Published online Jun 26, 2025. doi: 10.4330/wjc.v17.i6.106525
Revised: April 13, 2025
Accepted: May 15, 2025
Published online: June 26, 2025
Processing time: 105 Days and 16.6 Hours
Noonan syndrome is a relatively common autosomal dominant genetic disorder characterized by cardiovascular defects owing to functional abnormalities in key genes such as RAF1. Mutations in RAF1 are typically associated with hypertrophic cardiomyopathy (HCM). However, in this case, the patient exhibited atrial and ventricular septal defects (VSDs).
This case report describes an 11-year-old boy diagnosed with Noonan syndrome, in whom genetic testing revealed a c.770C>T (p.Ser257 Leu) mutation in RAF1. The patient presented with intermittent chest discomfort and shortness of breath, symptoms that significantly worsened after physical activity. Clinical evaluation revealed marked growth retardation and multiple physical abnormalities. Electrocardiographic and echocardiographic assessments revealed VSDs, atrial septal defects, and left ventricular outflow tract obstruction. Following multidisciplinary consultation, the patient underwent cardiac surgical intervention, which led to cli
This case confirms the genotype-phenotype heterogeneity of Noonan syndrome and highlights the complex management requirements of RAF1 mutation-associated cardiac pathologies. Early surgical intervention can ameliorate structural defects, but it must be integrated with genetic counseling and lifelong monitoring to optimize patient outcomes.
Core Tip: This case report elucidates the unique clinical heterogeneity of the RAF1 c.770C>T (p.Ser257 Leu) mutation in Noonan syndrome. While this variant is classically associated with severe hypertrophic cardiomyopathy and pulmonary hypertension, our patient exhibited atypical congenital heart defects - including atrial septal defect and ventricular septal defect - coexisting with hypertrophic cardiomyopathy, suggesting potential dysregulation of alternative molecular pathways in cardiac morphogenesis. Notably, this case expands the phenotypic spectrum of RAF1 mutations, underscoring the necessity for comprehensive genetic counseling even in carriers of “classic” mutations, as genotype-phenotype correlations remain incompletely defined. Mechanistically, we propose that this mutation disrupts RAF1 protein-mediated mitogen-activated protein kinase signaling, thereby contributing to aberrant cardiac developmental pathways.
- Citation: Ma N, Li ZW, Liu JJ, Liu XG, Zhou X, Wang BW, Li YL, Zhang TC, Xie P. RAF1 mutation expands the cardiac phenotypic spectrum of Noonan syndrome: A case report. World J Cardiol 2025; 17(6): 106525
- URL: https://www.wjgnet.com/1949-8462/full/v17/i6/106525.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i6.106525
Noonan syndrome is a relatively common autosomal dominant genetic disorder characterized by short stature, thoracic deformities, congenital heart disease, and distinctive facial features[1]. Common manifestations of Noonan syndrome are cardiovascular defects, including atrial septal defects (ASDs), ventricular septal defects (VSDs), and pulmonary stenosis. These cardiac defects are closely related to the genetic background of Noonan syndrome and functional abnormalities in key genes. Mutations in genes such as PTPN11, KRAS, SOS1, RAF1, BRAF, and NRAS have been associated with Noonan syndrome. Approximately 50%-60%, 20%, 5%-15%, and 2% of Noonan syndrome cases are linked to PTPN11, SOS1,
An 11-year-old boy presented with intermittent chest tightness and shortness of breath.
The symptoms had persisted for 4 years and were exacerbated by physical exertion and alleviated during rest.
The patient had previously received treatment at a hospital in Beijing, where genetic testing revealed a RAF1 mutation [chromosomal location: Chr3: 12645699; transcript exon: NM_002880.3: Exon 7; nucleotide and amino acid change: C.770C>T (p.Ser257 Leu)]. The patient’s family was advised to consider septal defect repair (ventricular and atrial) and LVOT resection. However, the patient’s family opted for surgery and the patient was discharged. Over the following 4 years, the patient’s chest tightness and shortness of breath gradually worsened to a point where even mild physical activity could trigger symptoms.
The patient was born at full term via vaginal delivery but had a low Apgar score (exact value unknown) and experienced perinatal hypoxia. Since infancy, their food intake had been minimal, and complementary foods were introduced at 9 months. Their motor development was delayed, with no signs of rolling, crawling, or sitting during infancy. They began teething at 7 months and walking at 13 months, with an overall developmental delay compared to peers. Their intellec
The patient’s body temperature was 36.5 °C, pulse was 95 beats per minute, respiratory rate was 21 breaths per minute, and blood pressure was 87/54 mmHg. The patient had a height of 127 cm (< P3) and weight of 26 kg (< P3). Their father’s height was 173 cm, their mother’s height was 168 cm, and their older brother, aged 21 years, was 182 cm tall with normal growth and development. The patient had short stature, and their facial features included a prominent forehead, low posterior hairline, widened interocular distance, ptosis, broad nasal tip, low nasal bridge, thick auricles, low-set and posteriorly rotated ears, thick lips, misaligned teeth, missing teeth, protruding jaw, and short neck. A café-au-lait spot measuring approximately 2 mm × 4 mm was observed in the middle of their chest with scattered pigmented nevi across their body. Their lung sounds were clear bilaterally, with no dry or wet rales. Their cardiac rhythm was regular, with a 4/6 systolic murmur auscultated over all valve areas. Muscle strength in all four limbs was normal, and there was no edema in their lower extremities. The patient’s penis was approximately 4 cm long, and their testicular volume was approximately 2.5 mL.
Laboratory examinations are shown in Table 1.
| Test date | Test item | Result | Reference range | Note |
| July 11, 2024 | NT-proBNP (pg/mL) | 2487 | < 125 | Pre-surgery |
| July 11, 2024 | High-sensitivity troponin I (ng/mL) | 0.001 | < 0.0262 | Pre-surgery |
| July 13, 2024 | Growth hormone (ng/mL) | 6.58 | 0.09-1.95 | Pre-surgery |
| July 13, 2024 | Insulin-like growth factor 1 (ng/mL) | 147 | 50-286 | Pre-surgery |
| July 24, 2024 | NT-proBNP (pg/mL) | 6396 | < 125 | Post-surgery |
| July 24, 2024 | High-sensitivity troponin I (ng/mL) | 15.79 | < 0.0262 | Post-surgery |
| July 24, 2024 | Interleukin-6 (pg/mL) | 37.73 | < 7 | Post-surgery |
| July 24, 2024 | PCT (ng/mL) | 0.961 | < 0.065 | Post-surgery |
The following tests were performed on the patient echocardiography, cardiac magnetic resonance imaging, computed tomography angiography of thoracic great vessels, chest X-ray and electrocardiogram.
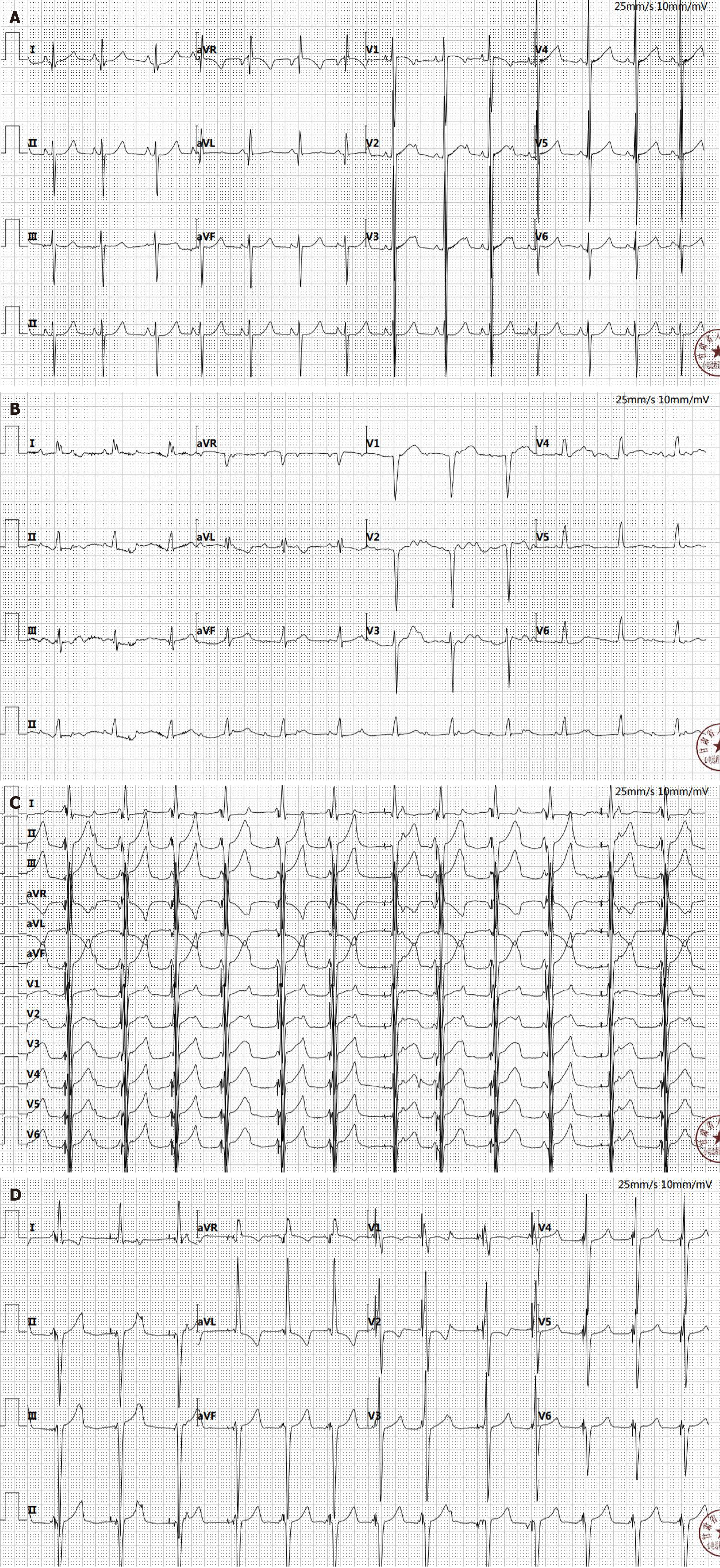
Patient was diagnosed with Noonan syndrome; ASD; VSD; ventricular septal hypertrophy; hypertrophic obstructive cardiomyopathy; post ASD repair; post VSD repair; residual stenosis after LVOT relief; third-degree atrioventricular block; sinus tachycardia; pacemaker implantation; atrial premature contraction; thyroid nodule; emphysema; and pulmo
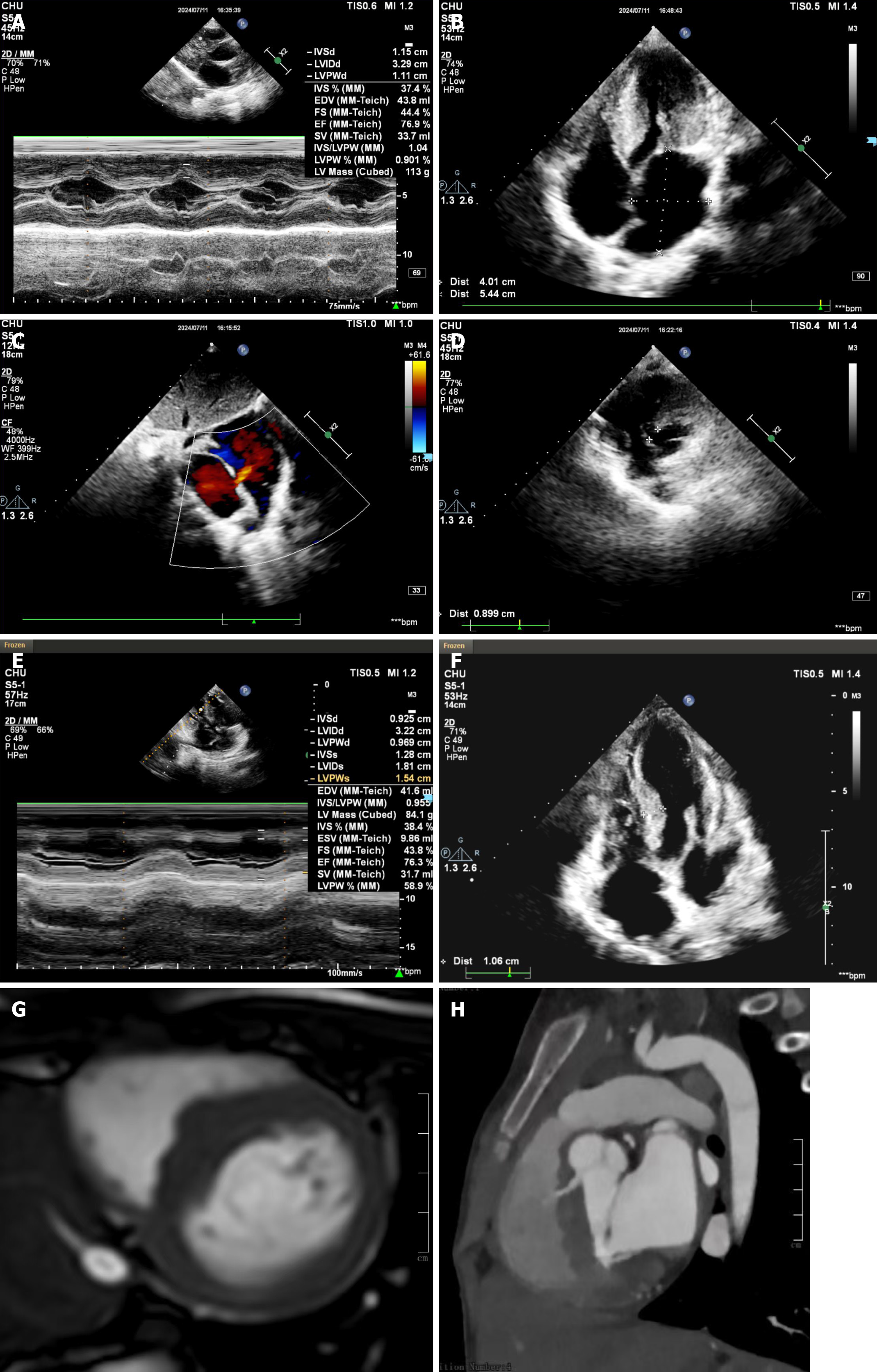
After consultation with a multidisciplinary team, it was unanimously recommended that the patient undergo cardiac surgery under general anesthesia, including ASD repair, VSD repair, and LVOT resection. Subsequently, the patient was transferred to the Department of Cardiovascular Surgery. Postoperative echocardiography showed that the shunts across the atrial and ventricular septa had almost disappeared; however, left ventricular wall thickening, LVOT obstruction, and reduced left ventricular diastolic function were observed. Specific measurements indicated that the thickness of the inter
The patient visited our center in July 2023. As of the date of this response (March 2024), a total of 8 months have passed, during which four follow-up visits have been conducted.
Preoperative echocardiography (on July 11, 2024) indicated the following findings: (1) The interventricular septum and the thickness of the left ventricular posterior wall were increased, with the thickened interventricular septum protruding into the LVOT, resulting in LVOT obstruction. No abnormalities were observed in the left ventricular wall echo or motion amplitude; (2) The anterior and posterior leaflets of the mitral valve were elongated and thickened (notably at the leaflet tips), with the anterior leaflet tip showing malalignment. The systolic anterior motion (SAM) phenomenon was observed at the mitral valve anterior leaflet and chordae tendineae during systole, with adequate leaflet opening but poor closure. The tricuspid valve leaflets appeared thickened, with adequate leaflet opening but suboptimal closure; no significant abnormalities were noted in the remaining membrane morphology; (3) In the parasternal four-chamber view, the echo of the atrial septum was interrupted by approximately 3.8 mm, with a residual end of 13.5 mm on the atrioventricular valve side and a residual end of 21.9 mm (soft residual end) at the top of the atrium; in the short-axis view of the great arteries: The echo of the atrial septum was interrupted by approximately 4.1 mm, with no residual end on the aortic valve side, and a residual end of 19.5 mm (soft residual end) on the opposite side of the aorta; in the subxiphoid two-chamber view, the echo of the atrial septum was interrupted by approximately 4.2 mm, with a residual end of 17.6 mm on the superior vena cava side and 24.0 mm on the inferior vena cava side, with a total length of the atrial septum of 41.5 mm. The echo of the membranous part of the interventricular septum was interrupted, with a left ventricular base of 9.5 mm, and the membranous part of the interventricular septum adhered to the tricuspid valve septal leaflet and chordae tendineae, presenting a tumor-like bulge, approximately 9.5 mm × 5.9 mm in size, with a rupture extending approximately 7 mm. There was almost no residual end at the defect site near the aortic valve, about 5.7 mm from the tricuspid septal leaflet. The aorta arose from the left ventricle; the pulmonary artery arose from the right ventricle; no abnormal channels were observed in the great vessels; and (4) There was a left-to-right shunt at the atrial level and a left-to-right shunt at the ventricular level, with velocity maximum (Vmax) = 4.4 m/second and peak gradient maximum (PGmax) = 77 mmHg, estimating the pulmonary artery systolic pressure within the normal range; under resting conditions, the forward blood flow velocity in the LVOT was significantly increased: Vmax = 4.7 m/second, PGmax = 89 mmHg; there was a small amount of regurgitation at the mitral valve; a small amount of regurgitation at the tricuspid valve, with Vmax = 3.2 m/second and PGmax = 40 mmHg.
The first postoperative echocardiography (on July 30, 2024) indicated the following findings: (1) Left atrial enlargement; (2) Increased thickness of the interventricular septum and left ventricular posterior wall: The thickened interventricular septum protruded into the LVOT, causing LVOT obstruction. The maximum thickness measurements of the interven
The second postoperative echocardiography (on August 31, 2024) indicated the following findings: (1) Strong echo from the pacemaker electrode was visible in the right atrium and right ventricle, with left atrial enlargement; (2) Increased thickness of the interventricular septum and left ventricular posterior wall: The thickened interventricular septum protruded into the LVOT, causing LVOT obstruction. The maximum thickness measurements of the interventricular septum are as follows: Zone I: 10.7 mm; zone II: 11.9 mm; zone III: 10.6 mm; zone IV: 12.6 mm; and left ventricular lateral wall: 10.6 mm; (3) The SAM phenomenon was observed during systole at the mitral valve anterior leaflet and chordae tendineae, with adequate leaflet opening but poor closure; (4) An echo of the patch used for atrial septal repair was observed, with a residual shunt approximately 0.8 mm wide on the inferior side of the patch. An echo of the patch was also noted on the interventricular septum, with no significant gaps around it; and (5) Atrial and ventricular shunts essen
The third postoperative echocardiography (on September 11, 2024) indicated the following findings: (1) Strong echo from the pacemaker electrode was visible in the right atrium and right ventricle, with left atrial enlargement; (2) Increased thickness of the interventricular septum and left ventricular posterior wall: The thickened interventricular septum protruded into the LVOT, causing LVOT obstruction. The maximum thickness measurements of the interventricular septum were as follows: Zone I: 10.7 mm; zone II: 11.9 mm; zone III: 10.6 mm; zone IV: 12.6 mm; and left ventricular lateral wall: 10.6 mm; (3) The SAM phenomenon was observed during systole at the mitral valve anterior leaflet and chordae tendineae, with adequate leaflet opening but poor closure; (4) An echo of the patch used for atrial septal repair was observed, with a residual shunt approximately 0.8 mm wide on the inferior side of the patch. An echo of the patch was also noted on the interventricular septum, with no significant gaps around it; and (5) Atrial and ventricular shunts essentially disappeared; under resting conditions, the forward blood flow velocity in the LVOT was increased: Vmax = 3.4 m/second, PGmax = 45 mmHg. There was a small amount of regurgitation at the mitral valve; a minimal amount of regurgitation at the aortic valve; and a small amount of regurgitation at the tricuspid valve, with Vmax = 2.0 m/second and PGmax = 17 mmHg, estimating the pulmonary artery systolic pressure at 22 mmHg.
The fourth postoperative echocardiography (on February 12, 2025) indicated the following findings: (1) Strong echo from the pacemaker electrode was visible in the right atrium and right ventricle, with left atrial enlargement; (2) Increased thickness of the interventricular septum and left ventricular posterior wall: The thickened interventricular septum protruded into the LVOT, causing LVOT obstruction. The maximum thickness measurements of the interventricular septum were as follows: Zone I: 9.1 mm; zone II: 11.5 mm; zone III: 11.7 mm; zone IV: 12.3 mm; (3) The anterior and posterior leaflets of the mitral valve were elongated and relaxed, adhering to the interventricular septum during systole, causing malalignment of the anterior leaflet. The “SAM” phenomenon was visible during systole, with adequate leaflet opening but poor closure; (4) An echo of the patch used for atrial septal repair was observed, and an echo of the patch was also noted on the interventricular septum, with no significant gaps around it; (5) Atrial and ventricular shunts disappeared; under resting conditions, the forward blood flow velocity in the LVOT was normal: Vmax = 1.8 m/second, PGmax = 13 mmHg. During the Valsalva maneuver, the forward blood flow velocity in the LVOT increased: Vmax = 2.7 m/second, PGmax = 29 mmHg. There was a small amount of regurgitation at the mitral valve; a minimal amount of regurgitation at the aortic valve; and a small amount of regurgitation at the tricuspid valve, with Vmax = 2.2 m/second and PGmax = 20 mmHg, estimating the pulmonary artery pressure at 25 mmHg; and (6) The child’s height was 130 cm, weight was 27 kg, and body surface area was 0.99 m².
Based on the results of the four echocardiograms, the highest pulmonary artery pressure was 32 mmHg, and the maximum forward blood flow velocity in the LVOT has been progressively decreasing under resting conditions, along with a decrease in pressure (Table 2). The velocity and pressure of tricuspid regurgitation have significantly reduced compared to preoperative levels. The observable conclusion is that the child has shown good recovery after the inter
| Date | Left ventricular outflow tract (resting state) | Vmax (m/second) | PGmax (mmHg) | Tricuspid regurgitation Vmax (m/second) | Tricuspid regurgitation PGmax (mmHg) | Pulmonary artery systolic pressure (mmHg) |
| July 11, 2024 | Significantly increased | 4.7 | 89 | 3.2 | 40 | - |
| July 30, 2024 | Increased | 3.8 | 57 | 2.6 | 27 | 32 |
| August 31, 2024 | Increased | 3.4 | 47 | 2.0 | 17 | 22 |
| September 11, 2024 | Increased | 3.4 | 45 | 2.0 | 17 | 22 |
| February 12, 2025 | Normal | 1.8 | 13 | 2.2 | 20 | 25 |
| Date | Zone I (mm) | Zone II (mm) | Zone III (mm) | Zone IV (mm) |
| July 11, 2024 | - | - | - | - |
| July 30, 2024 | 10.7 | 11.9 | 10.6 | 12.6 |
| August 31, 2024 | 10.7 | 11.9 | 10.6 | 12.6 |
| September 11, 2024 | 10.7 | 11.9 | 10.6 | 12.6 |
| February 12, 2025 | 9.1 | 11.5 | 11.7 | 12.3 |
The diagnosis of Noonan syndrome relies on clinical features and genetic testing results. During the initial screening phase, physicians primarily suspect the syndrome based on distinctive facial features, growth retardation, developmental abnormalities, and physical examination findings. Further diagnostic evaluation can be performed using echocardiography to identify specific cardiac anomalies such as ASDs, VSDs, or pulmonary valve stenosis. These findings further strengthen the suspicion of Noonan syndrome. Finally, a diagnosis is confirmed using genetic testing.
In this case, the patient harbored an RAF1 mutation. The RAF1 gene is located on chromosome 3p25. Patients with Noonan syndrome carrying RAF1 mutations typically exhibit a distinct set of phenotypic features, such as cardiovascular defects and growth retardation. These characteristics differ from the phenotypes caused by mutations in other genes, such as PTPN11 or KRAS, leading to varying clinical manifestations.
The patient’s preoperative electrocardiogram indicated sinus rhythm and left ventricular hypertrophy, with no third-degree atrioventricular block present. Previous case reports also did not show any instances of third-degree atrioven
RAF1 variants are commonly associated with HCM and pulmonary hypertension. An observational retrospective analysis revealed that among children with NM_002880.4: C.770C>T and NP_002871.1: P.Ser257 Leu mutations, 92% were diagnosed with HCM, with most receiving a definitive diagnosis within the first year of life[2]. The study found that 30% of these patients were premature, and 47% of newborns required treatment in the neonatal intensive care unit for complications related to HCM or pulmonary hypertension, with a mortality rate of approximately 13%. This study indicated that patients with the pathogenic variant c.770C>T in the RAF1 gene exhibited particularly severe phenotypes characterized by rapid progression of neonatal HCM and high mortality rates[2]. Further investigation of this mutation site revealed that the RAF1 gene mutation (c.770C>T, p.Ser257 Leu) affects patients with Noonan syndrome through alterations in cardiac ultrastructure, abnormal calcium handling, and excessive activation of signaling pathways[3]. For instance, mutations at this site significantly affect cardiac function, leading to prominent ultrastructural defects in cardio
The RAF1 mutation reported in this case (NM_002880.4: C.770C>T, p.Ser257 Leu) has also been reported in previous studies. Research has indicated that the most common pathogenic variant among RAF1 mutations is c.770C>T, which accounts for approximately 37.5% of the patients with RAF1 mutations[4]. The c.770C>T variant is typically associated with HCM and pulmonary hypertension[5]. However, in the present case, although HCM was present, pulmonary hyper
The RAF1: C.770C>T mutation is closely related to the mechanism of PAH through the excessive activation of the mitogen-activated protein kinase (MAPK) signaling pathway. This mutation is located in the conserved region of RAF1 (Ser257 Leu) and significantly enhances RAF1 kinase activity by relieving the self-inhibitory effect at the Ser259 phos
In this case, the child’s pulmonary artery pressure reached a maximum of 32 mmHg, which may be attributed to the presence of ASD and VSDs. In this context, the defects in the atrial and ventricular septa act as shunt valves, reducing pulmonary artery pressure. We aimed to identify the potential mechanisms by which the c.770C>T (p.Ser257 Leu) mutation causes ASDs and VSDs by analyzing and summarizing previous studies related to RAF1. Dysregulation of signal transduction caused by the c.770C > T (p.Ser257 Leu) mutation typically manifests as aberrant activation of the rat sarcoma viral oncogene homolog-mitogen-activated protein kinase (RAS)-MAPK signaling pathway, which is the core mechanism underlying the various symptoms in patients with Noonan syndrome[2]. This mutation generally acts as a positive regulator, enhancing signal transmission and subsequently influencing cell growth and differentiation.
The rapidly accelerated fibrosarcoma-mitogen-activated protein kinase kinase-extracellular signal-regulated kinase cascade within the RAS-MAPK signaling pathway is a core component of signal transduction and is involved in the regulation of cellular functions. RAF1 plays a crucial role in the RAS-MAPK signaling pathway through mechanisms such as signal amplification, kinase activity, gene expression regulation, and feedback modulation. Overactivation of RAF1 may lead to VSDs and myocardial hypertrophy. The patient presented with VSDs, ventricular septal hypertrophy, and LVOT obstruction.
The c.770C>T (p.Ser257 Leu) mutation in RAF1 is associated with HCM and pulmonary hypertension. This case report describes an 11-year-old patient with Noonan syndrome whose genetic testing revealed chromosome position chr3: 12645699, transcript exon NM_002880, and the nucleotide change c.770C>T (p.Ser257 Leu). The patient’s phenotypes included HCM, ASDs, and VSDs.
| 1. | Bell JM, Considine EM, McCallen LM, Chatfield KC. The Prevalence of Noonan Spectrum Disorders in Pediatric Patients with Pulmonary Valve Stenosis. J Pediatr. 2021;234:134-141.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (37)] |
| 2. | Gazzin A, Fornari F, Niceta M, Leoni C, Dentici ML, Carli D, Villar AM, Calcagni G, Banaudi E, Massuras S, Cardaropoli S, Airulo E, Daniele P, Monda E, Limongelli G, Riggi C, Zampino G, Digilio MC, De Luca A, Tartaglia M, Ferrero GB, Mussa A. Defining the variant-phenotype correlation in patients affected by Noonan syndrome with the RAF1:c.770C>T p.(Ser257Leu) variant. Eur J Hum Genet. 2024;32:964-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (37)] |
| 3. | Nakhaei-Rad S, Haghighi F, Bazgir F, Dahlmann J, Busley AV, Buchholzer M, Kleemann K, Schänzer A, Borchardt A, Hahn A, Kötter S, Schanze D, Anand R, Funk F, Kronenbitter AV, Scheller J, Piekorz RP, Reichert AS, Volleth M, Wolf MJ, Cirstea IC, Gelb BD, Tartaglia M, Schmitt JP, Krüger M, Kutschka I, Cyganek L, Zenker M, Kensah G, Ahmadian MR. Molecular and cellular evidence for the impact of a hypertrophic cardiomyopathy-associated RAF1 variant on the structure and function of contractile machinery in bioartificial cardiac tissues. Commun Biol. 2023;6:657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Li X, Yao R, Tan X, Li N, Ding Y, Li J, Chang G, Chen Y, Ma L, Wang J, Fu L, Wang X. Molecular and phenotypic spectrum of Noonan syndrome in Chinese patients. Clin Genet. 2019;96:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, López Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 495] [Article Influence: 27.5] [Reference Citation Analysis (0)] |