INTRODUCTION
Cardiac catheterization has been increasingly adopted for diagnostic coronary angiography and percutaneous coronary intervention (PCI) and is historically performed through the femoral artery. Although the femoral artery has been the default access site for many years, transradial access (TRA) has increased in popularity for diagnostic and interventional coronary procedures because of its advantages over the femoral approach, becoming the dominant vascular access site in many countries. Current data show a significant rise in TRA usage compared to previous years, underscoring its establishment as a preferred approach in contemporary practice. Over the last 10 years, the utilization of TRA throughout the entire range of cardiac catheterization indications has increased 2.8-fold, from 23.9% in 2013 to 68.2% in 2022, driven by evidence supporting its clinical advantages[1].
The adoption of TRA has also demonstrated a marked increase among patients presenting with complex coronary artery lesions, including chronic total occlusions, bifurcations and calcified lesions, or with prior coronary artery bypass grafting (CABG)[2,3]. In addition, the prevalence of TRA in coronary catheterization is notably higher in academic cardiovascular training programs as well as in institutions that prioritize a radial-first approach to procedural practice, with rates exceeding 90% in some centers, particularly among younger interventional cardiologists[4,5]. Beyond its widespread acceptance and preference for coronary catheterization, TRA is increasingly being recognized as a viable alternative to the more commonly used transfemoral access (TFA) for non-coronary procedures. TRA is progressively being integrated into procedures such as carotid artery stenting[6,7], stroke thrombectomy[8-10], and as a secondary ancillary access for structural cardiac interventions[11,12].
This widespread adoption reflects the numerous advantages of TRA over the traditional transfemoral approach, particularly in coronary interventions. TRA is associated with lower mortality, significantly reduced bleeding, and fewer vascular complications. Meta-analyses strongly support its safety profile. Maqsood et al[13] demonstrated a significant reduction in major bleeding [odds ratio (OR): 0.46; 95%CI: 0.35–0.59] and access-site hematoma (OR: 0.34; 95%CI: 0.24–0.48). Gargiulo et al[14] further highlighted a mortality benefit (hazard ratio (HR): 0.77; 95%CI: 0.63–0.95; P = 0.012), suggesting that TRA’s advantages extend beyond bleeding prevention. In chronic total occlusion PCI, Nguyen et al[15] reported reduced access-site complications (OR: 0.33) and major bleeding (OR: 0.34), with comparable procedural success. Similarly, Senguttuvan et al[16] found a mortality benefit in acute coronary syndrome, especially STEMI [relative risk (RR): 0.71; 95%CI: 0.56–0.90], alongside fewer major adverse cardiac events and vascular complications. These findings align with the latest European Society of Cardiology (ESC)[17,18] and American College of Cardiology/American Heart Association (AHA)/Society for Cardiovascular Angiography and Interventions[19] guidelines, which recommend radial access as the first choice for coronary catheterization due to its superior safety profile and clinical outcomes.
While the benefits of TRA in coronary procedures are well-established, its application has expanded to both diagnostic and therapeutic non-coronary interventions. In diagnostic cerebral angiography, a recent comprehensive meta-analysis demonstrated that TRA is associated with a significantly lower complication rate (OR: 0.5, P = 0.02) compared to TFA, supporting TRA as a safe and practical option following careful patients selection and procedural planning[20]. In therapeutic procedures, TRA has shown comparable efficacy with added safety in several domains. In stroke thrombectomy, TRA achieves similar recanalization success to TFA with shorter hospital stays[21]. In peripheral vascular interventions, TRA reduces access-site complications (OR: 0.64; 95%CI: 0.45–0.91) and minor bleeding (OR: 0.52; 95%CI: 0.31–0.86) but has higher procedural failure rates[22]. For posterior circulation neuro interventions, TRA lowers total complications (OR: 0.29; 95%CI: 0.12–0.73) and access-site complications (OR: 0.19; 95%CI: 0.06–0.62), though it requires longer catheter retention times[23]. In carotid artery stenting and intracranial aneurysm interventions, TRA achieves comparable outcomes to TFA, though higher crossover rates and technical challenges have been noted[24,25]. Lastly, in neuro endovascular procedures, TRA reduces access-site (1.62% vs 3.31%) and neurological complications (1.64% vs 3.82%) compared to TFA, despite higher rates of vascular spasm and wound infections[26]. These findings suggest that TRA is a safe alternative to TFA in non-coronary interventions, particularly for reducing access-site complications, though careful patient and procedure selection remains critical in certain contexts. This is further supported by recommendations from the ESC[27] and the AHA[28], which advocate for TRA as a safe and effective option in select non-coronary procedures, emphasizing its role in minimizing procedural risks.
While the benefits of TRA in interventional procedures are well-documented, its widespread adoption has also brought attention to its potential impact on the structural alterations of the radial artery. These changes not only raise concerns about long-term vascular health but also affect the radial artery's usability for future interventions. A deeper understanding of these changes is essential to mitigate complications and ensure the sustainability of TRA.
In this review, we summarize the existing literature on structural alterations of the radial artery following transradial procedures, incorporating a discussion of predisposing factors and potential prevention strategies.
STRUCTURAL ALTERATIONS OF THE RADIAL ARTERY FOLLOWING TRANSRADIAL PROCEDURES
The radial artery is a fundamental access site for interventional procedures, but mechanical and hemodynamic stresses can compromise its structural integrity through damage to the arterial wall layers, alterations in lumen diameter, and radial artery occlusion (RAO). Understanding TRA-induced structural changes and their predisposing factors is crucial for optimizing procedural outcomes by implementing appropriate prevention strategies.
Damage to the arterial wall layers
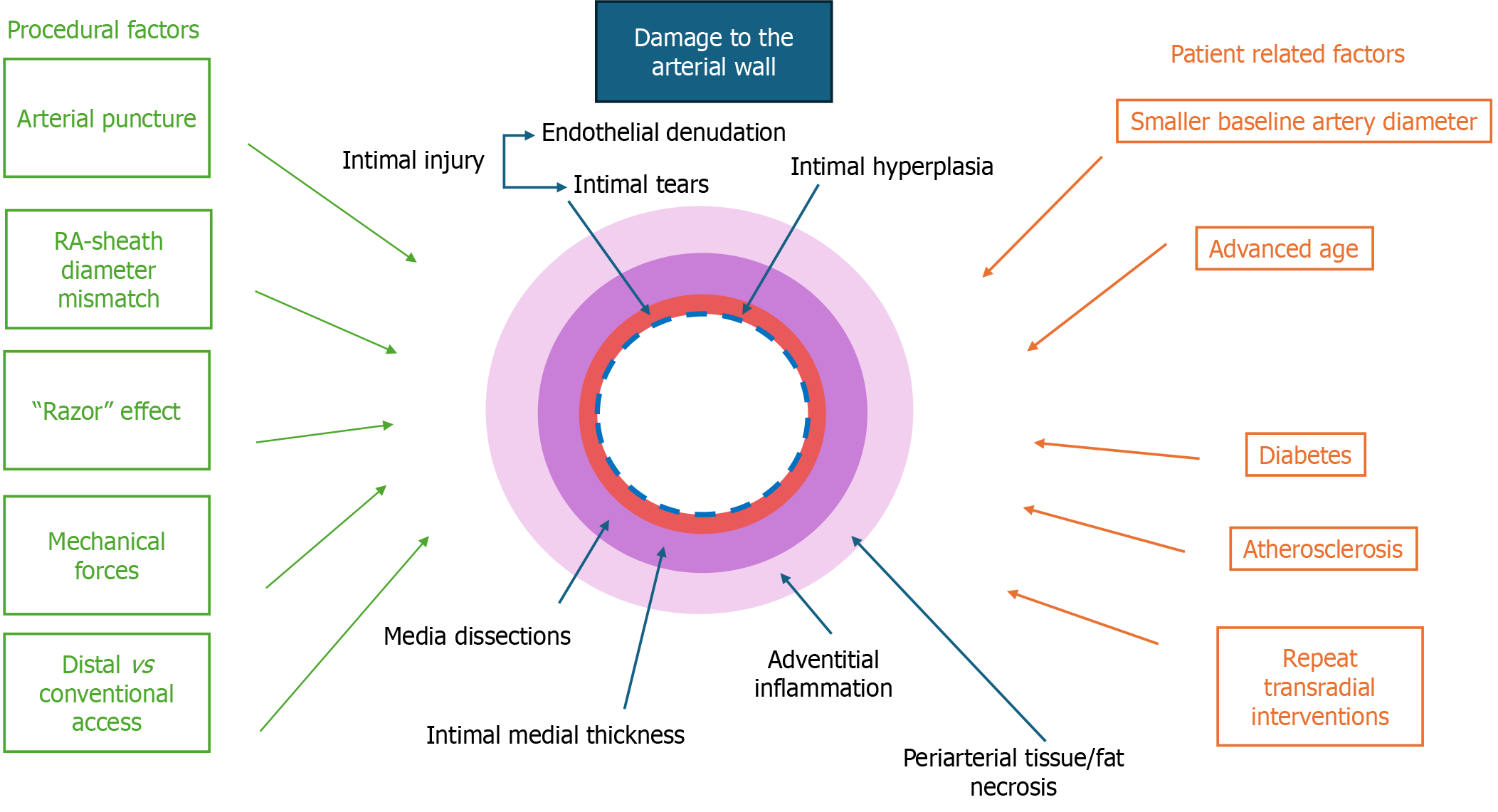
Despite its benefits, TRA can lead to structural damage in the radial artery due to arterial puncture, mechanical forces exerted during guidewire, sheath or catheter insertion and manipulation, sheath friction from the artery inner diameter-sheath outer diameter mismatch as well as damage caused by the space between the guidewire and the catheter tip, which shaves the vessel wall (“razor” effect)[29,30]. These mechanisms can cause significant structural damage to the radial artery, leading to both acute and chronic vascular alterations. The primary mechanisms of injury affect all layers of the arterial wall: The intima (endothelial layer), the media (smooth muscle layer), and the adventitia (outer connective tissue layer). Figure 1 demonstrates procedural and patient-related risk factors that impact all layers of the arterial wall.
Figure 1 Mechanisms and factors contributing to radial artery wall damage following transradial access.
Schematic representation of the factors contributing to arterial wall damage following transradial access (TRA). Procedural factors (green) such as arterial puncture, sheath diameter mismatch, mechanical forces and choice of access site (distal vs conventional) contribute to intimal injury and hyperplasia, medial dissections, intimal-medial thickening and adventitial injury. Patient-related factors (orange), including smaller baseline artery diameter, advanced age, diabetes, atherosclerosis and repeat TRA interventions, further exacerbate arterial remodeling. These structural changes may not only impact long-term vascular integrity but also have implications for the radial artery’s suitability as a conduit for coronary artery bypass grafting.
Acute intimal injuries: The most immediate structural alteration following transradial catheterization is acute intimal injury, primarily caused by mechanical friction between the guidewire, sheath, catheter and the radial arterial wall, leading to endothelial denudation and intimal tears. Immunohistochemical evaluation has demonstrated that TRA induces diffuse endothelial lesions. Although these lesions tend to heal over time, incomplete recovery of endothelial integrity persists even beyond 30 days post-procedure, potentially impacting the radial artery's long-term usability[31]. Intimal tears, a characteristic form of acute intimal injury following transradial intervention, represent a more advanced stage of intimal damage beyond endothelial denudation. Reports from optical coherence tomography (OCT) studies indicate substantial variation in the incidence of intimal tears (37.3%-67.1%)[32,33], though lower rates (2.2%-11.5%) have been observed following the distal TRA approach[29,34]. Importantly, repeat TRA has been associated with a higher incidence of intimal tears, with studies reporting rates as high as 44.1% vs 23.0% in first-time procedures (P = 0.002)[33], whereas other findings suggest no significant difference (46.2% vs 28%; P = 0.18)[32]. Additionally, Zhang et al[35] observed an even higher incidence of intimal tears in repeat transradial cases (48.3% vs 29.4%; P < 0.001), reinforcing the notion that procedural repetition exacerbates intimal trauma.
Yonetsu et al[33] found intimal tears to be significantly more frequent in the distal (43.8%) and mid- (34.2%) radial artery segments compared to the proximal radial artery (17.8%; P = 0.001 and P = 0.038, respectively), emphasizing the role of radial artery-sheath diameter mismatch in acute injury. Although intimal tears were more frequent in the distal and mid-radial artery, they were also observed near the radial artery ostium, suggesting additional contributing factors such as mechanical stretching during sheath manipulation and radial artery spasm, regardless of vessel diameter. Notably, Di Vito et al[32] reported a different distribution pattern, showing a trend toward a higher frequency in the proximal segment (31.4% vs 15.7%; P = 0.09). While they speculated that the use of a long hydrophilic-coated sheath might have influenced this distribution, their study did not provide conclusive evidence for a protective effect. Unlike previous findings that suggested a predominant localization of intimal tears in either proximal or distal segments, Niu et al[29] reported a lower incidence of intimal tears and found no significant difference among the proximal, mid-, and distal radial artery segments (2.5% vs 6.0% vs 5.0%; P = 0.183). They proposed that factors such as the distal TRA approach and combined vasodilator administration (nitroglycerin plus verapamil) may have contributed to these findings. However, further research is required to confirm any protective effect.
Chronic intimal changes: While intimal tears are frequently observed following transradial interventions, they are no longer detectable 90 days post-procedure[36]. However, even after acute disruptions in the intimal layer resolve, many patients develop intimal hyperplasia, a chronic vascular response triggered by inflammatory and proliferative mechanisms due to endothelial and vessel wall injury from sheath insertion and catheter manipulation. Intimal hyperplasia represents the initial response to endothelial injury, characterized by the proliferation and migration of medial smooth muscle cells into the intima, along with extracellular matrix deposition[37]. Notably, Staniloae et al[38] observed that intimal hyperplasia was already evident within 2-3 days post-catheterization, indicating an immediate vascular response. Furthermore, Kamiya et al[39] reported a mean interval of 46 days between preoperative transradial catheterization and radial artery harvesting for CABG, demonstrating that catheterization-induced structural changes persist over time. Additionally, Kala et al[40] performed OCT and demonstrated a significant increase in radial artery intimal volume even 9 months after transradial PCI. Further supporting this timeline, Costa et al[41] used high-resolution ultrasound to show that radial artery intimal thickening appeared within 3 hours post-catheterization and persisted 30 days later. Collectively, these studies indicate that intimal hyperplasia develops rapidly following transradial interventions and remains a persistent feature over time, potentially compromising the radial artery's suitability as a bypass conduit.
Considering the distribution of intimal hyperplasia in the radial artery, some studies did not specify a predominant pattern along its length[39]. In contrast, Staniloae et al[38] reported that intimal hyperplasia was more pronounced in the distal radial artery, possibly due to sheath-artery size mismatch. Additionally, they observed a statistical trend toward increased intimal hyperplasia in the proximal artery. Moreover, multivariate linear regression analysis discovered that external sheath diameter/vessel diameter > 1 was a clinically independent predictor of increased rates in intimal thickness[35]. These findings suggest that while sheath insertion and catheter manipulation affect the entire vessel, proximal injury may be less severe and more likely to undergo partial resolution over time.
The distribution and severity of intimal hyperplasia after transradial intervention appear to be influenced by procedural factors and the number of prior catheterizations. Di Vito et al[32] did not identify a significant difference in intimal hyperplasia between first-time and repeat transradial procedures. However, their study utilized long hydrophilic-coated sheaths, which may have reduced vessel trauma, though the extent of this protective effect remains uncertain. They also observed that intimal hyperplasia was more pronounced in the distal radial artery segment, suggesting that this region may be inherently more susceptible to atherosclerosis and intimal alterations. In contrast, Yonetsu et al[33] demonstrated that intimal thickening was significantly greater in repeat transradial interventions across all examined sites of the radial artery. Their findings suggest that repeated radial access leads to cumulative vascular remodeling, affecting both proximal and distal segments. Supporting this, Zhang et al[35] used very-high-frequency ultrasound to demonstrate significantly greater intimal thickness in patients undergoing repeat transradial procedures (P < 0.001). Multivariate analysis identified repeated TRA as an independent predictor of post-procedural intimal thickening, reinforcing the idea that cumulative vascular trauma accelerates structural remodeling. These contrasting results highlight the potential impact of sheath selection on arterial integrity and reinforce the notion that while the distal radial artery is particularly vulnerable, repeated interventions exacerbate intimal hyperplasia throughout the vessel.
Media: While both acute and chronic alterations of the intima are well-documented consequences of transradial procedures, growing evidence suggests that vascular injury may also extend into the media, resulting in structural changes such as medial dissections and increased intima-media thickness (IMT). Medial dissections, though less frequently reported than intimal tears, represent a significant form of vascular trauma following transradial procedures and have been identified as a predictor of increased intimal thickness. Notably, Costa et al[41] reported a much higher incidence of medial dissections in 89.8% of patients at 3 hours post-procedure. However, their analysis was limited to a narrow region of interest (4 mm proximally and distally from the puncture site), and these dissections did not correlate with postprocedural RAO or loss of pulsation. Moreover, repeated catheter and sheath manipulation may impact the incidence of medial dissections. In line with this notion, studies have shown that in patients undergoing a first transradial coronary intervention, their incidence ranges from 9.5% to 11.8%, while significantly higher rates up to 30.2% have been reported in those with repeated procedures[33,35].
Given the variability in reported incidence, procedural and anatomical factors have been investigated for their role in modulating the risk of medial dissection. Evidence from recent studies suggests that distal TRA may mitigate the incidence of medial dissections, particularly in patients with prior radial interventions. Niu et al[29] reported a 16.5% incidence of medial dissections following distal TRA in patients undergoing their first transradial procedure, while Poletti et al[42] observed a slightly lower rate of 12% in a cohort that included both first-time and repeat transradial interventions. These findings suggest that distal TRA alters the anatomical site of entry and potentially minimizes catheter-induced trauma, possibly offering a protective effect against medial injury, even in previously accessed arteries.
The anatomical distribution of medial dissections along the radial artery appears to be influenced by both mechanical interactions and sheath coverage. Yonetsu et al[33] reported that medial dissections tended to cluster in the proximal and distal portions of the radial artery, suggesting distinct mechanistic causes depending on the segment. Dissections in the distal segment were likely attributable to a stretching effect during sheath insertion, whereas those in the proximal portion, which does not have sheath protection, were thought to result from catheter advancement or withdrawal near the radial ostium. Similarly, Niu et al[29] observed a significantly higher incidence of medial dissections in the proximal radial artery compared to the distal segment (11.0% vs 4.5%; P < 0.05), supporting the notion that sheath presence may confer protection against catheter-induced trauma during distal transradial procedures. In contrast, Di Vito et al[32], who employed a long 25-cm hydrophilic-coated introducer sheath that covered the entire radial artery, found no significant difference in medial dissection rates between proximal and distal segments. This finding suggests that full-length sheath coverage may provide uniform protection along the artery and reduce site-specific variation in medial injury.
Beyond localized injuries such as medial dissections, transradial procedures also induce diffuse arterial wall remodeling, as reflected in increased IMT. Yan et al[43] observed a significant rise in IMT from 0.25 ± 0.12 mm pre-procedure to 0.69 ± 0.31 mm at 1 day, followed by partial regression to 0.38 ± 0.17 mm at 30 days (P < 0.05), indicating incomplete but ongoing vascular healing. Similarly, Tehrani et al[36] reported a mean increase in IMT of 0.07 mm at 90 days post-procedure, highlighting the persistence of arterial wall thickening following TRA. IMT appears to be an extremely common response to transradial procedures, with Zhang et al[35] reporting post-procedural thickening in 80.2% of first-time cases and 89.4% of repeat interventions (P < 0.001). These findings are supported by studies from Shen et al[44] and Yonetsu et al[33], who similarly demonstrated significantly greater intimal and medial thickening in patients undergoing repeat TRA, identifying procedural repetition as an independent predictor of arterial wall remodeling.
In addition to repeated TRA, several patient and procedural factors have been associated with IMT progression following transradial procedures. Shen et al[44] demonstrated that smaller baseline radial artery diameter and PCI were associated with increased post-procedural IMT. Similarly, Tehrani et al[36] found that advanced age and the presence of diabetes independently predicted greater forearm radial artery IMT at 90 days. These findings underscore the multifactorial nature of arterial remodeling and highlight the importance of pre-procedural risk stratification to preserve radial artery integrity, particularly in patients requiring multiple interventions. Tehrani et al[36] also reported no significant difference in the mean change of forearm radial artery IMT between distal and conventional TRA, but noted that distal TRA may limit the feasibility of future repeated access. This is due to its association with increased IMT in both the distal and forearm radial artery segments, whereas conventional access affects only the forearm segment.
Adventitia: Adventitial injury, although less frequently emphasized compared to intimal or medial damage, has also been documented as a consequence of transradial catheterization, particularly near the access site. Histopathological evaluation by Staniloae et al[38] revealed that the distal ends of previously catheterized radial arteries exhibited significantly greater adventitial inflammation (33.3% vs 0%; P = 0.01) and periarterial tissue or fat necrosis (26.7% vs 0%; P = 0.02) compared to non-catheterized arteries. Furthermore, within the same artery, the distal segment showed more adventitial inflammation than the proximal segment (33.3% vs 0%; P = 0.011), implicating localized trauma likely due to sheath-vessel diameter mismatch and repeated manipulation at the puncture site. Such adventitial inflammation may play a pivotal role in modulating the structural integrity of the intima and media and could contribute to radial artery shrinkage through sustained inflammatory signaling and vessel wall remodeling. Complementing these histological observations, Costa et al[41] used high-resolution ultrasound to demonstrate that total wall thickness, encompassing the intima, media, and adventitia, tripled within 3 hours of cannulation and further increased by 28% at 30 days post-procedure. This persistent thickening localized to the puncture site emphasizes the enduring inflammatory and remodeling response of the adventitial layer and highlights the importance of considering adventitial changes as part of the broader spectrum of radial artery remodeling following TRA.
Changes in radial artery lumen diameter
In addition to histological and structural alterations of the arterial wall layers, TRA can lead to changes in radial artery lumen diameter—an outcome that may influence vessel patency and long-term suitability for future access or grafting.
In patients undergoing their first transradial procedure, early changes in radial artery lumen diameter are common. Yan et al[43] reported that the mean inner diameter of the catheterized radial artery significantly decreased from 2.37 ± 0.57 mm pre-procedure to 1.95 ± 0.50 mm at 1 day post-procedure (P < 0.01). This acute reduction is likely driven by an inflammatory response to radial artery puncture and mechanical friction between the sheath and intima, which induces intimal injuries, intima-media thickening, and narrowing of the vessel lumen. Notably, the observed decrease in luminal diameter exceeded the degree of intima-media thickening, suggesting a functional component of vasoconstriction in addition to structural changes. Supporting this pattern, Shen et al[44] found that the mean radial artery diameter 1 day post-procedure was significantly smaller in repeat-TRA patients compared to first-time patients (1.79 ± 0.54 mm vs 1.93 ± 0.57 mm; P < 0.05), indicating that prior interventions may exacerbate early luminal narrowing. At 1-month post-procedure, a partial recovery in radial artery diameter was observed, with the mean inner diameter increasing to 2.23 ± 0.41 mm. However, it remained significantly reduced compared to baseline (P < 0.05), indicating that full luminal restoration does not occur within the 1st month. IMT also remained elevated, suggesting that the partial luminal recovery may be attributed to improved endothelial function and reduced local vasoconstrictive tone rather than structural normalization. Aykan et al[45] similarly reported a statistically significant reduction in radial artery diameter 1 month post-procedure (2.97 ± 0.46 mm to 2.82 ± 0.51 mm; P < 0.001), providing further evidence that radial diameter does not fully normalize in the short term.
Extending this observation, Buturak et al[46] demonstrated that this reduction persists at 6 months, with mean diameter decreasing from 2.85 ± 0.44 mm pre-procedure to 2.74 ± 0.42 mm (P = 0.0001). While the clinical implications of this persistent narrowing remain to be fully clarified, these results highlight the potential for long-lasting alterations in radial artery following TRA. Reinforcing these findings, Fan et al[47] demonstrated that radial artery diameter remained significantly reduced even 1 year after TRA, with mean diameter decreasing from 2.44 ± 0.38 mm to 2.30 ± 0.41 mm (P = 0.002), indicating long-term persistence of radial narrowing beyond the expected recovery window. Beyond these localized assessments, Kala et al[40] conducted a serial volumetric analysis using OCT and observed a significant reduction in arterial lumen volume from 356.3 mm³ at baseline to 304.7 mm³ at 9 months (P < 0.001). Lumen volume decreased in 79.2% of patients, while 20.8% showed no change or an increase, highlighting interindividual variability. These findings demonstrate that radial artery narrowing following TRA extends beyond a single site and persists over time, reinforcing the concept of sustained arterial alterations. Importantly, the study also showed that even uncomplicated and relatively short transradial procedures can affect the radial artery as part of a broader vascular response. The authors suggested that unmeasured factors such as genetic predisposition, variability in catheter manipulation, or differences in antiplatelet therapy might account for the observed heterogeneity in lumen responses.
Although radial artery narrowing is frequently reported after transradial procedures, findings from structured access protocols suggest that careful pre-procedural analysis of vascular architecture and sheath selection may help preserve vessel diameter and reduce the risk of long-term remodeling. In two studies employing Doppler ultrasound for procedural planning, Yoon et al[48] and Koziński et al[49] did not observe significant reduction in radial artery diameter at 7 days and 60 days post-procedure, respectively. Both studies systematically excluded patients with unsuitable radial artery anatomy prior to intervention, ensuring an optimal sheath-to-artery size match. The combination of this tailored-access strategy with the use of only 5 Fr or 6 Fr sheaths may have reduced mechanical stress on the vessel wall and helped preserve radial artery structure. These results underscore the potential value of individualized radial access planning in reducing vascular injury and maintaining vessel patency over time.
RAO
While structural alterations involving all layers of the radial artery wall, along with lumen narrowing, are frequently observed after TRA, a more clinically significant consequence is RAO—a complication that may compromise future access and conduit suitability. The incidence of RAO varies across studies, but it is generally reported to range between 9% and 14%. A prospective study involving 427 patients found a RAO incidence of 11.24%, with spontaneous recanalization observed in 32.6% of cases within 3 months[50]. A multicenter study of 1357 patients reported an overall RAO incidence of 9.5%, with higher rates in the angiography-only group (10.6%) compared to the PCI group (6.2%)[51]. Additionally, a systematic review and meta-analysis of 41 studies comprising 30020 patients reported an overall RAO incidence of 13%, with early RAO (within 24 hours) occurring in 14% of cases and late RAO (after 24 hours) in 10% of cases[52].
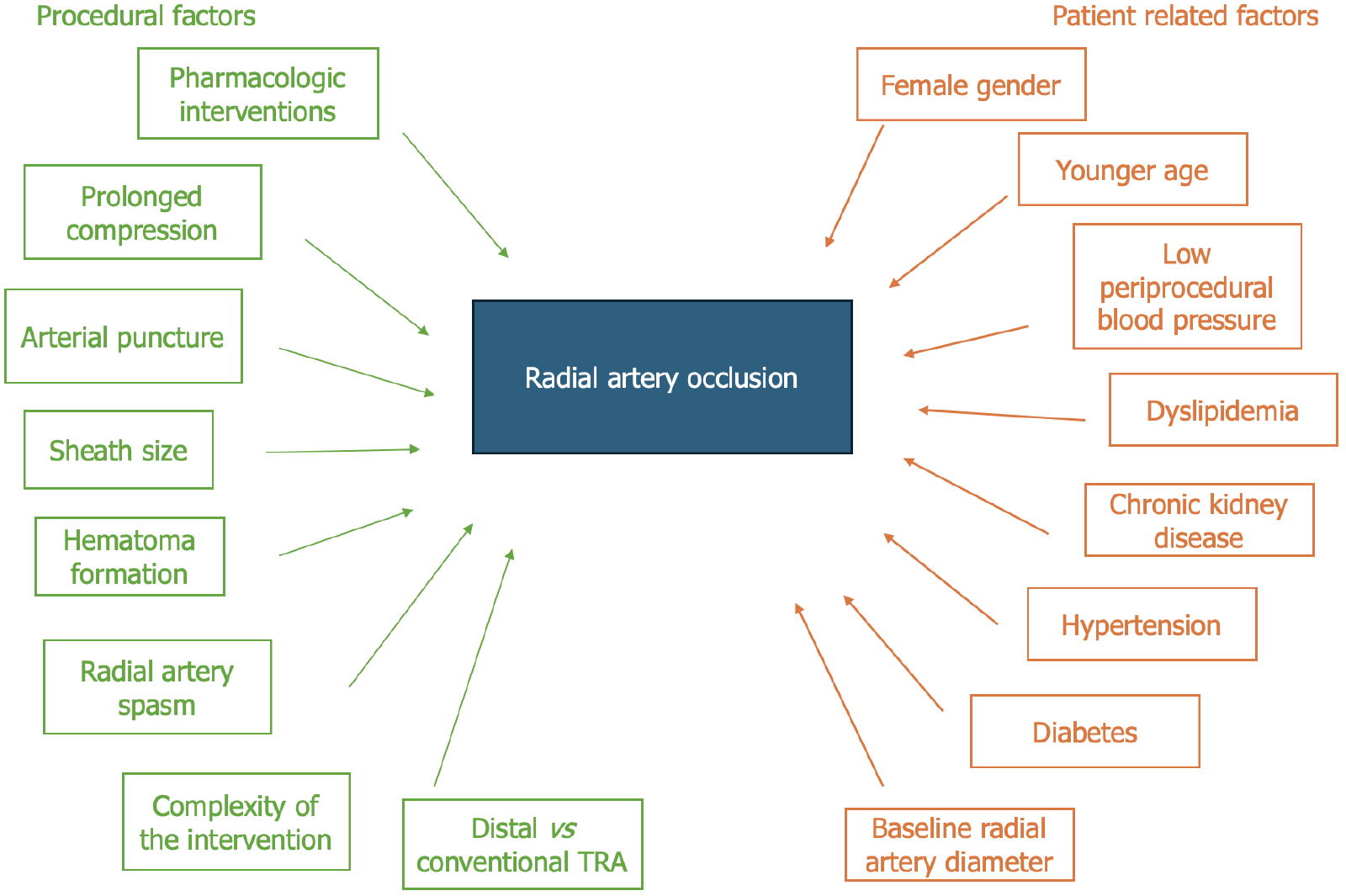
The development of RAO is influenced by a combination of patient-related and procedural-related risk factors, many of which are potentially modifiable with proper technique and planning. These risk factors are depicted in detail in Figure 2.
Figure 2 Procedural and patient-related factors associated with radial occlusion artery after transradial access.
Schematic representation of the factors contributing to radial occlusion artery (RAO) following transradial access (TRA). Procedural factors (green), including periprocedural pharmacologic interventions, prolonged compression, multiple arterial punctures, sheath size, hematoma formation, radial artery spasm, procedural complexity and choice of access site (distal vs conventional) impact the incidence of RAO. Patient-related factors (orange), such as female sex, younger age, low periprocedural blood pressure, dyslipidemia, chronic kidney disease, hypertension, diabetes, and baseline radial artery diameter, further predispose individuals to RAO. Understanding these factors is crucial for optimizing strategies to minimize RAO risk and preserve radial artery integrity. TRA: Transradial access.
Among patient-related factors, female sex has consistently been identified as an independent predictor of RAO following TRA. In a large retrospective study involving 1307 patients, Munir et al[53] found that female sex was significantly associated with increased RAO risk (OR: 1.79; 95%CI: 1.21–2.64; P = 0.003), with women representing 31.8% of RAO cases compared to 20.3% in the non-RAO group. Similarly, Tuncez et al[54] observed a higher RAO incidence in females, reporting that 8 out of 10 RAO cases occurred in women (P = 0.019), suggesting that anatomical factors such as smaller radial artery diameters in women may lead to an unfavorable sheath-to-artery ratio. This association was further supported by Didagelos et al[51], who identified female sex as an independent predictor of RAO in multivariate analysis, reinforcing the need for meticulous sheath selection and pre-procedural vessel assessment in women to mitigate occlusion risk. Additionally, a recent meta-analysis by Khalid et al[52] that encompassed data from 41 studies confirmed female sex as a significant predictor of RAO, with subgroup analysis showing an overall effect size of 0.22 (95%CI: 0.00–0.44). Age has also been identified as a significant patient-related risk factor for RAO. In a comprehensive meta-analysis by Khalid et al[52], increasing age was associated with a higher incidence of RAO, reinforcing its role as a consistent predictor across diverse populations. These findings are consistent with an earlier meta-analysis by Rashid et al[55], which also reported older age as a predictor of RAO following TRA. Although the precise mechanisms remain uncertain, this association may reflect the cumulative effects of age-related vascular and functional changes, which could predispose elderly patients to post-procedural occlusion. Additionally, cardiovascular instability during the procedure has been identified as an independent predictor of RAO. Lounes et al[50] reported a significant association between low periprocedural systolic blood pressure and RAO (OR: 0.598; P = 0.007), while Munir et al[53] found cardiovascular instability to double the odds of occlusion (OR: 2.51; 95%CI: 1.23–5.12; P = 0.012). These findings underscore the importance of hemodynamic optimization in minimizing the risk of RAO.
Traditional cardiovascular risk factors have also been associated with an increased risk of RAO following TRA. Munir et al[53] identified dyslipidemia as an independent predictor of RAO, highlighting its role in post-procedural vascular outcomes. Complementing this, Wang et al[56] reported that patients with elevated low-density lipoprotein cholesterol had a significantly higher incidence of RAO, with multivariate analysis confirming it as an independent risk factor (OR: 3.799; 95%CI: 1.292–11.166; P = 0.015), underscoring the importance of lipid management in patients undergoing repeated TRA. Dwivedi et al[57] demonstrated that chronic kidney disease, defined as a glomerular filtration rate below 60 mL/minute/1.73 m², significantly elevated RAO risk (OR: 1.504; 95%CI: 1.085–2.083; P = 0.014). Ay et al[58] reported hypertension as an independent risk factor for radial artery thrombosis, a key precursor to RAO. Additionally, diabetes mellitus has emerged as another significant contributor to RAO. In a study of 497 patients undergoing repeated right radial interventions, Wang et al[56] found a significantly higher prevalence of diabetes in the RAO group compared to those without occlusion (33.3% vs 19.2%; P = 0.021), and multivariate analysis confirmed diabetes as an independent predictor of RAO (OR: 2.186; 95%CI: 1.014–4.714; P = 0.046). Further supporting the link between cumulative cardiovascular risk and RAO, Nadir et al[59] demonstrated that a CHA2DS2-VASc score ≥ 3 was an independent predictor of RAO (OR: 1.44; 95%CI: 1.17–1.78; P < 0.001), and also predicted persistent occlusion despite treatment (OR: 1.37; 95%CI: 1.01–1.85; P = 0.03). Because this score incorporates multiple common comorbidities, such as hypertension, diabetes, vascular disease, and advanced age, it underscores the importance of assessing overall cardiovascular burden rather than isolated risk factors when predicting RAO. This highlights the need for comprehensive risk evaluation and tailored procedural planning in patients undergoing TRA.
Beyond demographic and clinical risk factors, emerging evidence suggests that specific blood biomarkers may also help predict the risk of RAO following transradial procedures. Ay et al[58] reported that lower hematocrit levels were independently associated with a higher risk of radial artery thrombosis, potentially due to compromised hemodynamic stability. In parallel, Wang et al[56] identified elevated D-dimer levels as an independent predictor of RAO, reflecting a prothrombotic state that may predispose patients to vascular occlusion. Additionally, inflammatory markers such as C-reactive protein, platelet count, and platelet distribution width have been linked to RAO occurrence[59], as suggested by Inci et al[60], highlighting the role of systemic inflammation in post-procedural vascular injury. Collectively, these findings support the utility of a targeted laboratory panel, including hematocrit, D-dimer, C-reactive protein, and platelet indices, to identify high-risk patients prior to TRA and facilitate tailored preventive strategies.
While patient-related risk factors provide important insight into RAO susceptibility, procedural factors play a critical and often modifiable role in determining vascular outcomes. These begin at the point of arterial access, where technique and precision significantly influence the extent of arterial wall injury and subsequent thrombotic risk. One of the earliest procedural hazards is multiple puncture attempts during radial artery cannulation. Dwivedi et al[57] found that repeated punctures were significantly associated with an increased incidence of RAO, likely due to compounded vascular trauma and heightened arterial spasm. Mechanical injury from multiple attempts can disrupt all arterial wall layers and provoke vasospastic responses, reducing radial artery flow and increasing thrombotic risk. These findings underscore the importance of ultrasound guidance, meticulous technique, and operator experience in minimizing access-related vascular complications. Beyond puncture attempts, the anatomical site of access may also influence RAO risk. Several studies have explored the benefits of distal TRA compared to conventional TRA. A large multicenter randomized trial by Aminian et al[61] did not identify any significant difference in RAO incidence between distal TRA and conventional TRA when a rigorous hemostasis protocol was implemented (0.91% vs 0.31%; P = 0.29). However, other studies suggest a potential protective effect of distal TRA. Wang et al[62] reported a significantly lower incidence of RAO in the distal TRA group compared to the conventional TRA group (2.0% vs 9.0%; P = 0.030), alongside a shorter hemostasis time. Similarly, in a propensity-matched study of 1163 patients, Pacchioni et al[63] found RAO rates of 0% with distal TRA vs 4.8% with conventional TRA (P < 0.0001), highlighting improved radial artery patency with distal access. These findings were further supported by a meta-analysis of randomized controlled trials by Barbarawi et al[64], which concluded that distal TRA is associated with a significantly lower risk of RAO. Collectively, these studies indicate that when feasible, distal TRA may offer a safer alternative for radial access by reducing mechanical injury and preserving arterial patency.
Following initial arterial access, one of the most critical procedural determinants of RAO is the size of the arterial sheath relative to the radial artery diameter. Dwivedi et al[57] identified larger sheath size as an independent predictor of RAO, with multivariate analysis showing significantly higher occlusion rates in patients receiving 7 Fr sheaths compared to those with 5 Fr or 6 Fr (P < 0.001). This finding underscores the pathophysiological rationale that greater sheath-to-artery mismatch increases vascular trauma, vasospasm, and subsequent RAO. Supporting this, a meta-analysis by Rashid et al[55] encompassing 66 studies demonstrated a stepwise rise in RAO incidence with increasing sheath size—0% for 4 Fr, 2% for 5 Fr, 11% for 6 Fr, and up to 19.5% for 7 Fr—highlighting a size-dependent relationship between sheath and vascular injury. Beyond sheath dimensions, the intrinsic size of the radial artery itself plays a pivotal role. Rashid et al[55] also identified small radial artery diameter as a consistent RAO predictor across studies, suggesting heightened procedural vulnerability in such patients. Lounes et al[50] further confirmed that reduced radial diameter significantly increased occlusion risk (OR: 0.371; 95%CI: 0.323–0.618; P = 0.031). Complementing these findings, Garg et al[65] reported that radial arteries with diameters below 2.5 mm were independently associated with higher RAO incidence, emphasizing the need for anatomical screening prior to cannulation. Collectively, these data highlight the importance of meticulous pre-procedural planning, including ultrasound-guided vessel sizing and tailored sheath selection, to minimize vascular trauma and improve long-term access preservation.
Post-procedural hemostasis techniques and compression duration are pivotal in influencing the risk of RAO following TRA. Didagelos et al[51] reported that compared to mechanical compression devices, manual compression was significantly associated with a higher incidence of RAO, likely due to inconsistent pressure application and prolonged artery occlusion. Dwivedi et al[57] further demonstrated that extended hemostasis duration was an independent predictor of RAO, emphasizing that prolonged compression may impair radial flow and promote thrombus formation. Similarly, Ay et al[58] identified the duration of compression as a key determinant of radial artery thrombosis, reinforcing the need for timely and controlled pressure release. Supporting these findings, Rashid et al[55] showed that shorter compression times were associated with a significantly reduced RAO risk (RR: 0.28), highlighting the protective effect of early restoration of radial artery patency. Collectively, these findings advocate for the use of patent hemostasis techniques and time-guided compression protocols to minimize vascular complications and enhance radial artery patency.
Pharmacologic interventions during TRA can significantly influence the likelihood of RAO. In a large prospective study, Didagelos et al[51] demonstrated that the use of intravenous unfractionated heparin at doses exceeding 50 IU/kg was independently associated with a lower risk of RAO [adjusted OR (aOR): 0.56; 95%CI: 0.31–1.00], supporting the thrombotic pathophysiology of RAO and the protective role of adequate anticoagulation. Moreover, intra-arterial administration of verapamil also favored radial artery patency (aOR: 0.17; 95%CI: 0.04–0.76), likely through its vasodilatory and antispasmodic properties that reduce arterial trauma and flow compromise. In contrast, the use of intra-arterial nitroglycerin was associated with a markedly increased risk of RAO in the PCI subgroup (aOR: 7.40; 95%CI: 1.67–32.79), a finding that may reflect differential vascular responses or interactions with procedural factors. These results underscore the importance of individualized pharmacologic strategies, suggesting that verapamil may be a safer vasodilator option than nitroglycerin in the context of TRA, and that weight-adjusted unfractionated heparin dosing above 50 IU/kg should be considered standard to minimize thrombotic complications.
CONCLUSION
TRA has transformed the landscape of cardiovascular and neurovascular procedures, offering significant advantages over traditional TFA in terms of patient safety, comfort, and reduced vascular complications. As TRA becomes increasingly adopted across both diagnostic and therapeutic settings, greater attention is being directed toward its long-term impact on radial artery structure. Evidence demonstrates that TRA can induce both acute and chronic injuries to the arterial wall layers, lead to alterations in lumen diameter, and contribute to RAO. These structural changes may compromise the artery’s future usability, particularly in patients requiring repeated access or radial artery harvesting for CABG.
To mitigate these adverse outcomes, several procedural strategies have been identified. Meticulous pre-procedural ultrasound evaluation is essential to ensure appropriate patient selection and accurate sheath-to-artery size matching, thereby minimizing mechanical trauma. The use of smaller sheaths (≤ 6 Fr) and ultrasound-guided cannulation techniques has been associated with reduced arterial injury and improved procedural success. Intra-procedural pharmacologic measures—such as adequate anticoagulation (≥ 50 IU/kg of unfractionated heparin) and the administration of vasodilators like verapamil—can help reduce the risk of thrombosis, vasospasm, and trauma-induced vascular injury. Post-procedural care, particularly through patent hemostasis and structured, time-guided compression protocols, is critical to maintaining radial artery patency and limiting the incidence of RAO.
Integrating these preventive strategies throughout the procedural continuum is vital to preserving radial artery health, especially in high-risk populations such as those with smaller vessel calibers or multiple prior radial interventions. While existing evidence supports their efficacy, further research is needed to evaluate the long-term consequences of radial artery remodeling, optimize device and technique selection, and refine individualized risk stratification protocols.
Ultimately, balancing the procedural benefits of TRA with a proactive approach to vascular preservation will ensure that its growing use does not compromise long-term arterial integrity. As the field continues to evolve, a deeper understanding of the pathophysiological mechanisms underlying TRA-induced injury—and continued refinement of prevention strategies—will be essential to sustaining the advantages of this valuable access route.