Published online Jul 26, 2025. doi: 10.4330/wjc.v17.i7.107751
Revised: May 10, 2025
Accepted: July 8, 2025
Published online: July 26, 2025
Processing time: 116 Days and 1.9 Hours
Metabolic-associated steatotic liver disease (MASLD) is a global health burden in
Core Tip: Metabolic-associated steatotic liver disease (MASLD), a leading global liver disorder, is strongly linked to cardio
- Citation: Luong TV, Tran H, Hoang Thi BN, Vu HM, Le TT, Le TT, Tran Thi HT, Nguyen HM, Doan TC, Ho BA, Hoang TA, Dang HNN. Integrating liver and heart health: Cardiovascular risk reduction in patients with metabolic-associated steatotic liver disease. World J Cardiol 2025; 17(7): 107751
- URL: https://www.wjgnet.com/1949-8462/full/v17/i7/107751.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i7.107751
Metabolic-associated steatotic liver disease (MASLD), previously known as nonalcoholic fatty liver disease (NAFLD), is now recognized as a distinct clinical entity within the broader category of steatotic liver disease. According to a multiso
MASLD affects approximately 25%-30% of the adult population worldwide and is increasing in parallel with the increasing prevalence of obesity and T2DM[3-5]. Among its systemic consequences, cardiovascular disease (CVD) has emerged as the leading cause of morbidity and mortality in MASLD patients, surpassing liver-related complications[6,7]. Shared pathophysiological mechanisms, including chronic inflammation, insulin resistance, dyslipidemia, and endothe
Despite this well-established connection, cardiovascular risk assessment and management remain inadequately addressed in many MASLD patients[9]. Although current guidelines emphasize integrated care models that consider both hepatic and cardiovascular endpoints, MASLD is still often managed in isolation from broader cardiometabolic care[2].
This review aims to bridge that gap by providing an evidence-based overview of cardiovascular risk in MASLD, high
In addition to its hepatic manifestations, MASLD is a systemic disease intricately linked to cardiometabolic disorders, es
The elevated burden of CVD among patients with MASH strongly suggests that the two conditions share overlapping pathophysiological foundations. MASLD and CVD are linked through common CMRFs such as obesity, insulin resis
The mechanistic basis connecting MASLD and CVD is well established and includes endothelial dysfunction, dysregulated lipid metabolism, systemic inflammation, oxidative stress, insulin resistance, and the formation of unstable atherosclerotic plaques[11]. These disturbances contribute to structural and functional changes in the cardiovascular system, increasing susceptibility to complications such as hypertension, atherosclerosis, arrhythmias, myocardial dysfunction, valvular abnormalities, and thrombosis.
Insulin resistance in MASLD often leads to atherogenic dyslipidemia, characterized by elevated levels of triglycerides, increased concentrations of small dense low-density lipoprotein (LDL) particles, and reduced HDL cholesterol[12,13]. Together with systemic inflammation, these lipid abnormalities contribute to accelerated atherogenesis and impaired endothelial function[14].
When MASLD coexists with atherosclerosis, particularly in the context of MASH, the combined disease burden is greater than that of atherosclerosis alone. A systemetic review have demonstrated a robust association between MASH and early atherosclerotic changes, such as carotid artery thickening and subclinical atherosclerosis, regardless of diabetes status[15]. These include increased carotid intima-media thickness, arterial stiffness, impaired left ventricular function, endothelial dysfunction, reduced flow-mediated dilation, and coronary artery calcification[16].
In individuals with coexisting MASLD and T2DM, insulin resistance serves as a critical amplifier of cardiovascular risk, further increasing the incidence of adverse cardiovascular events[14]. Identifying MASLD in these patients may help clinicians identify a subgroup with elevated cardiovascular risk that could benefit from more intensive and targeted ma
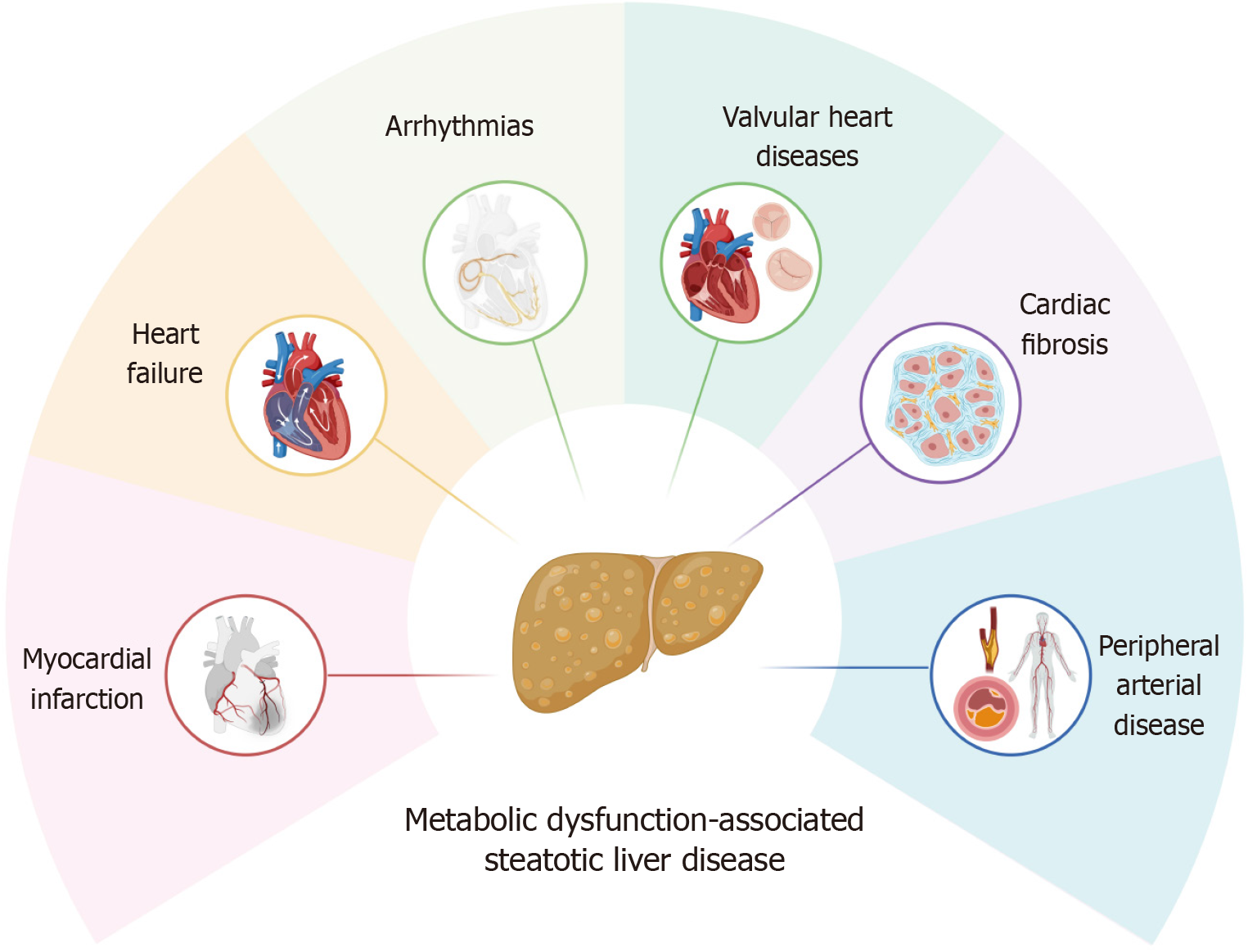
The interplay between MASLD and CVD gives rise to a broad spectrum of clinically relevant cardiac complications. These include major adverse cardiovascular events (MACEs), heart failure, arrhythmias, valvular heart disease, neuro-circula
Major adverse cardiac events: Recent evidence has demonstrated a robust association between MASLD and MACEs. In a comprehensive synthesis of data from 16 observational studies, individuals with MASLD presented a 64% greater like
The extent of hepatic fibrosis appears to stratify cardiovascular risk further. A multinational study involving 458 patients with advanced MASLD revealed that those with bridging fibrosis had a markedly higher incidence of cardiova
In addition, coronary artery disease (CAD) represents a key pathophysiological intersection between MASLD and cardiovascular morbidity. A large-scale cross-sectional study by Chang et al[21] revealed a significant correlation between MASLD and the presence of coronary artery calcium (CAC), an established marker of subclinical atherosclerosis. Particularly in nondiabetic MASLD patients, increased CAC burden has been linked to multivessel coronary involvement and heightened susceptibility to myocardial ischemia, including ST-segment elevation myocardial infarction[22].
Taken together, these data reinforce the concept of MASLD as an independent determinant of cardiovascular risk, highlighting the importance of integrating hepatic evaluation into the cardiovascular risk assessment paradigm, parti
Heart failure: MASLD has been increasingly recognized for its strong association with the risk of new-onset heart failure. A comprehensive meta-analysis involving over 11 million individuals demonstrated that patients with MASLD face a 1.5-fold increased risk of developing heart failure. This risk escalates in direct correlation with the degree of cirrhosis, as reflected by the fibrosis-4 (FIB-4) index, alongside a higher incidence of hospitalization due to heart failure[23,24]. The study conducted by Fudim et al[25] demonstrates a stronger association between MASLD and heart failure with pre
These findings reinforce the hypothesis that heart failure, particularly HFpEF, is not simply an associated comorbidity of MASLD, but may also be a direct consequence of the disease, driven by histological changes and subsequent cardio
Arrhythmia: Cardiac arrhythmias, particularly atrial fibrillation (AF), are increasingly recognized as significant cardio
Cardiac valvular complications: MASLD has been associated with a higher incidence of aortic valve sclerosis, as shown in a meta-analysis of over 2600 patients[43]. Recent findings from the MESA study also linked MASLD to an increased risk of aortic valve calcification and incident aortic stenosis, independent of genetic predisposition[44]. These associations suggest a possible role of MASLD in valvular heart disease, warranting further investigation.
Peripheral artery complications: Peripheral artery complications are increasingly recognized in patients with MASLD and are likely associated with progressive atherosclerosis, endothelial dysfunction, and arterial stiffness[45,46]. Patients with MASLD, including those without T2DM, often exhibit a low ankle-brachial index (< 0.9), indicating an elevated cardiovascular risk[47]. Measures of arterial stiffness, such as brachial–ankle pulse wave velocity and cardio-ankle vascular index, have also been found to be significantly higher in these patients[47]. The underlying pathophysiological mechanisms include chronic low-grade inflammation and oxidative stress, leading to endothelial injury and reduced nitric oxide production, thereby promoting vasoconstriction and atherosclerosis[48]. Insulin resistance and dyslipidemia in MASLD contribute to extracellular matrix accumulation and arterial wall fibrosis, reducing vascular elasticity[49]. Furthermore, increased levels of pro-inflammatory cytokines such as TNF-α and IL-6 activate endothelial cells, upregulate adhesion molecules, recruit leukocytes, and promote atheroma formation[50]. In addition, lipid metabolism disorders lead to lipid deposition in peripheral arterial walls, and impaired endothelial progenitor cell function limits vascular repair capacity[51,52]. Given the strong association with metabolic syndrome, MASLD substantially increases the risk of peripheral artery disease even in patients without T2DM[53].
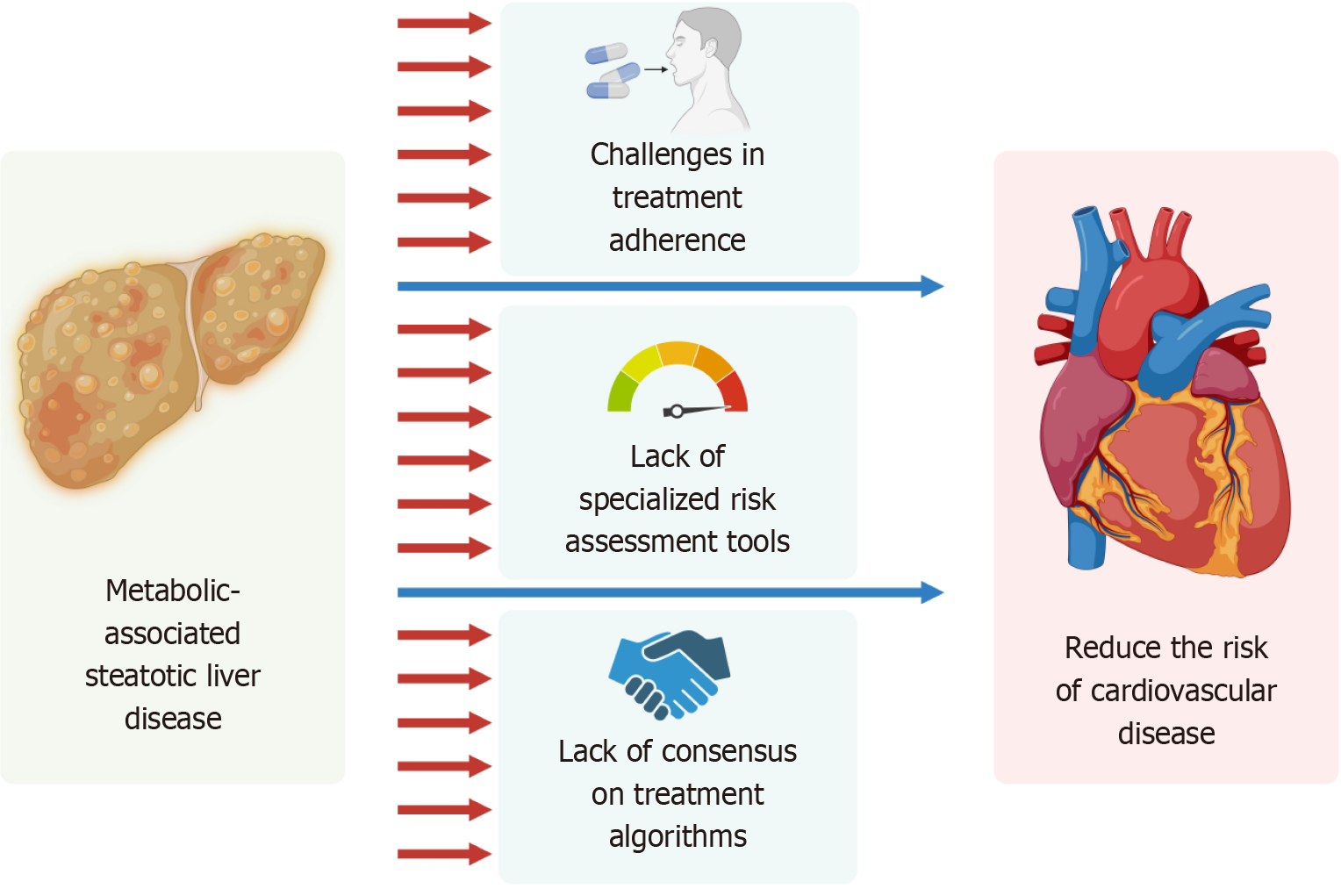
Managing MASLD and its cardiovascular complications presents several challenges that hinder optimal patient care, as illustrated in Figure 2. Addressing these barriers is essential to improving outcomes.
The most recent guidelines of the ESC, ACC/AHA, AASLD and EASL have shown that patients with MASLD are at increased risk of developing cardiovascular problems, including atherosclerosis, hypertension, and CAD[2,54,55]. This may be due to the chronic inflammation, insulin resistance, and metabolic factors associated with MASLD, which increase the burden on the cardiovascular system.
Current cardiovascular risk assessment tools, such as the Framingham Risk Score or ASCVD Risk Calculator, are not specifically designed for MASLD patients. These tools often underestimate cardiovascular risk in this population because they do not account for liver-specific factors, such as hepatic fibrosis, which is a strong predictor of adverse outcomes[56-58].
However, there is still a need for validated, MASLD-specific risk assessment tools that integrate hepatic and cardiova
There is no universally accepted treatment algorithm for managing cardiovascular risk in MASLD patients. While guidelines exist for individual conditions like T2DM, hypertension, and dyslipidemia, they do not provide clear, inte
As it is unclear if MASLD independently increases the CVD risk, neither EASL, the European Association for the Study of Diabetes and Obesity[70,71], the AACE[72], AASLD[69], ESC[68], or ACC/AHA[55] recommend any specific treatment of CVD risk factors in the setting of MASLD.
The current treatment of MASLD/MASH is hampered by the lack of uniform standards, which leads to differences in clinical practice and complicates physician decision-making and international collaboration. In addition, the genetic, metabolic, and lifestyle diversity of patients means that a treatment may work for some but not others, requiring the development of individualized strategies. Although some therapies are effective in the short term, their long-term effectiveness-particularly with medications and lifestyle interventions-requires further investigation, where patient adherence is a major challenge[73].
This lack of consensus leads to variability in clinical practice and highlights the need for evidence-based, multidisciplinary guidelines tailored to this high-risk population.
Long-term adherence to lifestyle modifications and pharmacotherapy remains a significant challenge in MASLD patients. Many patients struggle to maintain dietary changes, regular exercise, and medication regimens due to socioeconomic barriers, lack of motivation, or inadequate education about the disease. Improving adherence requires a patient-centered approach that includes education, behavioral support, and regular follow-up.
The primary objective of lifestyle care is to provide the education, resources and motivation for people with MASLD to adopt and adhere to lifestyle behaviors that will improve and sustain health and wellbeing. Improving diet quality, increasing physical activity, decreasing or abstinence from alcohol consumption, and smoking cessation can have multi
Given the predominance of cardiovascular mortality in patients with MASLD, current guidelines consistently emphasize the importance of early risk assessment and aggressive management of cardiometabolic comorbidities. Rather than addressing MASLD in isolation, an integrated strategy should target the overlapping metabolic and inflammatory me
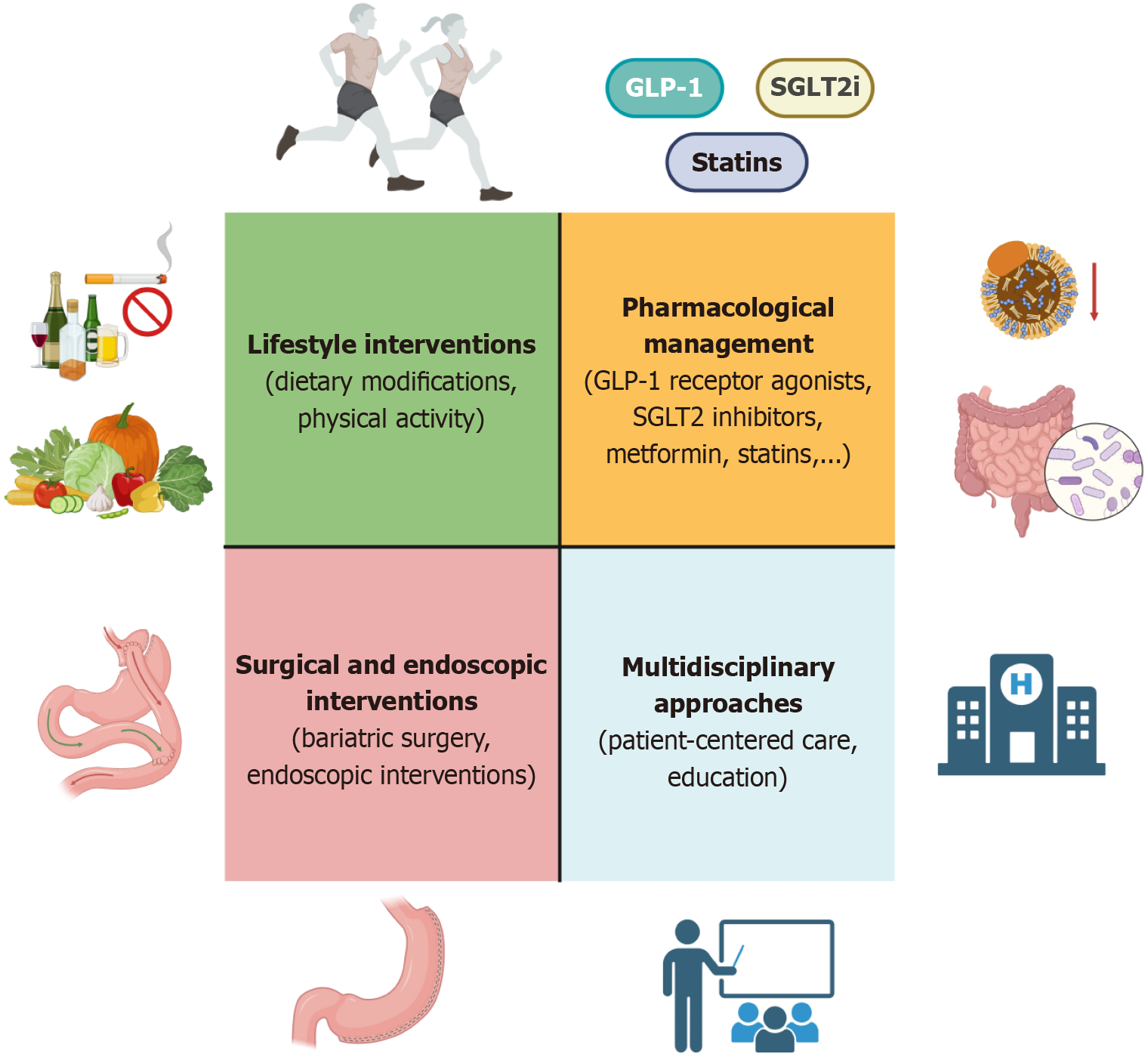
Lifestyle modifications remain the cornerstone of MASLD management, with proven benefits for both hepatic and cardiovascular health. These interventions target the root causes of metabolic dysfunction and are supported by robust clinical evidence.
Dietary modifications: A reduction of 7%-10% in body weight can significantly improve hepatic steatosis, inflammation, and fibrosis[76]. Furthermore, sustained weight loss helps reverse hepatic steatosis, improve insulin sensitivity, and reduce systemic inflammation, thereby lowering cardiovascular risk, a major comorbidity in patients with MASLD[55]. Therefore, dietary modification plays a central role in comprehensive cardiovascular risk management strategies in MASLD patients.
Diets should be individualized according to the patient’s nutritional and metabolic status, while ensuring energy control-typically reducing 500-1000 kcal/day in overweight or obese patients-to achieve a ≥ 7%-10% reduction in initial body weight, which significantly improves hepatic steatosis and related cardiovascular risks[55,77]. Meal composition should prioritize: Complex carbohydrates from whole grains (45%-50% of total energy intake), protein from fish, soy, and lean white meat (20%-25%), and unsaturated fats from olive oil, nuts, and fatty fish like salmon and mackerel (25-30%)[76,78]. Elimination or drastic reduction of simple sugars, trans fats, and ultra-processed foods is essential[77].
Healthcare professionals should actively encourage smoking cessation and the adoption of healthy dietary patterns focused on vegetables, fruits, nuts, and minimally processed whole grains. Increased consumption of leafy greens, lean animal protein, and fish is recommended, while intake of trans fats, red and processed meats, refined carbohydrates, sucrose, fructose, and sugar-sweetened beverages should be limited[55].
Among dietary models, the Mediterranean diet has been shown to be the most effective for patients with MASLD. This diet, rich in leafy vegetables, fruits, legumes, fish, and olive oil, has demonstrated benefits in improving hepatic steatosis, insulin resistance, and cardiovascular risk factors[77,79]. Additionally, the DASH diet is suitable for patients with coexis
Beyond diet, the role of alcohol remains controversial. While some studies suggest that light daily alcohol intake may offer cardiovascular benefits, current data indicate that even light to moderate alcohol consumption can increase the risk of liver disease progression in MASLD patients[77]. Systems biology analyses suggest a synergistic interaction between alcohol consumption and metabolic syndrome that exacerbates the pathogenic pathways of hepatic steatosis[77]. There
In addition, intermittent fasting-including time-restricted eating or alternate-day fasting-has emerged as a promising adjunct strategy. This approach may reduce hepatic triglycerides, improve insulin sensitivity, and regulate blood lipids, thereby enhancing the effectiveness of nutritional interventions[2,77]. Dietary plans should be regularly monitored and adjusted based on clinical and laboratory markers such as weight, waist circumference, liver enzymes [aspartate aminotransferase (AST), alanine aminotransferase (ALT)], blood glucose, lipid profile, and glycated hemoglobin (HbA1c). Patient education on maintaining consistent and long-term healthy eating behaviors is crucial to the effective manage
Physical activity: Physical activity is one of the key strategies with comprehensive effects in managing cardiovascular risk in patients with MASLD. A growing body of evidence indicates that regular physical activity not only supports weight loss but also confers direct metabolic benefits independent of weight reduction.
Regular exercise reduces cardiovascular risk by lowering blood pressure and improving lipid profiles[55]. In patients with type 2 diabetes and NAFLD, it also reduces liver fat content and visceral adiposity, improves body composition, and enhances insulin sensitivity[80]. Additionally, in patients with type 2 diabetes, structured physical activity has been shown to significantly reduce HbA1c levels, further supporting its role in metabolic control[81].
In terms of exercise modalities, both aerobic training (such as brisk walking, running, cycling) and resistance training (such as weightlifting or muscle-strengthening exercises) have demonstrated effectiveness in reducing hepatic fat and improving metabolic parameters. In the RAED2 study, aerobic or resistance training performed three times per week for four months significantly reduced liver fat in patients with T2DM and NAFLD, even in the absence of significant weight loss[80]. According to current ACC/AHA guidelines, adults should engage in at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity activity per week, along with at least two sessions of muscle-streng
In MASLD patients, physical activity offers specific benefits, including reduction of hepatic fat accumulation, slowing progression to steatohepatitis (MASH) and liver fibrosis, and improvement in overall cardiovascular function, largely through enhanced endothelial function and reduced vascular inflammation[80,81]. Moreover, maintaining regular physical activity helps enhance weight loss outcomes and boost the effectiveness of dietary interventions and/or pharmacologic therapy. In lifestyle intervention programs such as the LOOK-AHEAD trial, or in studies combining nutrition and exercise, patients achieved better outcomes in terms of weight reduction, glycemic control, and liver fat reduction than those who received diet modification alone[82,83].
Finally, physical activity plans must be individualized based on each patient's functional capacity and comorbid con
Pharmacological therapies play a critical role in managing MASLD and reducing cardiovascular risk, particularly in patients with comorbidities such as T2DM, hypertension, and dyslipidemia.
GLP-1 receptor agonists: GLP-1 receptor agonists (GLP-1RAs) have evolved beyond their original role in glucose regula
Emerging dual- and multiagonist therapies, such as tirzepatide (GLP-1/GIP agonist) and survodutide (GLP-1/gluca
Interestingly, recent data suggest that GLP-1RAs may also exert their benefits through modulation of the gut-liver axis. These agents appear to influence the gut microbiota composition, thereby improving metabolic health and reducing hepa
Reflecting this evolving evidence base, the EASL–EASD-EASO 2024 Clinical Practice Guidelines recommend GLP-1RAs for patients with MASLD who have comorbid T2DM or obesity. These guidelines highlight the safety of GLP-1RAs even in patients with MASH and compensated cirrhosis and emphasize their positive impact on cardiometabolic out
In summary, GLP-1 receptor agonists offer a unique opportunity to address both hepatic and cardiovascular risks in MASLD through a combination of metabolic regulation, anti-inflammatory effects, cardiovascular protection, and gut–liver axis modulation. The use of these agents should be strongly considered in patients with MASLD who meet standard indications for these agents.
SGLT2 inhibitors: SGLT2 inhibitors are widely recommended for the treatment of T2DM, heart failure, and chronic kidney disease owing to their proven cardiovascular and renal benefits[54,76,96,97]. Specifically, agents such as empa
In addition to their cardiovascular benefits, SGLT2 inhibitors have also demonstrated potential hepatic effects. Several studies have reported reductions in liver fat content and improvements in aminotransferase levels in T2DM patients treated with empagliflozin, dapagliflozin, and licogliflozin[98-102]. Furthermore, in a large Korean cohort involving over 80000 individuals with T2DM and MASLD, SGLT2 inhibitor use was associated with a lower incidence of liver-related events and MASLD regression[103].
However, as no randomized controlled trials (RCTs) with histological liver endpoints are currently available, the EASL-EASD-EASO Clinical Practice Guidelines (2024) do not recommend SGLT2 inhibitors as MASH-specific therapies. Nevertheless, these findings confirm that these agents are safe for use in MASLD patients within their approved indi
Emerging evidence supports the combined use of GLP-1 receptor agonists and SGLT2 inhibitors as a complementary strategy for managing type 2 diabetes with coexisting NAFLD or NASH and elevated cardiovascular risk[104,105]. The SUSTAIN-8 trial further showed that semaglutide and canagliflozin both improved body composition, including reduc
In summary, while SGLT2 inhibitors are not currently indicated as liver-directed therapies, they-especially when used in combination with GLP-1 receptor agonists-offer substantial promise in addressing both cardiovascular and hepatic risk in MASLD.
Metformin: Metformin remains the first-line pharmacologic agent for patients with T2DM, many of whom have co
Several studies have demonstrated that metformin can lower liver fat content and improve liver enzyme levels in patients with MASLD and T2DM[112,113]. However, its effects on reversing fibrosis remain limited. Importantly, metformin has demonstrated cardiovascular protective effects, as supported by major trials such as UKPDS and HOME, which reported significant reductions in cardiovascular events and all-cause mortality in overweight patients with T2DM[114,115]. These benefits are attributed to the ability of metformin to reduce systemic inflammation, improve endothelial function, and lower atherogenic risk factors-mechanisms that are highly relevant in MASLD, where CVD is the leading cause of mortality[114,116].
While current guidelines such as those from the AASLD do not recommend metformin as a treatment for MASLD itself[69], its widespread use in patients with T2DM and metabolic syndrome makes it a pragmatic therapeutic option in MASLD patients with elevated cardiovascular risk. Thus, metformin remains a valuable cornerstone therapy in this population, as it targets both metabolic and vascular pathways that contribute to disease burden.
Statins: Statins play a pivotal role in cardiovascular risk reduction for patients with MASLD, who frequently present with an atherogenic lipid profile and other CMRFs. Clinical guidelines recommend moderate- to high-intensity statin therapy in MASLD patients with dyslipidemia or elevated cardiovascular risk, regardless of liver disease severity-except in cases of decompensated cirrhosis or acute liver failure[2,69,117,118].
Despite these recommendations, statins remain significantly underutilized in clinical practice. Observational studies across multiple healthcare systems report that up to 50% of eligible MASLD patients do not receive statin therapy, often owing to concerns over hepatotoxicity in the setting of elevated transaminases[119-121]. However, these enzyme eleva
The GREACE study demonstrated that patients with elevated baseline transaminases-presumed secondary to MASLD-who received statins experienced significant improvements in liver function tests and reduced cardiovascular events, with < 1% discontinuing therapy owing to hepatotoxicity[123]. A meta-analysis of 13 studies further revealed that statin therapy improved liver enzymes and histological features without worsening fibrosis[125].
In addition to cardiovascular protection, recent evidence supports a potential hepatoprotective role for statins. In a large cohort study by Choi et al[126] involving over 16500 patients with chronic liver disease, statin use was significantly associated with slower progression of liver fibrosis-measured via longitudinal FIB-4 scores-as well as a reduced risk of hepatic decompensation and HCC. These benefits were independent of baseline fibrosis stage and persisted after adjustment for multiple confounders. This real-world evidence highlights the dual benefit of statins in preventing both cardiovascular and liver-related complications in MASLD patients. In support of this, a meta-analysis of over 2 million individuals reported a 46% lower incidence of HCC among statin users, likely attributed to the anti-inflammatory and pleiotropic effects of statins[126].
Although RCTs with histological endpoints are still lacking, accumulating data from real-world studies and meta-analyses consistently support the efficacy and safety of statins in MASLD populations. In cases where LDL-C targets are not met with statin monotherapy, adjunctive agents such as ezetimibe or PCSK9 inhibitors may be considered, although dedicated data in MASLD remain limited[2,69,125].
Proprotein convertase subtilisin/kexin type 9 inhibitors: Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) are recommended for patients at very high cardiovascular risk who either fail to achieve LDL-C targets despite statin-ezetimibe therapy or are intolerant to statins[127]. Major trials such as FOURIER and ODYSSEY OUTCOMES demonstrated significant reductions in MACE with PCSK9i therapy[128,129]. Although evidence in MASLD remains limited, emerging studies suggest potential hepatic benefits. A randomized trial in 40 patients with familial hyperlipidemia showed complete resolution of MASLD after one year of PCSK9i treatment[130]. Similarly, a retrospective review found that 8 of 11 MASLD patients achieved radiologic resolution alongside significant ALT reduction following PCSK9i therapy[131]. These findings suggest a promising, albeit preliminary, role for PCSK9 inhibitors in addressing both hepatic and cardiovascular risks in MASLD.
Peroxisome proliferator-activated receptor agonists: Peroxisome proliferator-activated receptor (PPAR) agonists, including pioglitazone, lanifibranor, and elafibranor, have been explored for their dual benefits on liver and cardiova
Among newer agents, lanifibranor, a pan-PPAR agonist, has shown promising results in a phase IIb trial, with improvements in liver fibrosis and cardiometabolic markers[133,134]. In contrast, elafibranor, another pan-PPAR agonist, failed to demonstrate significant histological benefits in a RESOLVE-IT (NCT02704403) study-a phase III trial[135].
Current EASL-EASD-EASO guidelines consider pioglitazone safe in noncirrhotic MASH and acknowledge the poten
Liver-directed thyroid hormone receptor agonists: Resmetirom, a selective thyroid hormone receptor-β (THR-β) agonist, has emerged as a major breakthrough in the treatment of MASLD. It is the first and currently only agent to demonstrate positive results in a registrational phase III clinical trial (MAESTRO-NASH trial), showing significant histological improvements in non-cirrhotic patients with significant fibrosis (F2-F3)[136].
Based on these robust findings, the United States Food and Drug Administration granted accelerated approval to resmetirom in 2024 for the treatment of non-cirrhotic MASLD patients with fibrosis stages F2–F3[137].
Beyond its histological benefits in the liver, resmetirom has also demonstrated favorable effects on cardiovascular risk markers. In the MAESTRO-NAFLD trial, resmetirom significantly reduced atherogenic lipoproteins, including apoCIII, lipoprotein(a), and VLDL-cholesterol, compared to placebo[138]. These improvements in lipid profiles were consistently observed in the MAESTRO-NASH trial as well[136]. This dual impact-targeting both hepatic pathology and CMRFs-highlights resmetirom’s potential not only to slow liver disease progression but also to improve cardiovascular outcomes in MASLD patients. Although long-term cardiovascular endpoint data are still awaited, current evidence positions resmetirom as a key therapeutic advance in the integrated management of MASLD.
Modulating the gut microbiota is an emerging strategy to improve metabolic health and reduce cardiovascular risk in MASLD. Prebiotics can promote the growth of beneficial bacteria and increase the production of short-chain fatty acids, which help reduce inflammation, improve insulin sensitivity, and lower hepatic lipid accumulation[139]. Additionally, rifaximin-a nonabsorbable antibiotic-has shown beneficial effects on MASLD by reducing AST, ALT, LDL cholesterol, and body mass index (BMI), suggesting systemic cardiometabolic improvements[140,141]. While further studies are needed, gut-targeted therapies may represent a supportive approach for reducing cardiovascular risk in MASLD patients.
The differential hepatocardiac effects of major pharmacologic agents are systematically compared in Table 1, high
| Drug class | Hepatic effects | Cardiovascular effects | Clinical notes |
| GLP-1 receptor agonists | Improves liver histology, reduces inflammation; supports weight loss | Reduces major adverse cardiovascular events | First-line in MASLD with type 2 diabetes mellitus or obesity; safe in compensated cirrhosis |
| SGLT2 inhibitors | Reduces hepatic steatosis, improves liver enzymes | Decreases heart failure hospitalizations, cardiorenal protection | Superior cardiovascular benefits; potential synergy with GLP-1RAs |
| Statins | Safe, may improve liver enzymes | Significantly reduces cardiovascular events | Preferred in MASLD with CV risk; avoid in decompensated cirrhosis |
| THR-β agonists | Markedly improves liver fibrosis | Limited cardiovascular data available | Promising but requires further research |
| PPAR agonists | Improves hepatic inflammation | Variable effects depending on specific agent | Pioglitazone beneficial in diabetic patients |
| Metformin | No improvement in liver histology | Beneficial for diabetic patients | Not recommended for MASLD alone |
Bariatric (metabolic) surgery is increasingly recognized as an effective strategy for improving both hepatic and cardiova
Current guidelines recommend considering bariatric surgery in noncirrhotic MASLD patients with a BMI ≥ 40 kg/m² or ≥ 35 kg/m² with comorbidities and even in patients with a BMI ≥ 30 kg/m² with poorly controlled T2DM or hyper
In contrast, endoscopic bariatric therapies-such as intragastric balloon placement, endoscopic sleeve gastroplasty, or duodenal mucosal resurfacing-offer a minimally invasive alternative for patients who are not surgical candidates or prefer nonsurgical options. These procedures have resulted in moderate improvements in hepatic steatosis, insulin resistance, and cardiovascular risk markers[148,149]. However, owing to limited long-term and histology-based data, they are not yet recommended as standard therapies for MASLD, and further studies are warranted.
In summary, bariatric and emerging endoscopic interventions offer promising cardiometabolic benefits in MASLD and should be considered in appropriately selected patients to reduce cardiovascular risk and liver disease progression.
To effectively manage MASLD and its cardiovascular complications, an integrated and multidisciplinary approach is essential. This strategy ensures that both hepatic and cardiovascular health are addressed simultaneously, optimizing patient outcomes.
The need for multidisciplinary collaboration: Effective management of MASLD and CVD requires close collaboration between hepatologists, cardiologists, endocrinologists, and primary care providers. A multidisciplinary team can develop comprehensive care plans that address the full spectrum of metabolic, hepatic, and cardiovascular risks. For example, hepatologists can monitor liver health and fibrosis progression, whereas cardiologists focus on reducing cardiovascular risk through targeted therapies.
The link between MASLD and cardiometabolic disease is gaining attention through articles, research groups, and awa
Patient-centered care: A patient-centered approach is essential for improving outcomes in MASLD management. This begins with a comprehensive assessment that not only evaluates liver and cardiovascular health but also considers psychosocial factors such as mental well-being, social support, and lifestyle constraints-all of which can influence treatment adherence.
Personalized treatment plans should be developed on the basis of the individual’s specific clinical profile, preferences, and comorbidities. For example, patients with advanced fibrosis may benefit from more aggressive monitoring and therapy than patients with simple steatosis. Tailoring the approach in this way ensures that interventions are both clinically appropriate and aligned with the patient’s goals.
Equally important is shared decision-making. Engaging patients in discussions about their treatment options and expected outcomes empowers them to take an active role in managing their condition, which is linked to improved satisfaction and long-term adherence.
The evidence supports the role of behavioral therapy in helping patients modify their dietary habits, increase their physical activity, and strengthen their self-management skills. These strategies are effective in achieving sustainable weight loss and improving the histological features of MASH. Moreover, addressing psychosocial barriers—such as anxiety, depression, or lack of support-can further enhance motivation and treatment success[73,150,151].
The role of education and awareness: Educating patients about the interconnection between MASLD and CVD is essential to promote self-management, improve treatment adherence, and empower individuals to take an active role in reducing their cardiometabolic risk. Rather than offering generic advice to lose weight, healthcare providers should support patients in adopting sustainable lifestyles and behavioral changes-starting with the early identification of barriers to effective weight management[152].
Dietary and physical activity recommendations should be personalized to align with each patient’s medical condition, preferences, and socioeconomic context, thereby increasing the likelihood of long-term adherence[152]. Patients should be clearly informed that lifestyle modifications and appropriate pharmacotherapy can yield dual benefits-ameliorating liver steatosis while also improving cardiovascular health. Equally important is ensuring that healthcare professionals are equipped with up-to-date knowledge and clinical guidelines to provide consistent, evidence-based care.
Closing the gaps in MASLD and CVD management requires ongoing research to address unanswered questions and develop innovative solutions, with several areas identified as key opportunities for advancing care and improving patient outcomes.
Future research should focus on creating MASLD-specific risk assessment tools that incorporate liver-specific markers
New noninvasive prediction methods, such as high-resolution computed tomography, magnetic resonance imaging, and biomarkers (FIB-4, FAST), are opening new opportunities for the early detection of MASLD and the assessment of complications such as osteoporosis, cirrhosis, and hepatitis[153-156]. However, the effectiveness and stability of these technologies need to be further validated through large-scale studies.
While several emerging pharmacologic agents-such as GLP-1 receptor agonists, SGLT2 inhibitors, PPAR agonists, and THR-β agonists-have demonstrated promising dual benefits in terms of liver histology and cardiometabolic parameters, their long-term efficacy in reducing cardiovascular events in patients with MASLD remains uncertain. Most available data are derived from short- to midterm studies, surrogate markers, or extrapolated cardiovascular outcomes in broader diabetic or obese populations.
Currently, there is a critical lack of large-scale RCTs that directly evaluate the impact of these therapies on cardiova
Future research must prioritize well-designed, long-term outcome trials that include both hepatic and cardiovascular end points. This is essential not only for confirming the dual-organ efficacy of emerging treatments but also for guiding integrated therapeutic strategies for MASLD patients, who remain at high residual cardiovascular risk despite advances in pharmacotherapy.
Research is needed to identify the most effective lifestyle interventions for MASLD patients. A "one-size-fits-all" approach to lifestyle interventions is unlikely to be effective. Lifestyle intervention for MAFLD should be based on a comprehensive 24-hour strategy that simultaneously integrates diet, physical activity and exercise; reducing sedentary time; smoking; alcohol restriction; and improved sleep[157]. Research should explore the role of culturally tailored interventions and behavioral support programs in promoting long-term adherence to dietary and exercise recommendations.
Digital health technologies present promising opportunities to transform the management of MASLD by supporting behavior change, facilitating risk stratification, and enabling continuous care. Mobile applications and wearable devices allow real-time tracking of dietary intake, physical activity, and medication adherence, thereby offering patients personalized feedback and motivation. Systematic reviews and meta-analyses have demonstrated the effectiveness of these digital interventions in improving weight-related outcomes and promoting lifestyle modification[158-160].
In addition, artificial intelligence (AI) has potential for developing risk prediction models by integrating clinical, genetic, and behavioral data to identify high-risk individuals and personalize treatment strategies. Such AI-based tools may support earlier diagnosis and tailored interventions, improving both hepatic and cardiovascular outcomes. Fur
MASLD has emerged as a pivotal metabolic disorder with far-reaching cardiovascular implications, necessitating a fundamental rethinking of its clinical management. The recognition of shared pathophysiological pathways has catalyzed the development of dual-purpose therapies, from established agents like GLP-1RAs and SGLT2 inhibitors to groundbreaking liver-targeted treatments such as resmetirom. However, the field continues to grapple with significant challenges-particularly the absence of validated cardiovascular risk stratification tools specific to MASLD populations and insufficient long-term outcome data for emerging pharmacotherapies. These knowledge gaps underscore the urgent need for collaborative, multidisciplinary frameworks that integrate hepatology and cardiology perspectives. Moving forward, the research and clinical communities must prioritize the development of precision risk assessment methodologies, rigorous evaluation of therapeutic interventions through prospective trials, and implementation of integrated care pathways. By addressing these priorities, we can transform the current fragmented approach into a cohesive strategy that effectively mitigates the substantial cardiovascular burden borne by MASLD patients worldwide.
We would like to express our sincere gratitude to all the authors for their dedication and efforts in completing this study. We are especially thankful to Duong Hung Tran for his valuable support in formatting and editing the manuscript. We also extend our appreciation to the Heart and Metabolic Innovations Research Team (HAMIRT) for their collaborative spirit and meaningful contributions to this research.
| 1. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1212] [Cited by in RCA: 1313] [Article Influence: 656.5] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 437] [Article Influence: 437.0] [Reference Citation Analysis (1)] |
| 3. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1428] [Article Influence: 714.0] [Reference Citation Analysis (2)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7533] [Article Influence: 837.0] [Reference Citation Analysis (0)] |
| 5. | Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Liu C, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809-2817.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 398] [Article Influence: 132.7] [Reference Citation Analysis (2)] |
| 6. | Mellemkjær A, Kjær MB, Haldrup D, Grønbæk H, Thomsen KL. Management of cardiovascular risk in patients with metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med. 2024;122:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 7. | Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 803] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 8. | Zhou XD, Targher G, Byrne CD, Somers V, Kim SU, Chahal CAA, Wong VW, Cai J, Shapiro MD, Eslam M, Steg PG, Sung KC, Misra A, Li JJ, Brotons C, Huang Y, Papatheodoridis GV, Sun A, Yilmaz Y, Chan WK, Huang H, Méndez-Sánchez N, Alqahtani SA, Cortez-Pinto H, Lip GYH, de Knegt RJ, Ocama P, Romero-Gomez M, Fudim M, Sebastiani G, Son JW, Ryan JD, Ikonomidis I, Treeprasertsuk S, Pastori D, Lupsor-Platon M, Tilg H, Ghazinyan H, Boursier J, Hamaguchi M, Nguyen MH, Fan JG, Goh GB, Al Mahtab M, Hamid S, Perera N, George J, Zheng MH. An international multidisciplinary consensus statement on MAFLD and the risk of CVD. Hepatol Int. 2023;17:773-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 9. | Dang HNN, Luong TV, Tran TT, Hoang TA. The correlation between liver fibrosis and the 10-year estimated risk of cardiovascular disease in adults with metabolic-associated fatty liver disease: A cross-sectional study in Vietnam. Health Sci Rep. 2024;7:e2102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Fiorentino TV, Succurro E, Sciacqua A, Andreozzi F, Perticone F, Sesti G. Non-alcoholic fatty liver disease is associated with cardiovascular disease in subjects with different glucose tolerance. Diabetes Metab Res Rev. 2020;36:e3333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Hassen G, Singh A, Belete G, Jain N, De la Hoz I, Camacho-Leon GP, Dargie NK, Carrera KG, Alemu T, Jhaveri S, Solomon N. Nonalcoholic Fatty Liver Disease: An Emerging Modern-Day Risk Factor for Cardiovascular Disease. Cureus. 2022;14:e25495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 12. | Cai J, Zhang XJ, Ji YX, Zhang P, She ZG, Li H. Nonalcoholic Fatty Liver Disease Pandemic Fuels the Upsurge in Cardiovascular Diseases. Circ Res. 2020;126:679-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 13. | Yanai H, Adachi H, Hakoshima M, Iida S, Katsuyama H. Metabolic-Dysfunction-Associated Steatotic Liver Disease-Its Pathophysiology, Association with Atherosclerosis and Cardiovascular Disease, and Treatments. Int J Mol Sci. 2023;24:15473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 14. | Al Hashmi K, Giglio RV, Pantea Stoian A, Patti AM, Al Waili K, Al Rasadi K, Ciaccio M, Rizzo M. Metabolic dysfunction-associated fatty liver disease: current therapeutic strategies. Front Nutr. 2024;11:1355732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 16. | Galatou E, Mourelatou E, Hatziantoniou S, Vizirianakis IS. Nonalcoholic Steatohepatitis (NASH) and Atherosclerosis: Explaining Their Pathophysiology, Association and the Role of Incretin-Based Drugs. Antioxidants (Basel). 2022;11:1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 18. | Jamalinia M, Zare F, Lankarani KB. Systematic review and meta-analysis: Association between liver fibrosis and subclinical atherosclerosis in nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2023;58:384-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Kim H, Lee CJ, Ahn SH, Lee KS, Lee BK, Baik SJ, Kim SU, Lee JI. MAFLD Predicts the Risk of Cardiovascular Disease Better than NAFLD in Asymptomatic Subjects with Health Check-Ups. Dig Dis Sci. 2022;67:4919-4928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, Gonzalez-Fabian L, Alvarez-Quiñones Sanz M, Conde-Martin AF, De Boer B, McLeod D, Hung Chan AW, Chalasani N, George J, Adams LA, Romero-Gomez M. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155:443-457.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 600] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 21. | Chang Y, Ryu S, Sung KC, Cho YK, Sung E, Kim HN, Jung HS, Yun KE, Ahn J, Shin H, Wild SH, Byrne CD. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the Kangbuk Samsung Health Study. Gut. 2019;68:1667-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 22. | Lv Q, Zhao H. The association of metabolic dysfunction-associated steatotic liver disease (MASLD) with the risk of myocardial infarction: a systematic review and meta-analysis. Ann Med. 2024;56:2306192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 23. | Mantovani A, Petracca G, Csermely A, Beatrice G, Bonapace S, Rossi A, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut. 2022;gutjnl-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 24. | Vieira Barbosa J, Milligan S, Frick A, Broestl J, Younossi Z, Afdhal N, Lai M. Fibrosis-4 Index Can Independently Predict Major Adverse Cardiovascular Events in Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2022;117:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Fudim M, Zhong L, Patel KV, Khera R, Abdelmalek MF, Diehl AM, McGarrah RW, Molinger J, Moylan CA, Rao VN, Wegermann K, Neeland IJ, Halm EA, Das SR, Pandey A. Nonalcoholic Fatty Liver Disease and Risk of Heart Failure Among Medicare Beneficiaries. J Am Heart Assoc. 2021;10:e021654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Chang KC, Su TH, Wu CK, Huang SC, Tseng TC, Hong CM, Hsu SJ, Liu CH, Yang HC, Liu CJ, Kao JH. Metabolic dysfunction-associated steatotic liver disease is associated with increased risks of heart failure. Eur J Heart Fail. 2025;27:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 27. | VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME, Shah SJ, Lima JAC, Lloyd-Jones DM. Longitudinal Association of Non-Alcoholic Fatty Liver Disease With Changes in Myocardial Structure and Function: The CARDIA Study. J Am Heart Assoc. 2020;9:e014279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 28. | Wijarnpreecha K, Lou S, Panjawatanan P, Cheungpasitporn W, Pungpapong S, Lukens FJ, Ungprasert P. Association between diastolic cardiac dysfunction and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig Liver Dis. 2018;50:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Lee YH, Kim KJ, Yoo ME, Kim G, Yoon HJ, Jo K, Youn JC, Yun M, Park JY, Shim CY, Lee BW, Kang SM, Ha JW, Cha BS, Kang ES. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J Hepatol. 2018;68:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Peters AE, Pandey A, Ayers C, Wegermann K, McGarrah RW, Grodin JL, Abdelmalek MF, Bekfani T, Blumer V, Diehl AM, Moylan CA, Fudim M. Association of liver fibrosis risk scores with clinical outcomes in patients with heart failure with preserved ejection fraction: findings from TOPCAT. ESC Heart Fail. 2021;8:842-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, Lewis CE, Rinella ME, Shah SJ. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology. 2015;62:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 32. | Byrne CD, Targher G. Non-alcoholic fatty liver disease-related risk of cardiovascular disease and other cardiac complications. Diabetes Obes Metab. 2022;24 Suppl 2:28-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 33. | Mantovani A, Rigamonti A, Bonapace S, Bolzan B, Pernigo M, Morani G, Franceschini L, Bergamini C, Bertolini L, Valbusa F, Rigolon R, Pichiri I, Zoppini G, Bonora E, Violi F, Targher G. Nonalcoholic Fatty Liver Disease Is Associated With Ventricular Arrhythmias in Patients With Type 2 Diabetes Referred for Clinically Indicated 24-Hour Holter Monitoring. Diabetes Care. 2016;39:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Ionescu VA, Gheorghe G, Bacalbasa N, Diaconu CC. Metabolic Dysfunction-Associated Steatotic Liver Disease: Pathogenetic Links to Cardiovascular Risk. Biomolecules. 2025;15:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 35. | Min BH, Devi S, Kwon GH, Gupta H, Jeong JJ, Sharma SP, Won SM, Oh KK, Yoon SJ, Park HJ, Eom JA, Jeong MK, Hyun JY, Stalin N, Park TS, Choi J, Lee DY, Han SH, Kim DJ, Suk KT. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes. 2024;16:2307568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 36. | Abdallah LR, de Matos RC, E Souza YPDM, Vieira-Soares D, Muller-Machado G, Pollo-Flores P. Non-alcoholic Fatty Liver Disease and Its Links with Inflammation and Atherosclerosis. Curr Atheroscler Rep. 2020;22:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Cho YK, Kang YM, Yoo JH, Lee J, Lee SE, Yang DH, Kang JW, Park JY, Jung CH, Kim HK, Lee WJ. The impact of non-alcoholic fatty liver disease and metabolic syndrome on the progression of coronary artery calcification. Sci Rep. 2018;8:12004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Tang Y, Fan J, Hou X, Wu H, Zhang J, Wu J, Wang Y, Zhang Z, Lu B, Zheng J. Metabolic dysfunction-associated steatotic liver disease and increased risk of atrial fibrillation in the elderly: A longitudinal cohort study. Int J Cardiol Heart Vasc. 2025;58:101676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Mahfouz RA, Gouda M, Galal I, Ghareb MS. Interatrial septal fat thickness and left atrial stiffness are mechanistic links between nonalcoholic fatty liver disease and incident atrial fibrillation. Echocardiography. 2019;36:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Ferenc K, Jarmakiewicz-Czaja S, Sokal-Dembowska A, Stasik K, Filip R. Common Denominator of MASLD and Some Non-Communicable Diseases. Curr Issues Mol Biol. 2024;46:6690-6709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Wijarnpreecha K, Panjawatanan P, Kroner PT, Cheungpasitporn W, Ungprasert P. Association between cardiac conduction defect and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Ann Gastroenterol. 2020;33:661-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Chen Z, Liu J, Zhou F, Li H, Zhang XJ, She ZG, Lu Z, Cai J, Li H. Nonalcoholic Fatty Liver Disease: An Emerging Driver of Cardiac Arrhythmia. Circ Res. 2021;128:1747-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 43. | Di Minno MN, Di Minno A, Ambrosino P, Songia P, Tremoli E, Poggio P. Aortic valve sclerosis as a marker of atherosclerosis: Novel insights from hepatic steatosis. Int J Cardiol. 2016;217:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Hao QY, Zeng YH, Lin Y, Guo JB, Li SC, Yang PZ, Gao JW, Li ZH. Observational and genetic association of non-alcoholic fatty liver disease and calcific aortic valve disease. Front Endocrinol (Lausanne). 2024;15:1421642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Long MT, Wang N, Larson MG, Mitchell GF, Palmisano J, Vasan RS, Hoffmann U, Speliotes EK, Vita JA, Benjamin EJ, Fox CS, Hamburg NM. Nonalcoholic fatty liver disease and vascular function: cross-sectional analysis in the Framingham heart study. Arterioscler Thromb Vasc Biol. 2015;35:1284-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Boccatonda A, D'Ardes D, Moronti V, Santilli J, Cipollone A, Lessiani G, Di Gregorio N, Serra C, Piscaglia F, Ferri C, Cipollone F. From MASLD to PAD: Looking for Cardiovascular Disease Starting from Metabolic Status. Medicina (Kaunas). 2024;60:1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 47. | Taharboucht S, Guermaz R, Brouri M, Bengherbia L, Chibane A. Ankle systolic pressure index in non-diabetic non-alcoholic fatty liver disease: A case-control study. J Med Vasc. 2023;48:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Ismaeel A, Brumberg RS, Kirk JS, Papoutsi E, Farmer PJ, Bohannon WT, Smith RS, Eidson JL, Sawicki I, Koutakis P. Oxidative Stress and Arterial Dysfunction in Peripheral Artery Disease. Antioxidants (Basel). 2018;7:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 49. | Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. 2016;65:425-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 369] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 50. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 51. | Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 52. | Peyter AC, Armengaud JB, Guillot E, Yzydorczyk C. Endothelial Progenitor Cells Dysfunctions and Cardiometabolic Disorders: From Mechanisms to Therapeutic Approaches. Int J Mol Sci. 2021;22:6667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 53. | Denimal D, Ponnaiah M, Phan F, Jeannin AC, Redheuil A, Salem JE, Boussouar S, Paulstephenraj P, Laroche S, Amouyal C, Hartemann A, Foufelle F, Bourron O. Metabolic dysfunction-associated steatotic liver disease (MASLD) biomarkers and progression of lower limb arterial calcification in patients with type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol. 2025;24:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 7266] [Article Influence: 1816.5] [Reference Citation Analysis (0)] |
| 55. | Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563-e595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 505] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 56. | Treeprasertsuk S, Leverage S, Adams LA, Lindor KD, St Sauver J, Angulo P. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int. 2012;32:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 57. | Golabi P, Fukui N, Paik J, Sayiner M, Mishra A, Younossi ZM. Mortality Risk Detected by Atherosclerotic Cardiovascular Disease Score in Patients With Nonalcoholic Fatty Liver Disease. Hepatol Commun. 2019;3:1050-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 58. | SCORE2 working group and ESC Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42:2439-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 770] [Article Influence: 192.5] [Reference Citation Analysis (0)] |
| 59. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1004] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 60. | Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11 Suppl 1:S209-S216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 61. | Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 62. | Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH, Santos RD, Budoff MJ, Nasir K. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 63. | Faasse S, Braun H, Vos M. The role of NAFLD in cardiometabolic disease: an update. F1000Res. 2018;7:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Pais R, Giral P, Khan JF, Rosenbaum D, Housset C, Poynard T, Ratziu V; LIDO Study Group. Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol. 2016;65:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 65. | Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B; ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3739] [Cited by in RCA: 3312] [Article Influence: 828.0] [Reference Citation Analysis (0)] |
| 66. | Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, Cohen DE, Horton JD, Pressman GS, Toth PP; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; Council on the Kidney in Cardiovascular Disease; Council on Lifestyle and Cardiometabolic Health; and Council on Peripheral Vascular Disease. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e168-e185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 350] [Article Influence: 116.7] [Reference Citation Analysis (0)] |
| 67. | Nabi O, Lapidus N, Boursier J, de Ledinghen V, Petit JM, Kab S, Renuy A, Zins M, Lacombe K, Serfaty L. Lean individuals with NAFLD have more severe liver disease and poorer clinical outcomes (NASH-CO Study). Hepatology. 2023;78:272-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 68. | Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Angelantonio ED, Franco OH, Halvorsen S, Richard Hobbs FD, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Rev Esp Cardiol (Engl Ed). 2022;75:429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 69. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 1157] [Article Influence: 578.5] [Reference Citation Analysis (1)] |
| 70. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3179] [Article Influence: 353.2] [Reference Citation Analysis (4)] |
| 71. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 505] [Article Influence: 56.1] [Reference Citation Analysis (2)] |
| 72. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 569] [Article Influence: 189.7] [Reference Citation Analysis (1)] |
| 73. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1625] [Article Influence: 162.5] [Reference Citation Analysis (1)] |
| 74. | Younossi ZM, Zelber-Sagi S, Henry L, Gerber LH. Lifestyle interventions in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2023;20:708-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 143] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 75. | Koutoukidis DA, Astbury NM, Tudor KE, Morris E, Henry JA, Noreik M, Jebb SA, Aveyard P. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA Intern Med. 2019;179:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 76. | Van Gaal L, Dirinck E. Pharmacological Approaches in the Treatment and Maintenance of Weight Loss. Diabetes Care. 2016;39 Suppl 2:S260-S267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Åberg F, Byrne CD, Pirola CJ, Männistö V, Sookoian S. Alcohol consumption and metabolic syndrome: Clinical and epidemiological impact on liver disease. J Hepatol. 2023;78:191-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 139] [Reference Citation Analysis (0)] |
| 78. | Fappi A, Mittendorfer B. Dietary protein intake and obesity-associated cardiometabolic function. Curr Opin Clin Nutr Metab Care. 2020;23:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Abenavoli L, Boccuto L, Federico A, Dallio M, Loguercio C, Di Renzo L, De Lorenzo A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int J Environ Res Public Health. 2019;16:3011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 80. | Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, Zanolin E, Schena F, Bonora E, Moghetti P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology. 2013;58:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 81. | Lampsas S, Marinos G, Lambrinos D, Theofilis P, Gialamas I, Pantelidis P, Zakynthinos GE, Kalogera V, Pililis S, Korakas E, Lambadiari V, Papavassiliou KA, Oikonomou E, Siasos G. Physical Activity Habits Among Physicians: Data From the Athens Medical Association. Am J Lifestyle Med. 2024;15598276241267213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 82. | Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 340] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 83. | Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, Wagenknecht LE, Pi-Sunyer FX, Kahn SE, Clark JM; Fatty Liver Subgroup of the Look AHEAD Research Group. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 84. | Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1574] [Article Influence: 157.4] [Reference Citation Analysis (0)] |
| 85. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1469] [Article Influence: 163.2] [Reference Citation Analysis (1)] |
| 86. | Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 2192] [Article Influence: 548.0] [Reference Citation Analysis (0)] |
| 87. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3025] [Cited by in RCA: 4071] [Article Influence: 452.3] [Reference Citation Analysis (1)] |
| 88. | Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, Hardt-Lindberg S, Hovingh GK, Kahn SE, Kushner RF, Lingvay I, Oral TK, Michelsen MM, Plutzky J, Tornøe CW, Ryan DH; SELECT Trial Investigators. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N Engl J Med. 2023;389:2221-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1295] [Cited by in RCA: 1099] [Article Influence: 549.5] [Reference Citation Analysis (0)] |
| 89. | Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 980] [Article Influence: 245.0] [Reference Citation Analysis (0)] |
| 90. | Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, Mao H, Zhang S, Ahmad NN, Bunck MC, Benabbad I, Zhang XM; SURMOUNT-2 investigators. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 291] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 91. | Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A; SURMOUNT-1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022;387:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 1515] [Article Influence: 505.0] [Reference Citation Analysis (0)] |
| 92. | Loomba R, Hartman ML, Lawitz EJ, Vuppalanchi R, Boursier J, Bugianesi E, Yoneda M, Behling C, Cummings OW, Tang Y, Brouwers B, Robins DA, Nikooie A, Bunck MC, Haupt A, Sanyal AJ; SYNERGY-NASH Investigators. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N Engl J Med. 2024;391:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 243] [Article Influence: 243.0] [Reference Citation Analysis (0)] |
| 93. | le Roux CW, Steen O, Lucas KJ, Startseva E, Unseld A, Hennige AM. Glucagon and GLP-1 receptor dual agonist survodutide for obesity: a randomised, double-blind, placebo-controlled, dose-finding phase 2 trial. Lancet Diabetes Endocrinol. 2024;12:162-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 94. | Sanyal AJ, Bedossa P, Fraessdorf M, Neff GW, Lawitz E, Bugianesi E, Anstee QM, Hussain SA, Newsome PN, Ratziu V, Hosseini-Tabatabaei A, Schattenberg JM, Noureddin M, Alkhouri N, Younes R; 1404-0043 Trial Investigators. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis. N Engl J Med. 2024;391:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 147] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 95. | Boutari C, DeMarsilis A, Mantzoros CS. Obesity and diabetes. Diabetes Res Clin Pract. 2023;202:110773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 96. | Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, Christodorescu RM, Crawford C, Di Angelantonio E, Eliasson B, Espinola-Klein C, Fauchier L, Halle M, Herrington WG, Kautzky-Willer A, Lambrinou E, Lesiak M, Lettino M, McGuire DK, Mullens W, Rocca B, Sattar N; ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44:4043-4140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 620] [Article Influence: 310.0] [Reference Citation Analysis (0)] |
| 97. | Kahl S, Gancheva S, Straßburger K, Herder C, Machann J, Katsuyama H, Kabisch S, Henkel E, Kopf S, Lagerpusch M, Kantartzis K, Kupriyanova Y, Markgraf D, van Gemert T, Knebel B, Wolkersdorfer MF, Kuss O, Hwang JH, Bornstein SR, Kasperk C, Stefan N, Pfeiffer A, Birkenfeld AL, Roden M. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care. 2020;43:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 98. | Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398:262-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 310] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 99. | Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care. 2018;41:1801-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 431] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 100. | Latva-Rasku A, Honka MJ, Kullberg J, Mononen N, Lehtimäki T, Saltevo J, Kirjavainen AK, Saunavaara V, Iozzo P, Johansson L, Oscarsson J, Hannukainen JC, Nuutila P. The SGLT2 Inhibitor Dapagliflozin Reduces Liver Fat but Does Not Affect Tissue Insulin Sensitivity: A Randomized, Double-Blind, Placebo-Controlled Study With 8-Week Treatment in Type 2 Diabetes Patients. Diabetes Care. 2019;42:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 101. | Harrison SA, Manghi FP, Smith WB, Alpenidze D, Aizenberg D, Klarenbeek N, Chen CY, Zuckerman E, Ravussin E, Charatcharoenwitthaya P, Cheng PN, Katchman H, Klein S, Ben-Ari Z, Mendonza AE, Zhang Y, Martic M, Ma S, Kao S, Tanner S, Pachori A, Badman MK, He Y, Ukomadu C, Sicard E. Licogliflozin for nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a study. Nat Med. 2022;28:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 102. | Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC; EMPA-REG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 103. | Jang H, Kim Y, Lee DH, Joo SK, Koo BK, Lim S, Lee W, Kim W. Outcomes of Various Classes of Oral Antidiabetic Drugs on Nonalcoholic Fatty Liver Disease. JAMA Intern Med. 2024;184:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 45] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 104. | Ludvik B, Frías JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, García-Pérez LE, Woodward DB, Milicevic Z. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 105. | Sumida Y, Yoneda M, Tokushige K, Kawanaka M, Fujii H, Yoneda M, Imajo K, Takahashi H, Eguchi Y, Ono M, Nozaki Y, Hyogo H, Koseki M, Yoshida Y, Kawaguchi T, Kamada Y, Okanoue T, Nakajima A, Jsg-Nafld JSGON. Antidiabetic Therapy in the Treatment of Nonalcoholic Steatohepatitis. Int J Mol Sci. 2020;21:1907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 106. | McCrimmon RJ, Catarig AM, Frias JP, Lausvig NL, le Roux CW, Thielke D, Lingvay I. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia. 2020;63:473-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 107. | Ozturk A, Olson MC, Samir AE, Venkatesh SK. Liver fibrosis assessment: MR and US elastography. Abdom Radiol (NY). 2022;47:3037-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 108. | Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - Where do we stand? World J Gastroenterol. 2016;22:7236-7251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 109. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 110. | Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: Past, present and future. J Hepatol. 2022;76:1362-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 141] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 111. | Mazza A, Fruci B, Garinis GA, Giuliano S, Malaguarnera R, Belfiore A. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012:716404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 112. | Lavine JE, Schwimmer JB, Molleston JP, Scheimann AO, Murray KF, Abrams SH, Rosenthal P, Sanyal AJ, Robuck PR, Brunt EM, Unalp A, Tonascia J; Nonalcoholic Steatohepatitis Clinical Research Network Research Group. Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemp Clin Trials. 2010;31:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 113. | Huang Y, Wang X, Yan C, Li C, Zhang L, Zhang L, Liang E, Liu T, Mao J. Effect of metformin on nonalcoholic fatty liver based on meta-analysis and network pharmacology. Medicine (Baltimore). 2022;101:e31437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 114. | King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 383] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 115. | De Jager J, Kooy A, Lehert P, Bets D, Wulffelé MG, Teerlink T, Scheffer PG, Schalkwijk CG, Donker AJ, Stehouwer CD. Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med. 2005;257:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 116. | Fruci B, Giuliano S, Mazza A, Malaguarnera R, Belfiore A. Nonalcoholic Fatty liver: a possible new target for type 2 diabetes prevention and treatment. Int J Mol Sci. 2013;14:22933-22966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 117. | Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 1171] [Article Influence: 167.3] [Reference Citation Analysis (0)] |
| 118. | Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 5336] [Article Influence: 1067.2] [Reference Citation Analysis (0)] |
| 119. | Thomson MJ, Serper M, Khungar V, Weiss LM, Trinh H, Firpi-Morell R, Roden M, Loomba R, Barritt AS 4th, Gazis D, Mospan AR, Fried MW, Reddy KR, Lok AS. Prevalence and Factors Associated With Statin Use Among Patients With Nonalcoholic Fatty Liver Disease in the TARGET-NASH Study. Clin Gastroenterol Hepatol. 2022;20:458-460.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 120. | Blais P, Lin M, Kramer JR, El-Serag HB, Kanwal F. Statins Are Underutilized in Patients with Nonalcoholic Fatty Liver Disease and Dyslipidemia. Dig Dis Sci. 2016;61:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 121. | Del Ben M, Baratta F, Polimeni L, Pastori D, Loffredo L, Averna M, Violi F, Angelico F. Under-prescription of statins in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2017;27:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 122. | Calderon RM, Cubeddu LX, Goldberg RB, Schiff ER. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: a therapeutic dilemma. Mayo Clin Proc. 2010;85:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 123. | Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 124. | Bays H, Cohen DE, Chalasani N, Harrison SA; The National Lipid Association's Statin Safety Task Force. An assessment by the Statin Liver Safety Task Force: 2014 update. J Clin Lipidol. 2014;8:S47-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 125. | Boutari C, Pappas PD, Anastasilakis D, Mantzoros CS. Statins' efficacy in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin Nutr. 2022;41:2195-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 126. | Choi J, Nguyen VH, Przybyszewski E, Song J, Carroll A, Michta M, Almazan E, Simon TG, Chung RT. Statin Use and Risk of Hepatocellular Carcinoma and Liver Fibrosis in Chronic Liver Disease. JAMA Intern Med. 2025;185:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 127. | Zeitouni M, Sabouret P, Kerneis M, Silvain J, Collet JP, Bruckert E, Montalescot G. 2019 ESC/EAS Guidelines for management of dyslipidaemia: strengths and limitations. Eur Heart J Cardiovasc Pharmacother. 2021;7:324-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 128. | Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3898] [Cited by in RCA: 4035] [Article Influence: 504.4] [Reference Citation Analysis (0)] |
| 129. | Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2585] [Cited by in RCA: 2287] [Article Influence: 326.7] [Reference Citation Analysis (0)] |
| 130. | Dimakopoulou A, Sfikas G, Athyros V. PCSK9 administration ameliorates non alcoholic fatty disease in patients with heterozygous familial hyperlipidemia. Hellenic J Atheroscler. 9;1-2. |
| 131. | Shafiq M, Walmann T, Nutalapati V, Gibson C, Zafar Y. Effects of proprotein convertase subtilisin/kexin type-9 inhibitors on fatty liver. World J Hepatol. 2020;12:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (2)] |
| 132. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1318] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 133. | Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, Loomba R, Harrison SA, Balabanska R, Mateva L, Lanthier N, Alkhouri N, Moreno C, Schattenberg JM, Stefanova-Petrova D, Vonghia L, Rouzier R, Guillaume M, Hodge A, Romero-Gómez M, Huot-Marchand P, Baudin M, Richard MP, Abitbol JL, Broqua P, Junien JL, Abdelmalek MF; NATIVE Study Group. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med. 2021;385:1547-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 448] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 134. | Cooreman MP, Butler J, Giugliano RP, Zannad F, Dzen L, Huot-Marchand P, Baudin M, Beard DR, Junien JL, Broqua P, Abdelmalek MF, Francque SM. The pan-PPAR agonist lanifibranor improves cardiometabolic health in patients with metabolic dysfunction-associated steatohepatitis. Nat Commun. 2024;15:3962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 135. | Albhaisi S, Sanyal AJ. Pharmacology of NASH. Compr Pharmacol 2022. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 136. | Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW, Rinella ME, Anstee QM, Abdelmalek MF, Younossi Z, Baum SJ, Francque S, Charlton MR, Newsome PN, Lanthier N, Schiefke I, Mangia A, Pericàs JM, Patil R, Sanyal AJ, Noureddin M, Bansal MB, Alkhouri N, Castera L, Rudraraju M, Ratziu V; MAESTRO-NASH Investigators. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med. 2024;390:497-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 723] [Article Influence: 723.0] [Reference Citation Analysis (0)] |
| 137. | Kingwell K. NASH field celebrates 'hurrah moment' with a first FDA drug approval for the liver disease. Nat Rev Drug Discov. 2024;23:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 138. | Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, Moussa SE, Alkhouri N, Bashir MR. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29:2919-2928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 208] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 139. | Khaznadar F, Petrovic A, Khaznadar O, Roguljic H, Bojanic K, Kuna Roguljic L, Siber S, Smolic R, Bilic-Curcic I, Wu GY, Smolic M. Biomarkers for Assessing Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus on Sodium-Glucose Cotransporter 2 Inhibitor Therapy. J Clin Med. 2023;12:6561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 140. | Janssen AWF, Houben T, Katiraei S, Dijk W, Boutens L, van der Bolt N, Wang Z, Brown JM, Hazen SL, Mandard S, Shiri-Sverdlov R, Kuipers F, Willems van Dijk K, Vervoort J, Stienstra R, Hooiveld GJEJ, Kersten S. Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids. J Lipid Res. 2017;58:1399-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 141. | Gangarapu V, Ince AT, Baysal B, Kayar Y, Kılıç U, Gök Ö, Uysal Ö, Şenturk H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 142. | Doumouras AG, Wong JA, Paterson JM, Lee Y, Sivapathasundaram B, Tarride JE, Thabane L, Hong D, Yusuf S, Anvari M. Bariatric Surgery and Cardiovascular Outcomes in Patients With Obesity and Cardiovascular Disease:: A Population-Based Retrospective Cohort Study. Circulation. 2021;143:1468-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 143. | Fisher DP, Johnson E, Haneuse S, Arterburn D, Coleman KJ, O'Connor PJ, O'Brien R, Bogart A, Theis MK, Anau J, Schroeder EB, Sidney S. Association Between Bariatric Surgery and Macrovascular Disease Outcomes in Patients With Type 2 Diabetes and Severe Obesity. JAMA. 2018;320:1570-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 144. | Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: A systematic review and meta-analysis. PLoS Med. 2020;17:e1003206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 145. | Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, Ningarhari M, Louvet A, Leteurtre E, Raverdy V, Dharancy S, Pattou F, Mathurin P. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159:1290-1301.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 146. | Verrastro O, Panunzi S, Castagneto-Gissey L, De Gaetano A, Lembo E, Capristo E, Guidone C, Angelini G, Pennestrì F, Sessa L, Vecchio FM, Riccardi L, Zocco MA, Boskoski I, Casella-Mariolo JR, Marini P, Pompili M, Casella G, Fiori E, Rubino F, Bornstein SR, Raffaelli M, Mingrone G. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): a multicentre, open-label, randomised trial. Lancet. 2023;401:1786-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 164] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 147. | Di Lorenzo N, Antoniou SA, Batterham RL, Busetto L, Godoroja D, Iossa A, Carrano FM, Agresta F, Alarçon I, Azran C, Bouvy N, Balaguè Ponz C, Buza M, Copaescu C, De Luca M, Dicker D, Di Vincenzo A, Felsenreich DM, Francis NK, Fried M, Gonzalo Prats B, Goitein D, Halford JCG, Herlesova J, Kalogridaki M, Ket H, Morales-Conde S, Piatto G, Prager G, Pruijssers S, Pucci A, Rayman S, Romano E, Sanchez-Cordero S, Vilallonga R, Silecchia G. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg Endosc. 2020;34:2332-2358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 300] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 148. | Jeeyavudeen MS, Khan SKA, Fouda S, Pappachan JM. Management of metabolic-associated fatty liver disease: The diabetology perspective. World J Gastroenterol. 2023;29:126-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 149. | Ji Y, Lee H, Kaura S, Yip J, Sun H, Guan L, Han W, Ding Y. Effect of Bariatric Surgery on Metabolic Diseases and Underlying Mechanisms. Biomolecules. 2021;11:1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 150. | Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, Harrison SA. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. 2013;6:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 151. | Newton JL, Hollingsworth KG, Taylor R, El-Sharkawy AM, Khan ZU, Pearce R, Sutcliffe K, Okonkwo O, Davidson A, Burt J, Blamire AM, Jones D. Cognitive impairment in primary biliary cirrhosis: symptom impact and potential etiology. Hepatology. 2008;48:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 152. | Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1717] [Cited by in RCA: 2037] [Article Influence: 169.8] [Reference Citation Analysis (0)] |
| 153. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1448] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 154. | Kang BK, Yu ES, Lee SS, Lee Y, Kim N, Sirlin CB, Cho EY, Yeom SK, Byun JH, Park SH, Lee MG. Hepatic fat quantification: a prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Invest Radiol. 2012;47:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 155. | Younes R, Caviglia GP, Govaere O, Rosso C, Armandi A, Sanavia T, Pennisi G, Liguori A, Francione P, Gallego-Durán R, Ampuero J, Garcia Blanco MJ, Aller R, Tiniakos D, Burt A, David E, Vecchio FM, Maggioni M, Cabibi D, Pareja MJ, Zaki MYW, Grieco A, Fracanzani AL, Valenti L, Miele L, Fariselli P, Petta S, Romero-Gomez M, Anstee QM, Bugianesi E. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol. 2021;75:786-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 156. | Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, Yilmaz Y, Czernichow S, Zheng MH, Wong VW, Allison M, Tsochatzis E, Anstee QM, Sheridan DA, Eddowes PJ, Guha IN, Cobbold JF, Paradis V, Bedossa P, Miette V, Fournier-Poizat C, Sandrin L, Harrison SA. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 549] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 157. | Keating SE, Chawla Y, De A, George ES. Lifestyle intervention for metabolic dysfunction-associated fatty liver disease: a 24-h integrated behavior perspective. Hepatol Int. 2024;18:959-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 158. | Schippers M, Adam PC, Smolenski DJ, Wong HT, de Wit JB. A meta-analysis of overall effects of weight loss interventions delivered via mobile phones and effect size differences according to delivery mode, personal contact, and intervention intensity and duration. Obes Rev. 2017;18:450-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 159. | Liu F, Kong X, Cao J, Chen S, Li C, Huang J, Gu D, Kelly TN. Mobile phone intervention and weight loss among overweight and obese adults: a meta-analysis of randomized controlled trials. Am J Epidemiol. 2015;181:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 160. | Flores Mateo G, Granado-Font E, Ferré-Grau C, Montaña-Carreras X. Mobile Phone Apps to Promote Weight Loss and Increase Physical Activity: A Systematic Review and Meta-Analysis. J Med Internet Res. 2015;17:e253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 321] [Article Influence: 32.1] [Reference Citation Analysis (0)] |