compared to WT mice given saline treatment, Ang II-infused animals showed elevated blood pressure, AF inducibility, atrial diameter, fibrosis, macrophage infiltration, and superoxide generation. CXCR-2 knockout animals, WT mice transplanted with CXCR-2-deficient bone marrow cells, and mice treated with SB225002 all showed a substantial reduction in these effects[38].
Additionally, in patients with sinus rhythm, circulating blood CXCL-1 levels and CXCR-2+ monocyte counts were lower in AF patients than in sinus rhythm patients, and these levels were linked to AF. Ultimately, this research revealed a new function for CXCR-2 in stimulating monocyte infiltration into the atria, which in turn speeds up atrial remodeling and AF when hypertension is present. AF in hypertensive situations may benefit from a novel treatment approach that involves blocking CXCR-2 activity[38].
AF recurrence after catheter ablation, inflammation, specifically linked to intermediate CD14++ CD16+ monocytes and atrial structural remodeling might play a significant role. However, the exact relationship between intermediate CD14++ CD16+ monocytes, structural remodeling, and AF recurrence remains unclear. Using flow cytometry, the percentage of intermediate monocytes (PIM) was assessed before ablation. The volume ratio (VR) of signal intensity larger than one standard deviation on late-gadolinium enhancement magnetic resonance imaging (LGE-MRI) was determined as a surrogate diagnostic for structural remodeling[39].
The goal was to ascertain if PIM and structural remodeling on LGE-MRI correlated, as well as to find the best cutoff value for AF recurrence prediction. Positive associations were found between PIM and B-type natriuretic peptide and structural remodeling using univariate analysis. PIM was shown to be independently correlated with VR on LGE-MRI by multivariable analysis. To sum up, there was a strong positive correlation between intermediate monocytes and structural remodeling. This raises the possibility that inflammatory indicators, atrial structural alterations, and the chance of AF recurrence are related, highlighting the need to evaluate these variables in post-ablation therapy[39].
It is well recognized that inflammation has a major part in the development and course of AF. It has been established that the lymphocyte-to-monocyte ratio (LMR) is a valid predictor in several disorders linked to inflammation. On the association between LMR and AF, however, little information is currently known. By utilizing information from the Medical Information Mart for Intensive Care-III database, the predictive efficacy of LMR in predicting all-cause death among AF patients was examined[40].
The X-tile analysis determined that 2.67 is the ideal cutoff value for LMR. Following propensity score matching (PSM), 1127 pairings in total with well-balanced covariates were produced. Patients with low LMR (≤ 2.67) had a greater 1-year all-cause mortality than those with high LMR (> 2.67), according to the Cox proportional-hazards model, both before and after PSM. Ultimately, a lower LMR (≤ 2.67) was linked to an increased risk of all-cause death after 28 days, 90 days, and 1 year, indicating that LMR may function as a separate predictor in individuals with AF[40].
Low-voltage zones (LVZs) are formed and increase as a result of inflammation that is mediated by toll-like receptor 4 (TLR4) and intermediate monocytes. Based on the expression of CD14/CD16, monocytes were classified into three subsets: Classical, intermediate, and non-classical. At the start of the ablation session, voltage mapping was carried out, with amplitudes of bipolar electrograms less than 0.5 mV being designated as LVZs[41].
Comparing patients with and without LVZs, it was discovered that the proportion of intermediate monocytes in the former group was larger. Compared to the other two categories, intermediate monocytes exhibited a considerably higher frequency of TLR4 expression. Additionally, a significant association was seen between the TLR4 expression level in intermediate monocytes and the total area of LVZs, with a stronger correlation observed in patients with paroxysmal AF[41].
In conclusion, the research indicates that the existence of expanding LVZs in patients with AF is positively correlated with intermediate monocytes and TLR4 expressions. This emphasizes how these biological components may promote inflammation in the pathophysiology of AF and the corresponding electrical remodeling in the atria[41]. Monocytes have a complex impact on thrombogenesis, structural remodeling, and inflammation in the pathophysiology of AF. There is potential for improving AF treatment and patient outcomes via ongoing study into monocyte behavior and its regulation. Interventions aimed at modulating monocyte activity or migration may offer novel approaches to prevent or treat AF.
One type of white blood cell called an eosinophil is essential to the body’s immune system, especially when it comes to protecting against allergic responses and parasites. They have a role in reducing inflammation as well. A smaller percentage of white blood cells than 5% are eosinophils. Together with mast cells and basophils, they also regulate asthmatic and allergy-related processes. Before moving into the bloodstream, these granulocytes undergo hematopoiesis in the bone marrow, where they undergo terminal differentiation and cease to proliferate[42].
Eosinophils are terminally developed cytotoxic effector cells, according to conventional wisdom. Understanding the molecular mechanisms governing eosinophil growth, trafficking, and degranulation has improved our understanding of the immune-regulating roles of these cells and how they affect homeostasis. This encompasses their participation in immunological control, tissue restoration, and preservation of equilibrium[43]. Eosinophils have more dynamic roles in the immune system than previously believed, and their immunomodulatory capabilities extend beyond their cytotoxic actions. Furthermore, the modern understanding of eosinophils has expanded to include their roles in the aetiology of several illnesses, including asthma and primary hypereosinophilic syndromes and others[43].
It has been determined that inflammation has a significant role in the initiation and progression of AF. Investigations were conducted on the impact of white blood cell and their differentials, with a particular emphasis on peripheral eosinophil count, on isolated AF. The results indicated that the lone AF group had substantially greater peripheral eosinophil count, neutrophil count, and LAD than the control group[44].
Patients with recurrent AF had increased LAD and eosinophil counts. A significantly significant correlation between eosinophil count, LAD and AF recurrence was found by univariable analysis. Using multivariate logistic regression analysis, two independent predictors of AF recurrence during antiarrhythmic medication therapy were found: Eosinophil count and LAD[44]. The findings substantiate the connection between lone AF and the white blood cell response and its constituent parts. In particular, peripheral eosinophil count and LAD could be crucial mediators of inflammation and remodeling of the atrium in AF. This demonstrates the possible importance of these inflammatory indicators for comprehending and treating AF[44].
According to emerging research, increased eosinophil levels may cause AF to develop and worsen through several interconnected molecular pathways, including reactive oxygen species (ROS) and pro-inflammatory cytokines can be released by eosinophils, which are important components of immune responses. These chemicals encourage atrial inflammation, which results in atrial tissue structural remodeling, a known predisposing factor for AF. Leukocyte infiltration, including eosinophils, has been found in the atrial tissue of AF patients, according to studies, which may play a part in the pathophysiology of the arrhythmia[45].
Eosinophils can cause atrial fibrosis by inducing collagen deposition and fibroblast activation. The maintenance of AF is aided by the disruption of normal electrical conduction in the atria caused by this fibrotic remodeling[46]. Fibrosis in cardiac tissues has been linked to inflammatory mediators generated by eosinophils, including transforming growth factor-beta. In individuals with AF, elevated eosinophil levels have been linked to increased thrombus development in the LA. A pro-thrombotic condition that increases the risk of stroke in AF is facilitated by eosinophils, which can increase platelet activation and aggregation[46]. In the atrial myocardium, oxidative stress is exacerbated by the ROS generated by eosinophils. In cardiomyocytes, this oxidative environment can disrupt calcium handling and ion channel function, resulting in electrical instability and heightened vulnerability to AF[47].
In conclusion, thrombogenesis, oxidative stress, inflammation, fibrosis, and direct myocardial damage are some of the ways that increased eosinophil levels might cause AF. These findings highlight the possibility of preventing and treating AF by focusing on eosinophil-mediated pathways.
The innate immune response, which is primarily caused by neutrophils, and adaptive immunity, which is supported by lymphocytes, are the two facets of the immune system that are conjugated by the NLR, which is a biomarker as a simple ratio between the neutrophil and lymphocyte counts measured in peripheral blood[48].
The function of the NLR in long-term follow-up for patients whose acute AF-related sinus rhythms were stabilized by amiodarone in terms of recurrence prediction. Acute AF patients who were effectively treated with amiodarone to transition to sinus rhythm were retrospectively added to the research[49].
According to univariate analysis, individuals with recurrent AF had lower platelet and lymphocyte counts, higher left atrial diameter and NLR, and all were statistically significant. Only NLR and left atrial diameter were shown to be independent predictors of AF recurrence in multivariate analysis. The results imply that during the long-term follow-up of patients treated with acute AF who have successfully converted to sinus rhythm with amiodarone, elevated NLR, showing increased inflammation, may act as a straightforward, affordable, and easily accessible predictor of recurrence[49].
After non-cardiac thoracic surgery, the relationship between the incidence of POAF and the neutrophil-lymphocyte ratio was examined. NLR did not, however, change significantly between individuals who developed POAF and those who did not. There was no change in the NLR before or just after surgery in this subgroup, and there was also no interaction about the likelihood of developing POAF. According to the results, there was no proof that preoperative or early postoperative NLR was connected to the emergence of POAF in patients following major non-cardiac thoracic surgery, in contrast to cardiac surgery[50].
NLR prognostic value for ischemic stroke and AF in individuals with type 2 diabetes mellitus was evaluated. After controlling for important variables, NLR is a significant predictor of new-onset ischemic stroke but not AF[51].
Fukuda et al[52] examined the relationship between atrial inflammatory alterations and the NLR in individuals with paroxysmal AF. One characteristic linked to thrombogenesis, SEC, is independently correlated with NLR.
When comparing NLR to LAA wall motion velocity in patients without SEC and to LAA wall motion velocity and LAA area in patients with SEC, significant associations were observed. Nevertheless, in both groups, NLR did not correlate with the left atrial volume index, mitral annular motion velocity, or mitral flow velocity. The results indicate that a high NLR in paroxysmal AF patients is directly connected to lower LAA contraction rather than poor left atrial body function and is associated with a greater chance of thrombogenesis[52]. According to Sahin et al[53], diabetes individuals with AF have higher NLRs than diabetic patients without AF. In individuals with diabetes, the NLR may function as a possible signal for the onset of AF[53].
In patients with AMI, Pan et al[54] evaluated the prognostic value of NLR and stress hyperglycemia ratio (SHR) for new-onset AF. It was discovered that, regardless of their status as diabetics, those who acquired new-onset AF after an MI had greater levels of SHR and NLR than those who did not. High SHR and high NLR were independently linked to the development of new-onset AF post-AMI after controlling for possible factors[54].
In addition, the research produced a unique nomogram for predicting the likelihood of new-onset AF in patients with AMI that included high NLR and high SHR. In terms of calibration, clinical usefulness, and prediction accuracy, the nomogram performed satisfactorily. In patients with AMI, SHR and NLR have separate associations with the incidence of new-onset AF. The created nomogram could help with new-onset AF risk categorization[54].
In patients with NVAF using warfarin, the study examined the relationship between the NLR and thrombosis and bleeding risk scores. The subjects were divided into two groups, group A [therapeutic range (TTR) ≥ 65%] and group B (TTR < 65%), according to how long they had been taking warfarin in the TTR. According to the results, patients in group B (low TTR) had greater levels of NLR than patients in group A (high TTR)[55].
Furthermore, NLR levels were considerably greater in patients categorized as high risk compared to low and intermediate risk based on the CHA2DS2-VASc score. Both the CHA2DS2-VASc and HAS-BLED scores showed a strong correlation with NLR levels. In individuals with NVAF who are at high risk of stroke and bleeding, NLR may be a helpful sign. Furthermore, with a sensitivity of 81% and specificity of 71%, NLR demonstrated predictive utility in identifying patients within the therapeutic international normalized ratio range. NLR may be a useful tool for NVAF patients using warfarin to measure risk[55].
The NLR has been emphasized in recent research as a potential biomarker in the setting of AF, providing new insights into its function in risk assessment, prognosis, and treatment approaches. Systemic inflammation and atrial structural alterations may be related, as evidenced by the correlation between NLR and the left atrial volume index. Individuals with greater NLR levels may be at a higher risk of developing new-onset AF, which might be explained by this con
The PLR is a blood test marker used to assess the severity of systemic inflammation. It’s calculated by dividing the absolute platelet count by the absolute lymphocyte count. In patients with non-valvular persistent AF, the predictive value of the inflammatory marker PLR was evaluated for recurrence following successful electrical cardioversion (ECV)[57].
The “AF recurrence group” had considerably higher mean PLR values than the “sinus rhythm maintenance” group. During the 6-month follow-up period following successful ECV, PLR was identified through multiple regression analyses as a risk factor linked to the recurrence of AF. PLR demonstrated a sensitivity of 83.3% and specificity of 84.5% for predicting AF recurrence when a cutoff value of 147 was applied. In patients with non-valvular persistent AF who have recovered sinus rhythm following successful ECV, elevated PLR, a sign of increased inflammation, may be a useful and affordable predictor of recurrence of AF[57].
Following coronary artery bypass graft (CABG) surgery, Gungor et al[58] examined the relationship between the PLR and AF. PLR, or PLR, was determined from blood samples provided by fasting patients before the CABG operation. AF was diagnosed based on established clinical criteria. The AF group had a mean age that was considerably higher than the non-AF group. The AF group had a substantially greater PLR compared to the non-AF group.
Age and PLR were linked to AF following CABG surgery, according to univariate analysis. Age and PLR were found to be independent predictors of AF following CABG surgery in a multivariate logistic regression model using the backward elimination approach. With 64% sensitivity and 56% specificity, PLR levels > 119.3 predicted POAF. Patients with greater preoperative PLR were more likely to experience AF following CABG surgery. Age and PLR level were independent predictors of AF after CABG surgery[58].
Cosansu et al[59] noted that in NVAF, the platelet-lymphocyte ratio plays a predictive effect in terms of bleeding risk. PLR, CHA2DS2-VASc, and HAS-BLED scores were found to have significant connections with each other. Using a cut-off value of 165.9, the receiver operating characteristic (ROC) analysis revealed that PLR predicted bleeding with a sensitivity of 83% and a specificity of 84%.
It was discovered that PLR has an area under the curve (AUC) of 0.88. Using a cut-off value of 125.3, PLR also identified patients within the therapeutic INR range in the ROC analysis with a sensitivity of 75%; its AUC was 0.73. PLR > 165.9 was identified as a significant indication for bleeding in the multivariate regression analysis, indicating a risk of bleeding that was more than twelve times higher[59].
Following coronary artery bypass grafting, POAF is a frequent complication that is linked to higher short- and long-term mortality. Preliminary research indicates that a higher preoperative PLR may be linked to an increased incidence of POAF following CABG. Inflammation is thought to be a contributing factor to POAF[60].
Between patients in the high-PLR and low-PLR groups, there was no statistically significant difference in the incidence of POAF. High PLR was not independently correlated with POAF, according to multivariable logistic regression analysis. Patients receiving isolated CABG do not exhibit an independent correlation between elevated preoperative PLR and POAF[60].
After isolated CABG surgery, the association between inflammation and the onset of AF was examined, with a particular emphasis on the indicators of inflammation, PLR and mean platelet volume (MPV). In comparison to preoperative values, postoperative PLR and MPV levels in the AF group were considerably greater. Higher postoperative PLR and MPV levels are linked to the development of AF following CABG surgery. This suggests that the presence of AF in this patient population may be linked to elevated inflammation as shown by these markers[61].
In individuals with nonvalvular AF, the link between the LAA flow velocity (LAA-FV) and the PLR was documented by Zuo and Yang[62]. It showed that, in contrast to individuals with normal LAA-FV, PLR was lower in those with diminished LAA-FV.
The PLR showed predictive significance with a sensitivity of 66.7% and specificity of 83.3% for decreasing LAA-FV. The AUC for PLR as a predictor of decreasing LAA-FV was 0.726 with a cut-off value of 88.16. Moreover, those with a PLR < 88.16 exhibited reduced left atrial strain in contrast to those with a PLR > 88.16, indicating a possible association between PLR and compromised left atrial strain. Based on these data, PLR may represent the inflammatory load in AF patients and may also be linked to atrial remodeling and prothrombotic status in these patients[62].
It has not been previously investigated by Tek and Kaplan Efe[63] how useful the PLR is for diagnosing the risk of LAA thrombogenic milieu (LAA TM) in patients with nonvalvular AF. AFCA or electrical cardioversion was performed on consecutive NVAF patients after transesophageal echocardiography[63].
To investigate the possible correlation between PLR and LAA TM which is defined as the presence of thrombus, sludge, and SEC in the LAA multivariate logistic regression analysis was done. Comparing patients with and without a thrombogenic milieu, those with LAA TM showed higher mean CHA2DS2-VASc scores, lower left ventricular ejection fraction, decreased LAA velocity, greater LAD, and a higher PLR value. The investigation found that PLR and LAA velocity were independent variables linked to LAA TM. PLR may function as a separate risk factor for LAA TM in individuals with nonvalvular AF[63].
In relation to AF, the PLR has become a useful biomarker that provides information on the thrombotic and inflammatory mechanisms that underlie the disorder. Its incorporation into clinical practice may improve risk assessment and guide therapy choices, especially with regard to surgical complications and AF recurrence.
The number of monocytes in a blood sample divided by the number of lymphocytes gives the MLR. It has been connected to a number of illnesses, such as cancer and cardiovascular ailments, and is thought to be a possible biomarker for inflammation. The inflammatory process and early recurrence of AF (ERAF) after PVI have been strongly correlated in previous research[64].
Given the correlation between systemic inflammation and alterations in peripheral blood leukocytes, the NLR and MLR have been suggested as markers of the systemic inflammatory response. It is yet unknown whether NLR or MLR is particularly connected to either ERAF or late recurrence of AF (LRAF), although several studies have connected ERAF with LRAF following PVI. While there was no significant difference in NLR and MLR before PVI between ERAF and no-ERAF groups, post-PVI NLR and MLR were notably higher in the ERAF group compared to the no-ERAF group[64].
Conversely, there was no significant difference in high-sensitivity C-reactive protein levels between both groups. Changes in NLR (ΔNLR) and MLR were significantly higher in the ERAF group than in the no-ERAF group. There were 147 people (23.6%) who were LRAF patients, and the ΔNLR was considerably greater in the LRAF group compared to the no-LRAF group. The research concludes that variations in NLR and MLR are related to ERAF, with a focus on how NLR variation affects LRAF prediction following PVI[64]. A useful biomarker for AF that provides information on the inflammatory mechanisms underlying the illness is the MLR. Especially regarding thromboembolic consequences and AF recurrence, its incorporation into clinical practice may improve risk assessment and guide therapeutic choices.
Red blood cells, often referred to as erythrocytes or red blood cells, are the most prevalent kind of blood cells and are in charge of returning carbon dioxide to the lungs and supplying oxygen to the body’s tissues. They are made in the bone marrow and contain the oxygen-binding protein hemoglobin. One important biomarker for AF is red blood cell distribution width. RDW is frequently indicated in complete blood count tests and quantifies the variation in the size of circulating red blood cells. In AF patients, elevated RDW levels have been linked to several negative consequences. The most common heart arrhythmia is AF, which dramatically increases the risk of stroke and death. People with a diagnosis of hypertension have a higher chance of having an arrhythmia. Acute coronary syndrome, heart failure, stable coronary disease, sluggish coronary flow, and stroke are among the cardiovascular conditions that are associated with elevated RDW values. In hypertensive individuals, the relationship between RDW and AF was looked at[65].
There was a significant difference in RDW levels between those with and without AF. The only independent risk variables linked to AF that were found using logistic regression analysis were left atrial dimension and RDW. RDW levels were greater in AF patients who were hypertensive. Therefore, in hypertensive individuals, an increased RDW level may indicate to medical professionals that AF may be developing or already present[65].
RDW is a readily quantifiable and affordable indicator that is associated with a range of cardiovascular problems. It reflects the variability in both the volume and size of red blood cells. Several research works suggest that RDW functions as a predictive factor for AF in various clinical settings. Zeng et al[66] conducted a review of the literature regarding the predictive value of RDW in the onset and progression of AF across various clinical stages, emphasizing its function in averting unfavourable consequences.
A measure of fluctuation in erythrocyte volume called red blood cell distribution width has been associated with several cardiovascular diseases, albeit its relationship to AF has not been fully established. In another study, the relationship between RDW and the first hospitalization for AF in a cohort drawn from the general community was investigated[67].
Once possible confounders such as risk factors for cardiovascular disease, dietary intake (iron, vitamin B12, and folate), and different haematological parameters (haemoglobin concentration, mean corpuscular volume, and corpuscular haemoglobin content) were taken into account, the hazard ratio for the incidence of AF was 1.33 (95%CI: 1.16-1.53) for the fourth quartile relative to the first quartile of RDW. Various haematological, dietary, and cardiovascular variables did not affect the independent link between RDW and AF incidence[67].
One of the main causes of morbidity and death in older patients, particularly for those in the intensive care unit, is AF. Prior research has demonstrated the significance of red blood cell distribution width in forecasting the onset of AF. RDW’s prognostic significance in critically ill AF patients is still mostly unclear, though. Therefore, the purpose of this research is to investigate the potential utility of forecasting both in- and out-of-hospital mortality in critically unwell AF patients[68].
Elevated RDW levels were associated with a higher risk of in-hospital death in severely sick AF patients. The high RDW group showed significantly reduced survival rates. To sum up, in critically sick patients with AF, greater RDW levels are linked to a higher risk of both in-hospital and out-of-hospital death[68].
Despite improvements in medical techniques, cardiac surgeons frequently encounter POAF after heart surgery. Various criteria have been investigated in numerous studies to identify people who are at risk of developing POAF. Different research looked at the capacities of RDW and the amount of EAT to predict POAF. Age, postoperative right heart width and tomography parameters (left atrial volume, left atrial diameters, and EAT volume) were found to be considerably greater in patients who experienced AF following cardiopulmonary bypass, according to the research[69].
On the other hand, these individuals had much lower hematocrit and haemoglobin levels. Age, postoperative RDW, and tomography variables (left atrial volume, left atrial diameters, and EAT volume) were all subjected to a logistic multivariate regression analysis. Remarkably, the only variable that stood out as an independent predictor of POAF development was age. The multivariate analysis highlighted that age was the only significant predictor of POAF, even though there was an elevation in EAT volume among patients suffering AF. In the context of POAF, it indicated the need for more studies to examine the predictive functions of RDW and epicardial fat[69].
Red blood cell distribution width, a sign of erythrocyte anisocytosis and a marker of inflammatory stress have recently been related to AF. Sick sinus syndrome (SSS) is frequently associated with AF. Korantzopoulos et al[70] evaluated whether patients with SSS receiving dual-chamber pacemaker implantation had a possible relationship between RDW and a history of AF. RDW and AF were independently correlated by multivariate analysis.
With an RDW cut-off point of 14, ROC curve analysis showed an AUC of 0.69, indicating a sensitivity of 70% and specificity of 69%. In conclusion, in individuals with SSS, RDW is associated with a history of AF. Additional investigation is necessary to investigate the predictive significance of RDW for the development of AF in the future and the persistence of arrhythmias in these individuals[70].
It has been established that a higher RDW is a reliable indicator of the prevalence and death from cardiovascular illnesses. On the other hand, less information is known about the relationship between RDW and altitude as well as the particular AF subtype. It was looked at how altitude affected RDW in patients with various forms of AF. A total of 303 nonvalvular AF patients were included; of these, 156 lived in low-altitude areas (77 exhibiting paroxysmal AF; 79 exhibiting persistent AF), and 147 in high-altitude areas (77 exhibiting paroxysmal AF; 70 exhibiting persistent AF). In these groups, the study evaluated echocardiography, serum biochemistry, complete blood counts, and baseline characteristics. Compared to control patients, people with AF showed greater RDW and LAD at both low and high elevations[71].
Furthermore, compared to paroxysmal AF, persistent AF had greater RDW and LAD levels. RDW and LAD were consistently greater in high-altitude groups when similar groups at low and high altitudes (paroxysmal AF, persistent AF, or control) were compared. RDW, mean corpuscular volume, and LAD levels were found to be independently correlated with AF patients in low-altitude locations by multivariate logistic regression analysis. Independent predictors of AF patients in high-altitude regions were creatinine, RDW, mean corpuscular volume, and LAD (RDW, odds ratio = 1.755, 95%CI: 1.179-2.613). The results imply that increased RDW levels may function as a separate risk indicator for nonvalvular AF, with the type of AF and residency altitude influencing this connection[71].
The association between AF and RDW in people with essential hypertension is poorly understood. Another research considered the relationship between RDW and AF in hypertensive individuals. Patients were then divided into subgroups for paroxysmal and chronic AF within the AF group. The RDW levels in the AF group were higher than in the controls, and the RDW levels in the persistent AF subgroup were higher than in the paroxysmal AF subgroup. The presence of AF rhythm was associated with greater RDW levels in the paroxysmal AF group than in the absence group[72].
There were no discernible variations in RDW between the paroxysmal AF subgroup’s absence group and the control group, or between the paroxysmal AF subgroup’s presence group and the persistent AF subgroup. An independent predictor of elevated RDW concentration in hypertensive individuals was shown by multivariate regression analysis to be the existence of an AF rhythm. In conclusion, it is important to preserve sinus rhythm in hypertension individuals since RDW may be linked to the occurrence of AF rhythm[72].
Vascular disorders, including AF and venous thromboembolism (VTE), have been linked to RDW as a possible marker. Unfortunately, little is known about RDW’s function as a risk predictor for thromboembolic events, particularly in patients with AF. In another investigation, using a population-based cohort, the relationship between RDW and the risk of AF, AF-related VTE, and ischemic stroke was examined[73].
Compared to those in the lowest quartile (RDW ≤ 12.3%), individuals with RDW in the highest quartile (RDW ≥ 13.3%) had a 30% increased risk of AF. The incidence of an ischemic stroke was twice in AF individuals with RDW in the higher tertile. Interestingly, in participants with AF, RDW did not significantly correlate with incident VTE. In a broad population, RDW was shown to be substantially correlated with incident AF. Elevated RDW levels were associated with a higher risk of ischemic stroke in AF patients, but not with VTE. These results highlight the possible use of RDW as a marker for cardiovascular risks, especially when AF and related thromboembolic events are present[73].
An increased risk of cardiovascular morbidity and death has been associated with RDW. The relationship between RDW and stroke in AF patients was investigated by Saliba et al[74]. The area under the ROC curve increased from 0.598 for the CHADS2 score alone to 0.618 when RDW was included in the model (P < 0.001), indicating that the addition of RDW in the model enhanced the prediction accuracy for stroke in patients with AF. In conclusion, regardless of the presence of anemia, RDW is directly linked to the risk of stroke in AF patients. Moreover, adding RDW to predictive models improves their accuracy in predicting stroke in this group[74].
One of the main causes of morbidity and death in older patients, particularly for those in the intensive care unit, is AF. Prior research has demonstrated the significance of red blood cell distribution width in forecasting the onset of AF. RDW’s prognostic significance in critically ill AF patients is still mostly unclear, though. Therefore, the purpose of this research is to investigate the potential utility of forecasting both in- and out-of-hospital mortality in critically unwell AF patients. Paroxysmal AF was shown to be related to LAD, RDW, and MPV/platelet[75].
Recent studies link inflammation to the development of AF and red blood cell transfusion is known to influence inflammation by increasing plasma levels of markers associated with inflammation. This research sought to test the theory that individuals having heart surgery have a higher chance of developing POAF after receiving a red blood cells transfusion. The results also showed that intensive care unit red blood cells transfusion significantly increased the risk for AF in addition to factors like older age, prior history of AF, higher preoperative hematocrit, β-blocker withdrawal, longer aortic clamp time, valve surgery, and inotropic usage within the unit[76].
To sum up, data shows that an increased risk of POAF after heart surgery has been associated with red blood cell transfusions in the critical care unit. This result highlights the need to take red blood cells transfusion into account when determining whether patients may benefit from prophylaxis to avoid this frequent postoperative complication[76]. The red blood cell distribution width has emerged as a valuable biomarker in the context of AF, offering insights into the inflammatory and thrombotic processes underlying the condition. Its integration into clinical practice could enhance risk assessment and inform management decisions, particularly concerning AF recurrence and associated complications.
These hematological parameters reflect underlying inflammatory processes, oxidative stress, and hemodynamic changes, all of which are increasingly recognized as important contributors to AF development and progression. Understanding these blood-based indicators not only enhances insights into AF mechanisms but also holds potential for improving early diagnosis, risk stratification, and therapeutic strategies. Future research should aim to further define the clinical utility of these hematological markers in the management of AF.
These hematological parameters may serve as valuable biomarkers for the early detection, risk stratification, and monitoring of AF progression. Hematological parameters play a supportive and important role in the diagnosis of AF by identifying underlying causes, triggers, or associated conditions. In AF, hematological parameters offer useful prognostic information by identifying markers linked to an elevated risk of complications, including death, heart failure, and stroke. The distribution width of red blood cells, alongside variations in platelet count, white blood cells, lymphocytes, neutrophils, monocytes, and derived inflammatory ratios such as the neutrophil-to-lymphocyte, platelet-to-lymphocyte, and MLRs, collectively play a significant role in the pathophysiology of AF as explained in figure and table. To understand the pathophysiology implications of hematocrit and hemoglobin in AF, the pathophysiologic mechanisms behind these relationships and any therapeutic implications, more study is necessary. A complete blood count may show leukocytosis, which may suggest infection or inflammation that might trigger AF, and anemia, which may worsen AF symptoms. The administration of anticoagulation and the choice of suitable medicines are two areas where hematological indicators play a critical role in directing the therapy of AF. To guarantee adequate anticoagulation and minimize bleeding risk, individuals taking warfarin must have their platelet count and coagulation profile regularly monitored. A complete blood count might also reveal thrombocytopenia or anemia, which can influence treatment choices or signal bleeding issues. By ensuring safe and customized therapy, routine evaluation of key hematological parameters maximizes the effectiveness and safety of AF treatment. Clinicians can more accurately monitor these hematological indicators, stratify risk, and create customized long-term care plans for AF patients.
| 1. | Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021;16:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 2. | Weymann A, Ali-Hasan-Al-Saegh S, Sabashnikov A, Popov AF, Mirhosseini SJ, Liu T, Lotfaliani M, Sá MPBO, Baker WLL, Yavuz S, Zeriouh M, Jang JS, Dehghan H, Meng L, Testa L, D'Ascenzo F, Benedetto U, Tse G, Nombela-Franco L, Dohmen PM, Deshmukh AJ, Linde C, Biondi-Zoccai G, Stone GW, Calkins H; Surgery And Cardiology-Group Imcsc-Group IMOC. Prediction of New-Onset and Recurrent Atrial Fibrillation by Complete Blood Count Tests: A Comprehensive Systematic Review with Meta-Analysis. Med Sci Monit Basic Res. 2017;23:179-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Rafaqat S, Sharif S, Naz S, Majeed M, Saqib M, Farzana Rashid, Ali Q. The Role of Hematological Parameters in Atrial Fibrillation Risk Assessment. Proc Pak Acad Sci B. 2023;60:635-641. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Distelmaier K, Maurer G, Goliasch G. Blood count in new onset atrial fibrillation after acute myocardial infarction - a hypothesis generating study. Indian J Med Res. 2014;139:579-584. [PubMed] |
| 5. | Lim WH, Choi EK, Han KD, Lee SR, Cha MJ, Oh S. Impact of Hemoglobin Levels and Their Dynamic Changes on the Risk of Atrial Fibrillation: A Nationwide Population-Based Study. Sci Rep. 2020;10:6762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Kim M, Hong M, Kim JY, Kim IS, Yu HT, Kim TH, Uhm JS, Joung B, Lee MH, Pak HN. Clinical relationship between anemia and atrial fibrillation recurrence after catheter ablation without genetic background. Int J Cardiol Heart Vasc. 2020;27:100507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Qin Z, Liao N, Lu X, Duan X, Zhou Q, Ge L. Relationship Between the Hemoglobin-to-Red Cell Distribution Width Ratio and All-Cause Mortality in Ischemic Stroke Patients with Atrial Fibrillation: An Analysis from the MIMIC-IV Database. Neuropsychiatr Dis Treat. 2022;18:341-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Sari S, Brooker J, Montalvo-Campana M, Shehata P, Pu X, Insler S, Ruetzler K, Troianos CA, Turan A. The association of hemoglobin with postoperative delirium and atrial fibrillation after cardiac surgery: a retrospective sub-study. Braz J Anesthesiol. 2024;74:744424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Sairaku A, Morishima N, Amioka M, Maeda J, Watanabe Y, Nakano Y. Does atrial fibrillation ablation worsen preexisting anemia? Another anemia paradox in DOAC era. J Cardiol. 2021;78:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Kodani E, Inoue H, Atarashi H, Okumura K, Yamashita T, Origasa H; J-RHYTHM Registry Investigators. Impact of hemoglobin concentration and platelet count on outcomes of patients with non-valvular atrial fibrillation: A subanalysis of the J-RHYTHM Registry. Int J Cardiol. 2020;302:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Wang J, Chen Z, Yang H, Li H, Chen R, Yu J. Relationship between the Hemoglobin-to-Red Cell Distribution Width Ratio and All-Cause Mortality in Septic Patients with Atrial Fibrillation: Based on Propensity Score Matching Method. J Cardiovasc Dev Dis. 2022;9:400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Öztürk İ, Öztürk S. Association between postoperative atrial fibrillation and the levels of hemoglobin or hematocrit: a systematic review and metaanalysis. Anaesth Pain Intensive Care. 2015;19:247-253. |
| 13. | Kamath S, Blann AD, Chin BS, Lanza F, Aleil B, Cazenave JP, Lip GY. A study of platelet activation in atrial fibrillation and the effects of antithrombotic therapy. Eur Heart J. 2002;23:1788-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Park J, Cha MJ, Choi YJ, Lee E, Moon I, Kwak S, Kwon S, Yang S, Lee S, Choi EK, Oh S. Prognostic efficacy of platelet count in patients with nonvalvular atrial fibrillation. Heart Rhythm. 2019;16:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Sohara H, Amitani S, Kurose M, Miyahara K. Atrial fibrillation activates platelets and coagulation in a time-dependent manner: a study in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 1997;29:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Pourtau L, Sellal JM, Lacroix R, Poncelet P, Bernus O, Clofent-Sanchez G, Hocini M, Haïssaguerre M, Dignat-George F, Sacher F, Nurden P. Platelet function and microparticle levels in atrial fibrillation: Changes during the acute episode. Int J Cardiol. 2017;243:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Lianos I, Varlamos C, Benetou DR, Mantis C, Kintis K, Dragona VM, Kanakakis I, Sionis D, Patsilinakos S, Alexopoulos D. Platelet function testing in atrial fibrillation patients undergoing percutaneous coronary intervention. J Thromb Thrombolysis. 2023;55:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Wysokinski WE, Tafur A, Ammash N, Asirvatham SJ, Wu Y, Gosk-Bierska I, Grill DE, Slusser JP, Mruk J, McBane RD. Impact of atrial fibrillation on platelet gene expression. Eur J Haematol. 2017;98:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Berezin A, Berezin A. Circulating platelet-derived vesicle in atrial fibrillation. Ann Clin Hypertens. 2019;3:031-038. [DOI] [Full Text] |
| 20. | Yang E, Zou T, Leichner TM, Zhang SL, Kambayashi T. Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. Eur J Immunol. 2014;44:2712-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Rienstra M, Sun JX, Magnani JW, Sinner MF, Lubitz SA, Sullivan LM, Ellinor PT, Benjamin EJ. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. 2012;109:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Jacob KA, Buijsrogge MP, Frencken JF, Ten Berg MJ, Suyker WJ, van Dijk D, Dieleman JM. White blood cell count and new-onset atrial fibrillation after cardiac surgery. Int J Cardiol. 2017;228:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Larosa DF, Orange JS. 1. Lymphocytes. J Allergy Clin Immunol. 2008;121:S364-9; quiz S412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Hammer A, Niessner A, Sulzgruber P. The impact of CD4(+)CD28(null) T lymphocytes on atrial fibrillation: a potential pathophysiological pathway. Inflamm Res. 2021;70:1011-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Malech HL, Deleo FR, Quinn MT. The role of neutrophils in the immune system: an overview. Methods Mol Biol. 2014;1124:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Friedrichs K, Adam M, Remane L, Mollenhauer M, Rudolph V, Rudolph TK, Andrié RP, Stöckigt F, Schrickel JW, Ravekes T, Deuschl F, Nickenig G, Willems S, Baldus S, Klinke A. Induction of atrial fibrillation by neutrophils critically depends on CD11b/CD18 integrins. PLoS One. 2014;9:e89307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Liu X, Li X, Xiong S, Zhang H, Suo R, Zhang X, Liu D, Fu H, Liu T, Li G. Neutrophil Extracellular Traps: Potential Prothrombotic State Markers and Therapeutic Targets for Atrial Fibrillation. Thromb Haemost. 2024;124:441-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Meulendijks ER, Fabrizi B, Bruns S, Boogert TV, Wesselink R, Boven WJV, Driessen A, Niessen H, de Vries TA, Eringa E, Planken N, Isgum I, Dey D, Krul SP, Hoebe R, de Groot JR, Al-shama R. PO-02-177 Epicardial Adipose Tissue Neutrophil Inflammation Relates to Severity But Not to Recurrence of Atrial Fibrillation and Is Reflected By Attenuation in CTA Scans. Heart Rhythm. 2023;20:S270-S271. [DOI] [Full Text] |
| 29. | Guo X, Zhang S, Yan X, Chen Y, Yu R, Long D, Sang C, Du X, Dong J, Ma C. Postablation neutrophil/lymphocyte ratio correlates with arrhythmia recurrence after catheter ablation of lone atrial fibrillation. Chin Med J (Engl). 2014;127:1033-1038. [PubMed] [DOI] [Full Text] |
| 30. | Bansal P, Verma D, Singh Tamber S, Sharma R. An update on pathogenesis of inflammatory disorders with its management. RPS Pharm Pharmacol Rep. 2023;2. [DOI] [Full Text] |
| 31. | Shahid F, Rahmat NA, Lip GYH, Shantsila E. Prognostic implication of monocytes in atrial fibrillation: The West Birmingham Atrial Fibrillation Project. PLoS One. 2018;13:e0200373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Miyosawa K, Iwata H, Minami-Takano A, Hayashi H, Tabuchi H, Sekita G, Kadoguchi T, Ishii K, Nozaki Y, Funamizu T, Chikata Y, Matsushita S, Amano A, Sumiyoshi M, Nakazato Y, Daida H, Minamino T. Enhanced monocyte migratory activity in the pathogenesis of structural remodeling in atrial fibrillation. PLoS One. 2020;15:e0240540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Chen SA, Zhang MM, Zheng M, Liu F, Sun L, Bao ZY, Chen FK, Li HX, Gu X. The preablation monocyte/ high density lipoprotein ratio predicts the late recurrence of paroxysmal atrial fibrillation after radiofrequency ablation. BMC Cardiovasc Disord. 2020;20:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Sveen KA, Smith G, Goncalves I, Edsfeldt A, Engstrom G, Bjorkbacka H, Bengtsson E. High levels of intermediate cd14++cd16+ monocytes predict incident atrial fibrillation in the general population. Eur Heart J. 2023;44. [DOI] [Full Text] |
| 35. | Canpolat U, Aytemir K, Yorgun H, Şahiner L, Kaya EB, Çay S, Topaloğlu S, Aras D, Oto A. The role of preprocedural monocyte-to-high-density lipoprotein ratio in prediction of atrial fibrillation recurrence after cryoballoon-based catheter ablation. Europace. 2015;17:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Satilmis S. Role of the monocyte-to-high-density lipoprotein ratio in predicting atrial high-rate episodes detected by cardiac implantable electronic devices. North Clin Istanb. 2018;5:96-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Kurt R, Şahin A. Increased Monocyte Count is Related to the Development of Atrial Fibrillation in Subjects with Heart Failure. Med Lab Tech J. 2020;6:100. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Zhang YL, Cao HJ, Han X, Teng F, Chen C, Yang J, Yan X, Li PB, Liu Y, Xia YL, Guo SB, Li HH. Chemokine Receptor CXCR-2 Initiates Atrial Fibrillation by Triggering Monocyte Mobilization in Mice. Hypertension. 2020;76:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Suehiro H, Kiuchi K, Fukuzawa K, Yoshida N, Takami M, Watanabe Y, Izawa Y, Akita T, Takemoto M, Sakai J, Nakamura T, Yatomi A, Takahara H, Sonoda Y, Nakasone K, Yamamoto K, Yamashita T, Hirata KI. Circulating intermediate monocytes and atrial structural remodeling associated with atrial fibrillation recurrence after catheter ablation. J Cardiovasc Electrophysiol. 2021;32:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Yu Y, Wang S, Wang P, Xu Q, Zhang Y, Xiao J, Xue X, Yang Q, Xi W, Wang J, Huang R, Liu M, Wang Z. Predictive value of lymphocyte-to-monocyte ratio in critically Ill patients with atrial fibrillation: A propensity score matching analysis. J Clin Lab Anal. 2022;36:e24217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Suehiro H, Fukuzawa K, Yoshida N, Kiuchi K, Takami M, Akita T, Tabata T, Takemoto M, Sakai J, Nakamura T, Yatomi A, Takahara H, Sonoda Y, Nakasone K, Yamamoto K, Suzuki A, Yamashita T, Hirata KI. Circulating intermediate monocytes and toll-like receptor 4 correlate with low-voltage zones in atrial fibrillation. Heart Vessels. 2020;35:1717-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Uhm TG, Kim BS, Chung IY. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol Res. 2012;4:68-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 679] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 44. | Chen P, Chen J, Xie X, Zhu J, Xia L. Eosinophils in patients with lone atrial fibrillation. Pacing Clin Electrophysiol. 2017;40:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 356] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 46. | Keçoğlu S, Demir M, Uyan U, Melek M. The effects of eosinophil on the left atrial thrombus in patients with atrial fibrillation. Clin Appl Thromb Hemost. 2014;20:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Friedrichs K, Baldus S, Klinke A. Fibrosis in Atrial Fibrillation - Role of Reactive Species and MPO. Front Physiol. 2012;3:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021;11:464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 49. | Karavelioğlu Y, Karapınar H, Yüksel M, Memiç K, Sarak T, Kurt R, Yilmaz A. Neutrophil to lymphocyte ratio is predictor of atrial fibrillation recurrence after cardioversion with amiodarone. Clin Appl Thromb Hemost. 2015;21:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Walsh K, Tan K, Zhang H, Desiderio D, Amar D. P24 - Neutrophil-lymphocyte ratio and risk of atrial fibrillation after thoracic surgery. J Cardiothor Vasc An. 2016;30:S28. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Chou OHI, Chang C, Zhou J, Chan J, Leung KSK, Lee TTL, Wong WT, Liu T, Zhang Q, Lee S, Wai AKC, Tse G. Predictive value of neutrophil-to-lymphocyte ratio for atrial fibrillation and stroke in type 2 diabetes mellitus: a population-based cohort study. Euro Heart J. 2022;43. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Fukuda Y, Tomomori S, Matsumura H, Tokuyama T, Hidaka T, Nakano Y, Kihara Y. O20-3 - In Paroxysmal Atrial Fibrillation Patients, Neutrophil-to-lymphocyte Ratio Is More Associated with Left Atrial Appendage Than with Left Atrial Body. J Card Fail. 2017;23:S38-S39. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Şahin Ş, Sarıkaya S, Alcelik A, Erdem A, Taşlıyurt T, Akyol L, Altunkaş F, Aktaş G, Karaman K. Neutrophil to lymphocyte ratio is a useful predictor of atrial fibrillation in patients with diabetes mellitus. Acta Med Mediterr. 2013;29:847-851. |
| 54. | Pan L, Li Z, Li C, Dong X, Hidru TH, Liu F, Xia Y, Yang X, Zhong L, Liu Y. Stress hyperglycemia ratio and neutrophil to lymphocyte ratio are reliable predictors of new-onset atrial fibrillation in patients with acute myocardial infarction. Front Cardiovasc Med. 2022;9:1051078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 55. | Cosansu K, Vatan MB, Gunduz H, Akdemir R. Use of neutrophil-lymphocyte ratio for risk stratification and relationship with time in therapeutic range in patients with nonvalvular atrial fibrillation: A pilot study. Clin Cardiol. 2018;41:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Soler-Espejo E, Marín F, López-Gálvez R, Ramos-Bratos MP, Sánchez-Villalobos M, Esteve-Pastor MA, Lip GYH, Rivera-Caravaca JM, Roldán V. The Neutrophil-to-Lymphocyte Ratio Is an Independent Inflammatory Biomarker for Adverse Events in Patients With Atrial Fibrillation: Insights From the Murcia AF Project II (MAFP-II) Cohort Study. Clin Cardiol. 2025;48:e70102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 57. | Dereli S, Bayramoğlu A, Yontar OC. Usefulness of platelet to lymphocyte ratio for predicting recurrence of atrial fibrillation after direct current cardioversion. Ann Noninvasive Electrocardiol. 2019;24:e12616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Gungor H, Babu AS, Zencir C, Akpek M, Selvi M, Erkan MH, Durmaz S. Association of Preoperative Platelet-to-Lymphocyte Ratio with Atrial Fibrillation after Coronary Artery Bypass Graft Surgery. Med Princ Pract. 2017;26:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Cosansu K, Ureyen CM, Kilic H, Karadag B, Cabbar A, Gunduz H, Akdemir R. New Uses of Platelet-Lymphocyte Ratio for Bleeding Risk Stratification in Patients with Nonvalvular Atrial Fibrillation: A Pilot Study. Dicle Tıp Dergisi. 2020. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Navani RV, Baradi A, Colin Huang KL, Jin D, Jiao Y, Nguyen JK, Ellis ZC, Newcomb AE, Wilson AM. Preoperative Platelet-to-Lymphocyte Ratio Is Not Associated With Postoperative Atrial Fibrillation. Ann Thorac Surg. 2020;110:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Altinbas O, Tanyeli O, Dereli Y, Ege E. Predictive value of platelet to lymphocyte ratio and mean platelet volume in atrial fibrillation after isolated coronary artery bypass graft operation. Ann Med Res. 2019;26:1033-1038. [DOI] [Full Text] |
| 62. | Zuo K, Yang X. Decreased platelet-to-lymphocyte ratio as predictor of thrombogenesis in nonvalvular atrial fibrillation. Herz. 2020;45:684-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Tek M, Kaplan Efe F. The association between platelet to lymphocyte ratio and left atrial appendage thrombogenic milieu in patients with non-valvular atrial fibrillation. Ankara Med J. 2022;22:260-269. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 64. | Yano M, Nishino M, Nohara H, Toyoshima T, Higashino N, Ukita K, Kawamura A, Yanagawa K, Nakamura H, Matsuhiro Y, Yasumoto K, Okamoto N, Tanaka A, Matsunage Y, Nakamura D, Yamato M, Egami Y, Shutta R, Tanouchi J. Elevation of Neutrophil to Lymphocyte Ratio and Monocyte to Lymphocyte Ratio Are Associated With Recurrence of Atrial Fibrillation After Pulmonary Vein Isolation. Circulation. 2019;140:A10659-. |
| 65. | Sarikaya S, Şahin Ş, Akyol L, Börekçi E, Yilmaz YK, Altunkaş F, Karaman K. Is there any relationship between RDW levels and atrial fibrillation in hypertensive patient? Afr Health Sci. 2014;14:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Wang Z, Korantzopoulos P, Roever L, Liu T. Red blood cell distribution width and atrial fibrillation. Biomark Med. 2020;14:1289-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Adamsson Eryd S, Borné Y, Melander O, Persson M, Smith JG, Hedblad B, Engström G. Red blood cell distribution width is associated with incidence of atrial fibrillation. J Intern Med. 2014;275:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 68. | Zeng H, Tao T, Ma Z, Wang M, Lu X, Zhao Y, Shen Z. Predictive value of red blood cell distribution width in critically ill patients with atrial fibrillation: a retrospective cohort study. Ann Palliat Med. 2021;10:2469-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 69. | Özbek K, Katlandur H, Arık B, Kuzgun A, Yıldız M, Keser A, Özdil H. Role of red blood cell distribution width and epicardial fat in atrial fibrillation after cardiopulmonary bypass. Electron J Gen Med. 2018;15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Korantzopoulos P, Kyrlas K, Liu T, Li G, Goudevenos JA. Red blood cell distribution width and atrial fibrillation in patients with sick sinus syndrome. J Cardiol. 2016;67:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Han K, Su X, Liu J, Yao F, Lu F. Red Cell Distribution Width as a Novel Marker for Different Types of Atrial Fibrillation in Low and High Altitude. Cardiol Res Pract. 2019;2019:6291964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Zheng LH, Liu SY, Hu F, Hu ZC, Shen LS, Wu LM, Yao Y. Relationship between red blood cell distribution width levels and atrial fibrillation in hypertensive patients. J Geriatr Cardiol. 2020;17:486-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Hald EM, Løchen ML, Lappegård J, Ellingsen TS, Mathiesen EB, Wilsgaard T, Njølstad I, Brækkan SK, Hansen JB. Red Cell Distribution Width and Risk of Atrial Fibrillation and Subsequent Thromboembolism: The Tromsø Study. TH Open. 2020;4:e280-e287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Saliba W, Barnett-Griness O, Elias M, Rennert G. The association between red cell distribution width and stroke in patients with atrial fibrillation. Am J Med. 2015;128:192.e11-192.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Lin G, Dai C, Xu K, Wu M. Predictive Value of Red Blood Cell Distribution Width and Atrial Diameter in Paroxysmal Atrial Fibrillation: A Cross-Sectional Study. Med Sci Monit. 2022;28:e937802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Koch CG, Li L, Van Wagoner DR, Duncan AI, Gillinov AM, Blackstone EH. Red cell transfusion is associated with an increased risk for postoperative atrial fibrillation. Ann Thorac Surg. 2006;82:1747-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |