Published online Apr 26, 2025. doi: 10.4330/wjc.v17.i4.104748
Revised: March 14, 2025
Accepted: April 3, 2025
Published online: April 26, 2025
Processing time: 111 Days and 20.1 Hours
Arteriovenous fistula is a rare cause of refractory heart failure, and corrective measures may lead to dramatic improvement; however, the long-term cardiac remodeling outcomes, particularly after delayed closure, remain unclear.
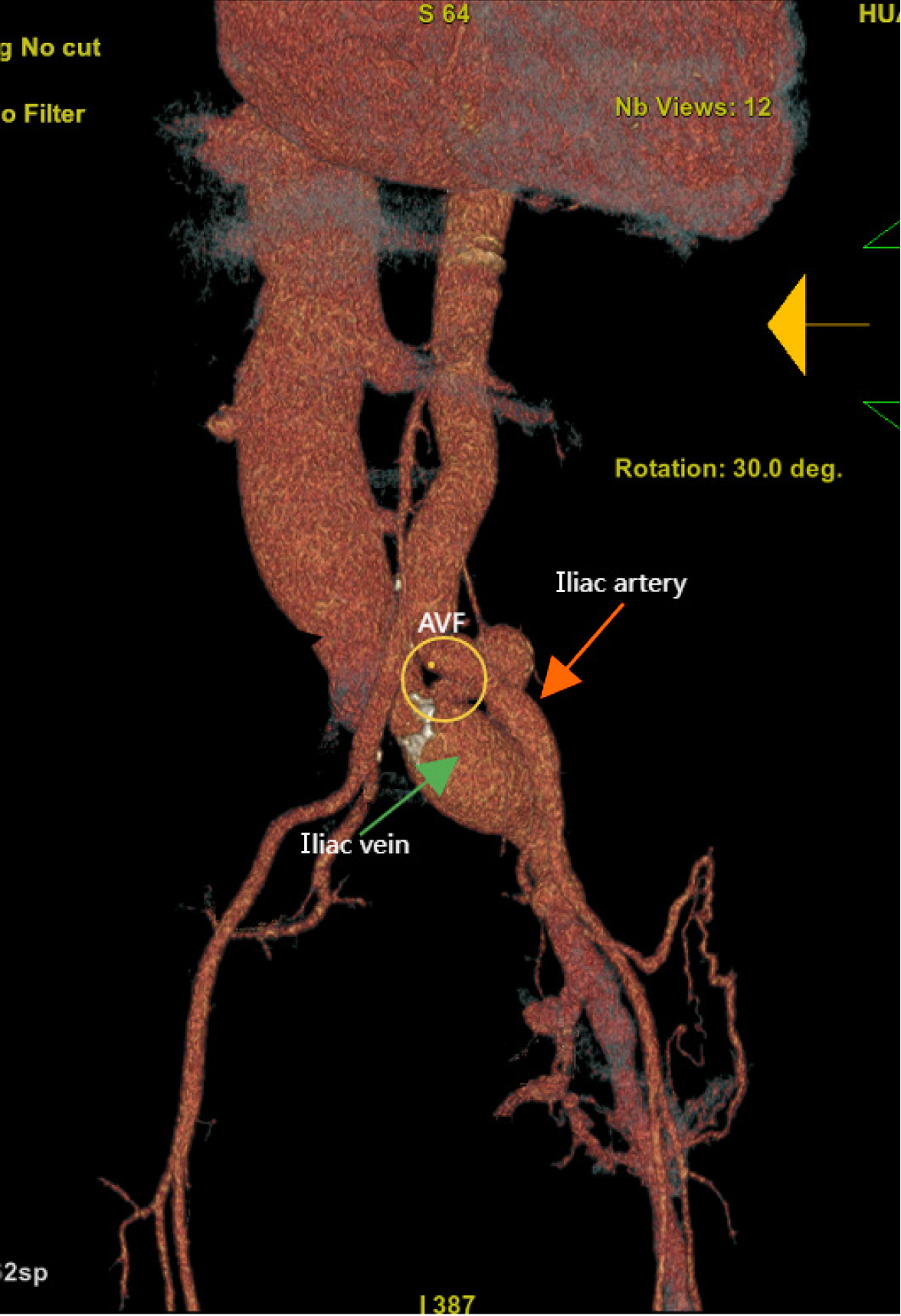
A 57-year-old man was admitted to the hospital with complaints of exertional dyspnea for more than 10 years. Physical examination revealed wet crackles in the lungs and a continuous machinery murmur in the left lower back and groin area. Asymmetric edema and varicose veins were observed in the lower limbs. Echocardiography revealed a dilated right ventricle with severe pulmonary hypertension. Computed tomography revealed a left common iliac arteriovenous fistula linked to prior lumbar disc surgery. Surgical repair resolved the symptoms, with echo
This case highlights that delayed arteriovenous fistula closure may result in in
Core Tip: Heart failure secondary to an arteriovenous fistula is a rare clinical condition. Our study reveals that delayed surgical repair may lead to incomplete reverse remodeling of the right heart, emphasizing the importance of timely intervention.
- Citation: He T, He X, Yuan XM. High-output heart failure secondary to iatrogenic arteriovenous fistula: A case report. World J Cardiol 2025; 17(4): 104748
- URL: https://www.wjgnet.com/1949-8462/full/v17/i4/104748.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i4.104748
Heart failure is a heterogeneous clinical syndrome caused by volume overload and myocardial damage, often resulting in considerable morbidity and mortality. Timely identification of the causes that can be addressed and corrected is thus important. Arteriovenous fistula (AVF), which creates a significant extracardiac left-right shunt, is a rare but treatable cause of refractory heart failure. While prompt closure typically restores cardiac function, the long-term consequences of delayed repair are poorly understood.
The hemodynamic burden of AVF arises from chronic high-output failure: (1) Reduced systemic vascular resistance increases cardiac output, leading to ventricular dilatation; and (2) Persistent volume overload significantly stresses the right heart because of elevated pulmonary arterial pressure from the shunt-driven pulmonary circulation overload. This mechanism is particularly pronounced in abdominopelvic AVFs, where direct venous return to the right atrium exa
In this study, we present a case of heart failure caused by an iatrogenic AVF, 19 years after lumbar disc surgery. Although surgical closure initially resolved the symptoms, progressive right heart recurrent redilatation emerged during the 2-year follow-up period—a novel finding underscoring the potential consequences of delayed repair. A literature review was conducted to identify predictors of incomplete cardiac recovery, with emphasis on intervention timing and RV adaptation patterns.
A 57-year-old man was admitted to our hospital with complaints of exertional dyspnea and asymmetric left-dominant lower limb edema for more than 10 years that had been aggravated for ten days.
The patient first exhibited chest tightness and shortness of breath approximately 2 years after lumbar disc surgery, accompanied by depressed edema of the lower limbs, abdominal distension and a poor appetite. The patient visited the cardiology and cardiac surgery clinic many times, and his heart failure was thought to be caused by valvular heart disease. For economic reasons, valve replacement surgery was not considered. Instead, long-term oral diuretics and other anti-heart failure treatments were administered, but the patient remained in New York Heart Association functional Class II-III.
The patient had a 4-year history of chronic hepatitis B and was treated with entecavir. During routine follow-up, liver tests revealed slight increases in transaminase and bilirubin levels, and hepatitis B virus-DNA could not be detected with real-time quantitative polymerase chain reaction. The patient underwent a lumbar disc operation 20 years ago without any reported intraoperative vascular injury.
The patient denied a history of alcohol consumption, drug addiction and a family history of premature coronary heart disease.
The patient’s blood pressure was 138/62 mmHg, indicating a wide pulse pressure. Physical examination revealed wet crackles in the lungs, and a 3/6-level systolic murmur could be heard in the mitral valve area and a continuous machinery murmur was evident over the left lower back. In addition, pitting edema, pigmentation and varicose veins were observed in both lower limbs, especially on the left.
The relevant laboratory findings included an increased brain natriuretic peptide (BNP) level of 3298 pg/mL (normal value < 100 pg/mL), a normal albumin level, an alanine aminotransferase level of 39 U/L (normal value < 40 U/L), and an aspartate aminotransferase level of 52 U/L (normal value < 40 U/L).
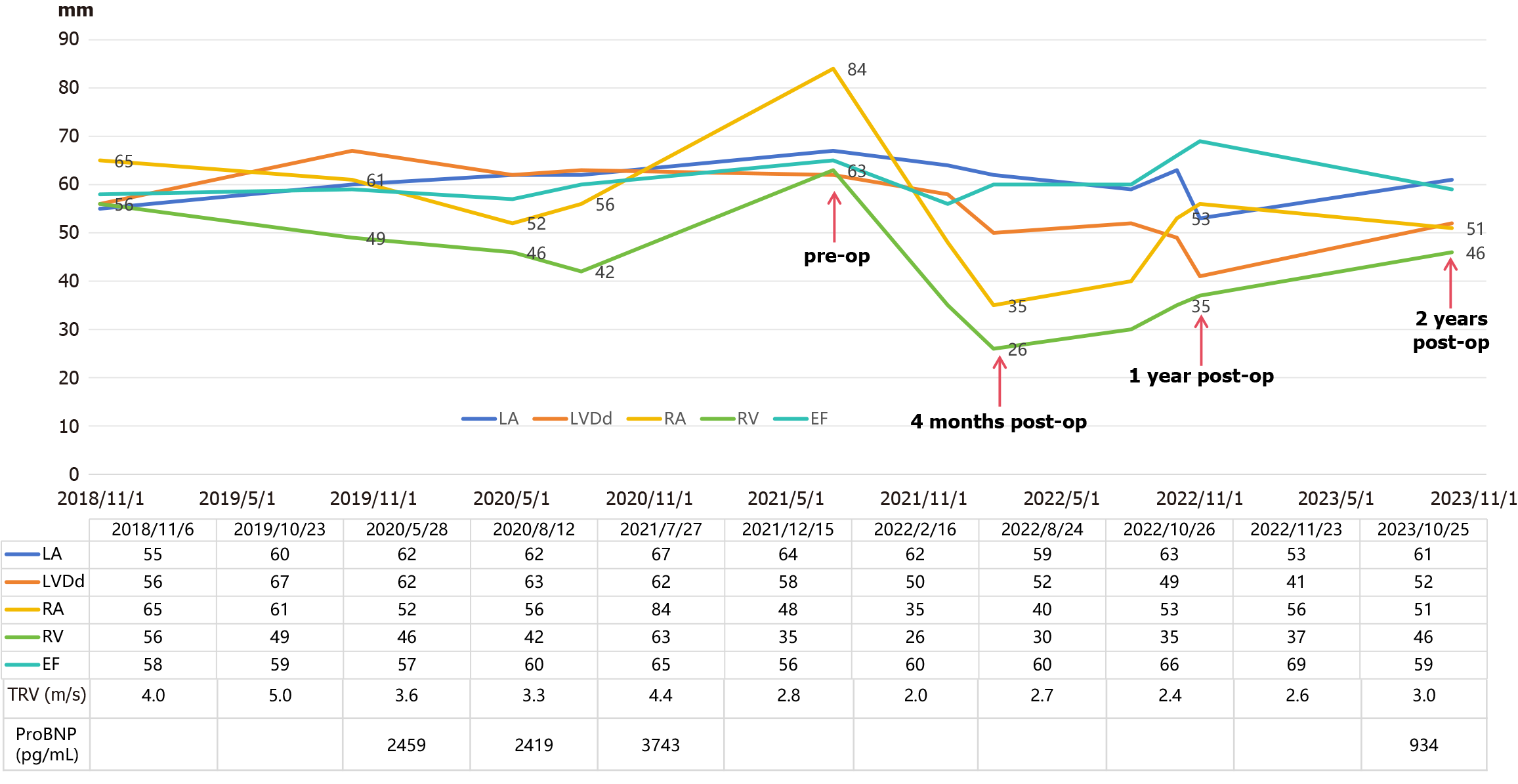
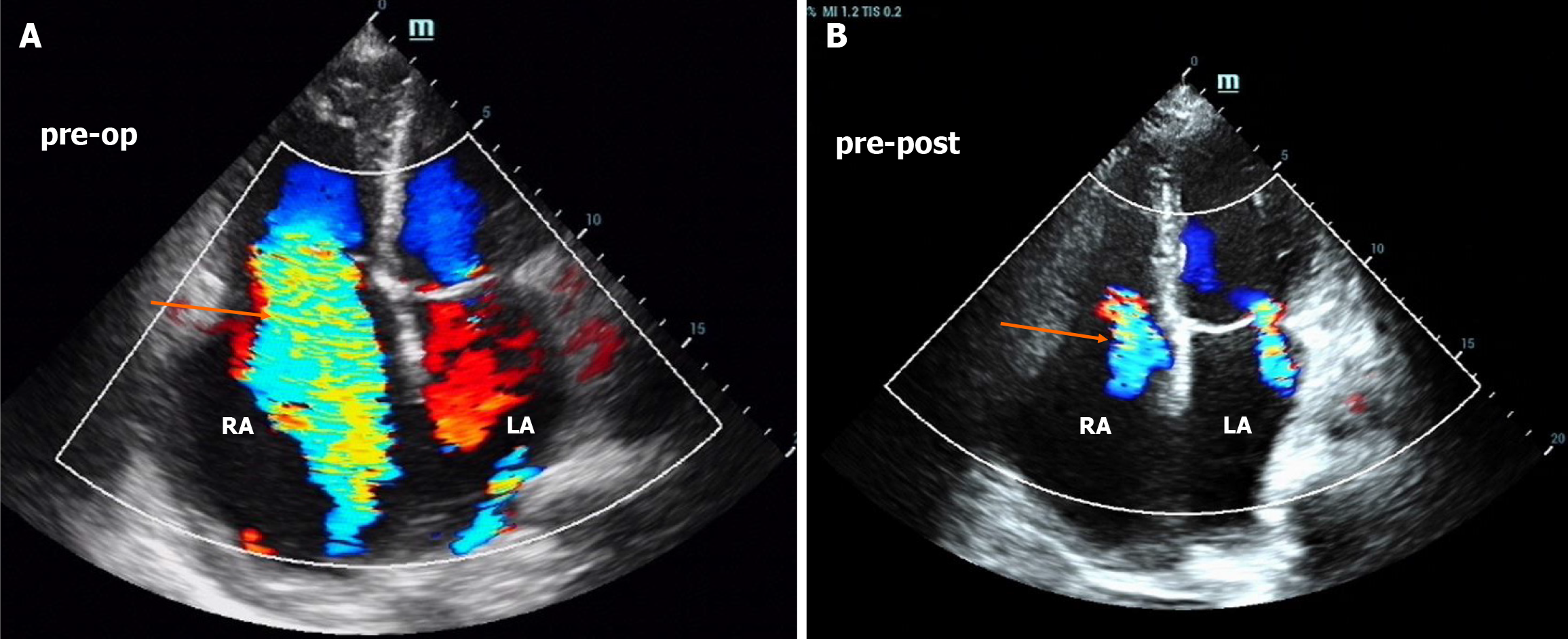
An electrocardiogram revealed atrial fibrillation and right bundle branch block. The echocardiogram results over the years are shown in Figure 1, and the most recent echocardiogram revealed a dilated whole heart, especially the right atrium, and a normal ejection fraction of 65%. In addition, mitral regurgitation and tricuspid regurgitation were observed, and the estimated high pulmonary pressure was 76 mmHg (Figure 2A).
On the basis of his symptoms, medical history, blood tests, and imaging examination, the patient was diagnosed with heart failure (unknown etiology), chronic hepatitis B and lumbar disc herniation (after surgery).
Considering the patient's young age of onset and suboptimal response to conventional anti-heart failure drug therapy, we performed cardiac magnetic resonance (CMR), but there were no positive findings. After admission, when the cardiova
We deliberated surgical vs endovascular intervention on the basis of anatomical feasibility and clinical evidence. Given the large arterial diameter, short fistula neck (Video 1) and lower medical reimbursement rate, endovascular stenting was deemed less suitable. Furthermore, the rarity of this condition and the limited institutional experience with endovascular approaches contrasted with the established efficacy of open surgery. Consequently, open surgery was performed under general anesthesia.
His symptoms and lower limb edema were significantly relieved one week after the operation, and a transthoracic echocardiography revealed a significant reduction in the right heart and tricuspid regurgitation (Figure 1 and Figure 2B) at four months. However, at the two-year follow-up, the patient showed significant enlargement of the right heart again, with the right atrium and right ventricle reaching 51 mm and 46 mm, respectively (Figure 1). However, he is still asymptomatic and has only mildly elevated BNP. To determine the cause and evaluate the presence of pulmonary hypertension, we conducted right heart catheterization, which revealed a normal cardiac output of 4.98 L/min, a cardiac index and pulmonary hypertension (mean pulmonary artery pressure of 14.7 mmHg and total lung resistance of 2.96 Wood units). We also conducted CMR late gadolinium enhancement and contrast-enhanced T1 mapping to determine whether there was fibrosis in the right atrium, but the results were normal. Potential mechanisms include residual hemodynamic stress from subclinical residual shunting and altered RV-pulmonary artery coupling, which could perpetuate wall stress. Chronic volume overload over 19 years may have induced extracellular matrix cross-linking, impairing reverse remodeling even after anatomical correction, despite the absence of late gadolinium enhancement on CMR. Given the recurrence of right heart enlargement with unclear etiology—potentially involving persistent myocardial remodeling—guideline-directed medical therapy was initiated to optimize the prognosis. In accordance with the 2022 ESC Heart Failure Guidelines, an angiotensin receptor/neprilysin inhibitor and beta blockers were selected to target neurohormonal activation and adverse cardiac remodeling. During follow-up, doses were carefully adjusted on the basis of blood pressure and heart rate to balance efficacy and tolerability.
Arteriovenous fistula (AVF) is an infrequent cause of heart failure. The pathophysiological mechanism is mainly volume overload. Specifically, AVF creation, which causes a significant extracardiac left-right shunt, leads to an increase in blood volume. Then, the right atrial pressure, pulmonary artery pressure, and left ventricle end-diastolic pressure gradually increase until the myocardium decompensates and then the patient develops symptoms of heart failure[5].
AVF can present with a variety of clinical symptoms and signs, including features of high output and right heart failure. The distinctive presenting symptoms include abdominal bruit, wide pulse pressure, and regional venous hy
AVF can occur at any location, most commonly between the iliac arteries and veins, and its causes can be both congenital and acquired. Congenital AVFs are mainly arteriovenous malformations[7], whereas acquired AVFs can be further divided into spontaneous AVFs and those secondary to vascular injury. The latter can be further categorized into traumatic (most commonly due to stab wounds or gunshot wounds) and iatrogenic (procedure-related) wounds, such as those occurring after lumbar disc surgery or endoscopic laser treatment of the great saphenous vein[8].
We reviewed several case reports of heart failure secondary to arteriovenous fistula in the past 20 years (Table 1). One report described a patient in which the time from the occurrence of AVF to the onset of heart failure was as long as 25 years after right nephrectomy[9], whereas another report described a patient with spontaneous rupture of an aneurysm who immediately developed heart failure and cardiac arrest[10]. The primary factors determining heart failure are the size and chronicity of the fistula and the pressure gradient across the shunt[6]. Iatrogenic AVFs are generally diagnosed later than other types of AVFs because of their smaller initial fistula orifice and low blood flow, which delay symptom onset.
| Time | Site | Age/sex | Time to diagnosis | Cause | Therapy | Prognosis |
| Shirai et al[15], 2025 | Spontaneous iliac AVF | 71/male | Immediately | Ruptured right common iliac artery aneurysm | Open abdominal aortic replacement | No follow up |
| Vranešić et al[16], 2024 | Iatrogenic iliac AVF | 52/female | Three months | Spine surgery | Endovascular treatment with a balloon-expandable stent graft | Excellent clinical condition at 5 years |
| Piraneo et al[10], 2024 | Spontaneous iliac AVF | 70/male | Immediately | Ruptured left common iliac artery aneurysm | NR | Death |
| Mach et al[7], 2024 | Renal arteriovenous | 69/female | 8 months | Renal arteriovenous malformation | Endovascular transcatheter embolization | Reduction in symptoms and overall health improvement (follow-up time not specified) |
| Kosum et al[2], 2023 | Iliac AVF | 44/male | 5 years | Lumbar discectomy | Endovascular closure by covered stent | Significant improvement in PH and high-output state at 6 weeks |
| Palić et al[4], 2023 | Left superficial femoral artery and left femoral vein | 33/male | 4 months | Gunshot injury and trauma | Open surgery | Significant reduction in right heart diameter with a resolution of tricuspid regurgitation at 3 months |
| Naouli et al[17], 2022 | Iliac AVF | 44/male | 4 years | Spine surgery | Open surgery | Significant regression of cardiac chambers at 3 months, asymptomatic at 6 years |
| Kumar et al[6], 2021 | Abdominal aortic aneurysm and IVC | 65/male | 8 months | An open abdominal aortic aneurysm repair | NR | No follow up |
| Charif et al[18], 2021 | Aorta and IVC | 24/male | 5 months | Abdominal shrapnel injury | Amplatzer septal occluder | Resolution of HOHF at 6 months |
| Kwon et al[19], 2019 | Right common iliac artery and IVC | 62/female | 18 months | Lumbar spine surgery | Endovascular stent graft | Reduction in symptoms (follow-up time not specified) |
| Petrov et al[9], 2019 | Right renal artery and IVC | 59/male | 25 years | Right nephrectomy | Coil embolization and LAA/PDA occluder | Reduction in symptoms and a resolution of tricuspid regurgitation at 2 years |
| Dahl et al[5], 2018 | Left common iliac artery and vein | 46/female | 8 years | Hysterectomy | Endovascular closure with a Medtronic stent graft | LV size and RV size normalized and tricuspid gradient decreased and even normalized after 3 months |
| Dahl et al[5], 2018 | Right common iliac artery and vein | 48/female | 12 years | Cholecystectomy | Endovascular closure with a Medtronic stent graft | Normalization of PH and complete resolution of symptoms at 6 months |
| Park et al[11], 2016 | Right common iliac artery and vein | 36/male | 16 years | Herniated disc repair | Endovascular sealing with a bifurcated stent graft | Resolution of the dilated heart chambers, IVC diameter and decrease in PH with a reduction in the TR V-max at 8 weeks |
| Kubelik et al[13], 2016 | Left common iliac artery and vein | 39/female | 12 years | Lumbar discectomy | Endovascular stent graft | Decreased cardiac output and cardiac index at 1 month |
| Nielsen-Kudsk et al[20], 2012 | Right common iliac artery and vein | 49/female | 1 year | Spine surgery (L5/S1) | Transcatheter insertion of a covered stent | No follow up |
Vascular complications of lumbar disc surgery are the most common cause of iatrogenic AVF, particularly at the L4-L5 disc level, where a broad vascular bed forms anatomically[11]. The incidence of vascular injury after disc surgery is approximately 0.039%-0.14%[12] but it can be life-threatening. The majority of cases reported in the literature present within the first year following lumbar surgery[13], but our patient was diagnosed 19 years later. The delayed diagnosis stemmed from insufficient physical examination, where clinicians overlooked the characteristic bruit of AVF and misattributed his symptoms to valvular heart disease. The diagnosis of AVF can be based on color Doppler ultrasound, computerized tomography angiography, and aortography, which remains the gold standard[6]. Acute cases of AVF require urgent surgical intervention, whereas for chronic cases, the best treatment option may not be as clear[6].
Open surgery demonstrates definitive efficacy in treating complex vascular pathologies by enabling direct excision of the fistula and vascular reconstruction, particularly in cases with limited interventional access (e.g., calcified fistulas or severe tissue adhesions). While this approach achieves low long-term recurrence rates, it requires open incisions and carries risks of tissue damage, resulting in prolonged hospitalization and higher rates of bleeding and mortality in patients with hemodynamic instability[3].
In contrast, interventional therapy employs minimally invasive embolization, facilitating rapid recovery and precise, imaging-guided targeting of lesions in high-risk anatomical regions[7]. However, its application may be limited by factors such as patient comorbidities, anatomical factors, and the availability of necessary equipment. Additionally, recurrence risks persist owing to potential migration or degradation of embolic materials, and the procedure demands advanced imaging equipment and specialized expertise, making it more costly than open surgery.
The role of medical therapy as the sole treatment is generally thought to be ineffective in the long term but may be supportive in patients awaiting corrective surgery. To achieve the best possible outcomes, multidisciplinary collaboration and discussion among cardiology, interventional radiology, and vascular surgery practitioners should be conducted to develop individualized treatment plans for patients. Chronic and potentially fatal sequelae of chronic AVF can be avoided by timely diagnosis and repair, as hemodynamic derangements usually improve after corrective surgery.
The current literature does not mention right heart redilatation following iliac AVF repair. However, studies on pulmonary hypertension (PH) associated with end-stage renal disease have revealed that pulmonary hypertension may persist or even worsen after the closure of arteriovenous fistulas. We hypothesize that the recurrence of right heart dilatation in this patient may be related to preexisting right heart dysfunction and residual hemodynamic stress. Long-term volume overload can induce myocardial apoptosis and extracellular matrix remodeling, manifesting as increased myocardial stiffness and functional impairment[14]. Even after anatomical correction, such matrix remodeling may limit cardiac chamber elasticity recovery and hinder reverse remodeling. Additionally, subclinical residual shunts could lead to localized arterial pressure elevation and venous pressure reduction, creating persistent hemodynamic stress. This imbalance disrupts ventricular-pulmonary coupling, resulting in increased sustained ventricular wall stress. Further
AVF should be considered in patients with unexplained right heart failure, especially in the setting of abdominal surgery or trauma—even decades after the inciting event. Delayed repair risks incomplete reverse remodeling despite anatomical success. Careful physical examination and medical history inquiry are particularly important for a timely diagnosis.
| 1. | Ingle K, Pham L, Lee V, Guo L, Isayeva-Waldrop T, Somarathna M, Lee T. Cardiac changes following arteriovenous fistula creation in a mouse model. J Vasc Access. 2023;24:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Kosum P, Chattranukulchai P, Theerasuwipakorn N, Sricholwattana S, Ariyachaipanich A, Tumkosit M, Wanlapakorn C, Srimahachota S, Boonyaratavej S. An unusual cause of high-output heart failure from the iliac arteriovenous fistula after lumbar discectomy: A case report. Radiol Case Rep. 2023;18:2140-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 3. | Benbrahim FZ, Ankri M, Fariyou A, El Aoufir O, Laamrani FZ, Laila J. Spontaneous ilio-iliac arteriovenous fistula: A rare complication of aorto-iliac aneurysm. Radiol Case Rep. 2024;19:2996-3000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Palić B, Mandić A, Prskalo Z, Brizić I. High-output heart failure following gunshot injury and traumatic arteriovenous fistula. Vascular. 2024;32:916-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Dahl JS, Andersen C, Duvnjak S, Moller JE. Two cases of high-output heart failure as initial presentation of iliac arteriovenous fistula. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Kumar S, Mogalapalli A, Milunski MR. Chronic Aortocaval Fistula Presenting as Right Heart Failure: A Case Report and Review of Literature. Cureus. 2021;13:e13528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Mach M, Maciejewski K, Ostrowski T, Maciąg R, Sajdek M, Gąsiorowski O, Gałązka Z. A Huge High-Flow Aneurysmal Renal Arteriovenous Malformation Treated With Endovascular Transcatheter Embolization. Cureus. 2024;16:e65487. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Gijsels S, Croo A, Randon C. Spontaneous Ilio-Iliac Arteriovenous Fistula from Rupture of an Iliac Aneurysm: A Systematic Review. Ann Vasc Surg. 2024;104:110-123. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Petrov I, Tasheva I, Stankov Z, Polomski P, Georgieva G, Marinov K. Uneventful Follow-Up 2 Years after Endovascular Treatment of a High-Flow Iatrogenic Aortocaval Fistula Causing Pulmonary Hypertension and Right Heart Failure. Methodist Debakey Cardiovasc J. 2019;15:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Piraneo JM, Mordecai R, Espinosa J, Lucerna A. Spontaneous Iliac Arteriovenous Fistula Leading to High-Output Heart Failure and Cardiac Arrest. Cureus. 2024;16:e51876. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Park T, Park SH, Arora A. Delayed High Output Heart Failure due to Arteriovenous Fistula Complicated with Herniated Disc Surgery. J Korean Med Sci. 2016;31:2051-2053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Gallerani M, Maida G, Boari B, Galeotti R, Rocca T, Gasbarro V. High output heart failure due to an iatrogenic arterio-venous fistula after lumbar disc surgery. Acta Neurochir (Wien). 2007;149:1243-7; discussion 1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Kubelik D, Morellato J, Jetty P, Brandys T, Hajjar G, Hill A, Nagpal S. Endovascular Repair of a Chronic AV Fistula Presenting as Post-Partum High Output Heart Failure. EJVES Short Rep. 2016;31:19-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Houston BA, Brittain EL, Tedford RJ. Right Ventricular Failure. N Engl J Med. 2023;388:1111-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 126] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 15. | Shirai Y, Saito A, Tanaka C, Moriyama Y, Ito Y, Ishibashi K, Motomura N. Ilio-Iliac Arteriovenous Fistula Secondary to a Ruptured Right Common Iliac Artery Aneurysm and Anomalous Anatomy of Inferior Vena Cava Resulting in an Arteriovenous Shunt Formation with Right-sided Cardiac Failure: A Case Report. Surg Case Rep. 2025;11:24-0094. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Vranešić II, Rotkvić PG, Jurca I, Perkov D, Kirhmajer MV. High Output Heart Failure Due to Iatrogenic Iliac Arteriovenous Fistula: Complication of Spine Surgery. JACC Case Rep. 2024;29:102260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Naouli H, Jiber H, Bouarhroum A. Iliac arteriovenous fistula following lumbar disc surgery. A case report. J Med Vasc. 2022;47:199-202. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Charif F, Nassar P, Youssef D, Neghawi Z, Saab M. High Output Heart Failure Secondary to Aorto-Caval Fistula Treated With an Amplatzer Septal Occluder: Case Report and Review of Literature. Cureus. 2021;13:e14430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Kwon SS, Park BW, Lee MH, Goo DE, Nam BD. Right heart failure due to arteriovenous fistula after lumbar spine surgery. Korean J Intern Med. 2020;35:1022-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Nielsen-Kudsk JE, Jóanesarson J, Bøttcher M. Pulmonary hypertension due to a large acquired systemic arteriovenous fistula. Heart. 2012;98:518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |