INTRODUCTION
Coronary microvascular dysfunction (CMD) refers to structural or functional abnormalities in the coronary microvascular system, leading to inadequate myocardial perfusion and persistent symptoms of myocardial ischemia[1]. Since its first report in 1967 of patients with normal coronary angiography but exhibiting coronary artery disease symptoms, the pathophysiology and clinical manifestations of CMD have garnered increasing attention[2,3]. Although percutaneous coronary intervention (PCI) has significantly improved the patency of coronary macrovascular blood flow, some patients continue to experience angina and myocardial ischemic symptoms[4,5]. This phenomenon has been attributed to CMD, which is closely associated with poor prognosis in PCI-treated patients[6].
The pathophysiology of CMD encompasses several mechanisms, including endothelial dysfunction, microvascular remodeling, and reperfusion injury[7]. These mechanisms significantly interact with the major components of metabolic syndrome (MetS)-obesity, hyperglycemia, hypertension, and dyslipidemia[8]. Patients with MetS are more prone to microvascular dysfunction, making them a high-risk population for microcirculatory disorders[9]. CMD not only increases the risk of adverse cardiovascular events post-PCI but also serves as a critical etiological factor in ischemia with non-obstructive coronary arteries (INOCA) and myocardial infarction with non-obstructive coronary arteries (MINOCA)[10]. Importantly, CMD significantly impacts patients' quality of life, leading to persistent angina and reduced exercise capacity, and is associated with increased long-term mortality rates[11]. The diagnosis of CMD currently relies on both invasive and non-invasive techniques. Coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR) are commonly used invasive tools for CMD assessment[12], while non-invasive imaging modalities such as positron emission tomography (PET) and cardiac magnetic resonance imaging (CMR) have demonstrated significant value in the early detection of CMD[13]. Regarding treatment, CMD management currently focuses on lifestyle modifications and conventional pharmacological therapies[14]; however, their effectiveness in improving microcirculatory function and long-term prognosis remains limited. Recent advancements in the diagnosis and management of CMD have introduced novel diagnostic tools and therapeutic strategies. For instance, the integration of artificial intelligence (AI) in imaging analysis has improved the accuracy of non-invasive diagnostic techniques such as CMR and PET[15,16]. Additionally, emerging therapies, including stem cell therapy and gene therapy, have shown promise in addressing the underlying mechanisms of CMD, such as endothelial dysfunction and oxidative stress[17,18]. These advancements underscore the importance of a multi-disciplinary approach to CMD management, combining cutting-edge diagnostics with personalized treatment strategies to improve patient outcomes.
Despite substantial progress in CMD diagnosis and treatment in recent years, challenges remain in areas such as the sensitivity of diagnostic tools, the personalization of therapeutic strategies, and the complex interactions between CMD and metabolic abnormalities[19,20]. This review aims to systematically summarize the latest advancements in the diagnosis and management of CMD following PCI, with a particular focus on the interaction between CMD and MetS. Our objective is to identify key challenges in CMD management, highlight novel therapeutic approaches, and provide insights into future research directions to optimize patient outcomes.
PATHOPHYSIOLOGICAL MECHANISMS OF CMD
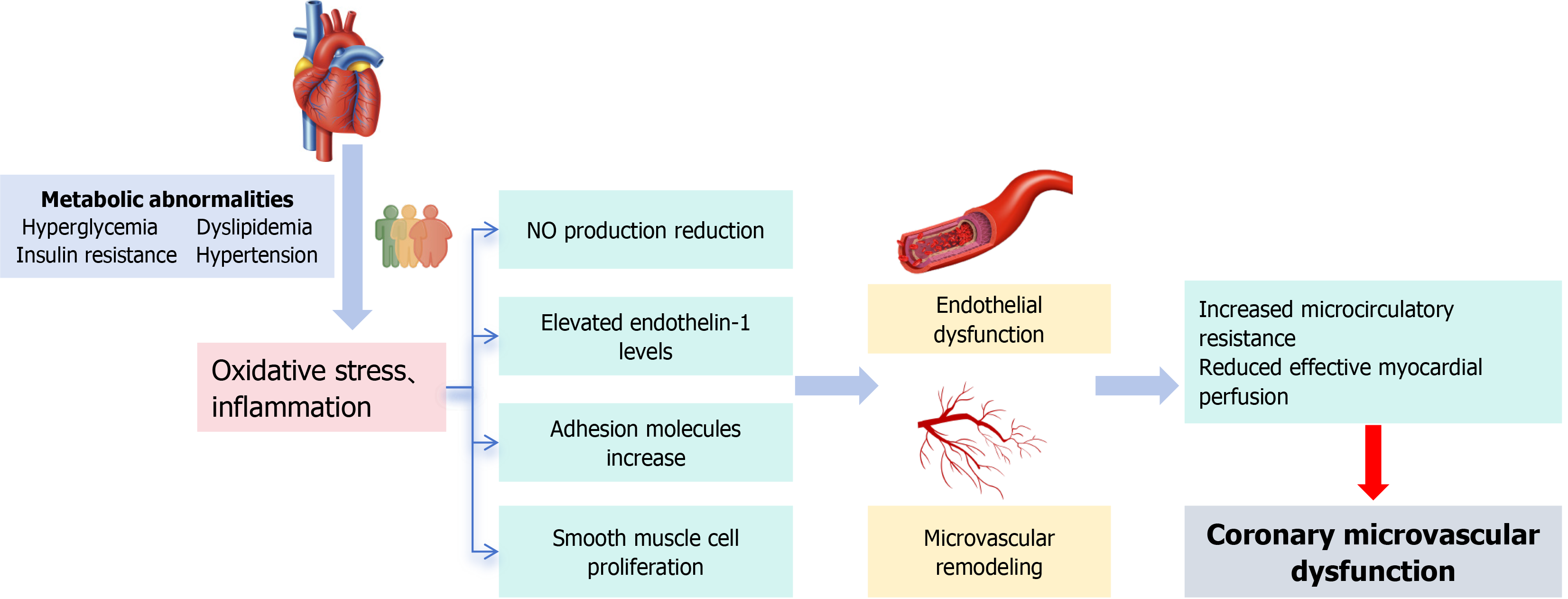
The primary pathophysiological mechanisms of CMD are illustrated in Figure 1. CMD involves complex and multifaceted pathophysiology, including abnormalities in vascular function regulation, compromised microvascular structural integrity, and systemic metabolic disorders[21,22]. These mechanisms may be further exacerbated after PCI, particularly in patients with MetS, who are more prone to developing a vicious cycle[23]. It is important to emphasize that CMD patients often exhibit multiple overlapping pathophysiological processes, which collectively impair the normal physiological function of the coronary arteries and, consequently, reduce myocardial blood flow perfusion.
Figure 1 The main pathophysiological mechanisms of coronary microvascular dysfunction.
NO: Nitric oxide.
Endothelial dysfunction
At the core of microcirculatory dysfunction lies endothelial dysfunction[24]. Endothelial cells play a vital role in maintaining vascular homeostasis by regulating the release of nitric oxide (NO), which promotes vasodilation and exerts anti-inflammatory effects[25]. However, the mechanical stress, oxidative stress, and localized inflammation caused by PCI can damage endothelial cells, leading to reduced NO production and increased levels of endothelin-1 (ET-1), significantly raising microvascular resistance and impairing myocardial perfusion[26]. For example, a study by Sorop et al[7] demonstrated that endothelial dysfunction is a key driver of CMD in animal models of CMD.
In the acute post-PCI phase, endothelial dysfunction is often accompanied by increased endothelial permeability, which can lead to interstitial edema. This phenomenon is driven by the disruption of endothelial tight junctions and the upregulation of vascular endothelial growth factor and other permeability factors. Studies have shown that reperfusion injury following PCI can exacerbate endothelial permeability, resulting in the leakage of plasma proteins and fluid into the interstitial space, thereby contributing to myocardial edema. For instance, a study by Heusch et al[27] demonstrated that reperfusion injury significantly increases endothelial permeability, leading to interstitial edema and impaired myocardial function. This interstitial edema not only exacerbates microvascular dysfunction but also contributes to the "no-reflow" phenomenon, further compromising myocardial perfusion.
Microvascular remodeling
Microvascular remodeling refers to structural changes in coronary microvessels, including smooth muscle cell proliferation, extracellular matrix deposition, and capillary rarefaction[28]. These alterations increase vascular stiffness and resistance, reducing microvascular perfusion efficiency. Chronic inflammation and mechanical stress stimulate vascular smooth muscle cell proliferation, leading to narrowing of the microvascular lumen[29]. Histological studies in patients with CMD have shown increased myocardial microvascular intimal thickness and decreased capillary density[30]. Excessive deposition of matrix proteins, such as collagen, causes perivascular fibrosis, impairing vascular compliance and further restricting blood flow[31]. The loss of capillary networks weakens myocardial oxygen supply, exacerbating ischemic injury. This phenomenon is particularly pronounced in patients with diabetes and MetS. Furthermore, hypertension increases mechanical stress on blood vessels, leading to endothelial injury and subsequent fibrosis, which results in endothelial dysfunction and microvascular remodeling, thereby promoting the development of CMD.
Metabolic abnormalities
Metabolic abnormalities, including hyperglycemia, insulin resistance, and dyslipidemia, significantly contribute to the development of CMD by inducing oxidative stress, inflammation, and endothelial dysfunction[32]. Adipokines, such as leptin and adiponectin, play a significant role in CMD by modulating inflammation and endothelial function. Leptin promotes inflammation and oxidative stress, while adiponectin has anti-inflammatory and vasoprotective effects. Additionally, elevated blood glucose levels accelerate the formation of advanced glycation end products (AGEs), which interact with their receptors to activate inflammatory and oxidative stress pathways[33]. This interaction further impairs NO production and disrupts endothelial cell integrity. Studies have demonstrated that AGEs are significantly elevated in diabetic CMD patients and are closely associated with the deterioration of microvascular function[34]. Insulin resistance inhibits the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway, reducing insulin-mediated vasodilation, promoting inflammation, impairing endothelial function, and further exacerbating microvascular dysfunction[35]. Simultaneously, elevated oxidized low-density lipoprotein (OxLDL) levels not only activate inflammatory pathways and induce endothelial cell apoptosis but also promote foam cell formation, accelerating microvascular atherosclerosis[36]. These metabolic abnormalities collectively worsen microvascular function.
The post-PCI phase is characterized by a robust inflammatory response, driven by the release of pro-inflammatory cytokines such as tumor necrosis factor-alpha, interleukin (IL)-6, and IL-1β. These cytokines promote the migration and proliferation of immune cells, including neutrophils, monocytes, and macrophages, into the coronary microvasculature. For example, a study by Konijnenberg et al[37] demonstrated that PCI-induced inflammation leads to the recruitment of monocytes and macrophages, which contribute to microvascular obstruction and endothelial dysfunction. Additionally, the activation of the complement system further amplifies the inflammatory response, leading to the release of additional cytokines and chemokines that perpetuate immune cell infiltration and microvascular damage. This cytokine-induced immune cell migration and proliferation play a critical role in the pathogenesis of CMD, particularly in the acute post-PCI phase.
Microvascular dysfunction in the post-PCI phase can persist both in the short-term and long-term, depending on the extent of endothelial injury, the severity of reperfusion injury, and the presence of underlying metabolic abnormalities. In the short-term, microvascular dysfunction is primarily driven by acute endothelial injury, oxidative stress, and inflammation, which can lead to transient microvascular obstruction and impaired myocardial perfusion. However, in patients with pre-existing conditions such as MetS or diabetes, microvascular dysfunction can persist long-term due to chronic endothelial dysfunction, microvascular remodeling, and ongoing inflammatory processes. For instance, a study by Hoshino et al[38] demonstrated that microvascular dysfunction can persist for months following PCI, particularly in patients with MetS, due to the chronic effects of oxidative stress and inflammation on endothelial and microvascular function. Therefore, both short-term and long-term changes in microvascular function are observed in the post-PCI phase, with long-term dysfunction being more prevalent in high-risk populations.
DIAGNOSIS OF CMD: CHALLENGES AND ADVANCEMENTS
The diagnosis of CMD is critical in the management of post-PCI patients[39]. However, its complexity and heterogeneity pose significant challenges. CMD is characterized by functional abnormalities of the coronary microvasculature, which cannot be directly visualized using traditional coronary angiography[40]. Consequently, accurate assessment of microcirculatory function relies on a combination of invasive and non-invasive techniques, offering a multidimensional evaluation of hemodynamic parameters and tissue perfusion status.
Invasive techniques remain the "gold standard" for CMD diagnosis[41], particularly during or after PCI, where CFR and IMR are commonly used. These methods utilize pressure wires and flow sensors to directly measure coronary blood flow and resistance, allowing precise quantification of microvascular function. CFR reflects the overall coronary artery's blood flow regulation capacity[42], while IMR specifically evaluates microvascular resistance and function[43]. Combining these parameters helps differentiate the contribution of macrovascular and microvascular dysfunction. In addition, the acetylcholine provocation test helps assess endothelial-dependent vasodilation and aids in determining whether CMD is associated with endothelial dysfunction[44]. Under normal endothelial function, acetylcholine induces vasodilation; however, in the case of endothelial dysfunction, acetylcholine may cause vasoconstriction, leading to vasospasm. In the test, acetylcholine is gradually injected into the coronary arteries after dilution, with each injection followed by a 1-minute waiting period before coronary angiography is performed. The coronary artery diameter is measured using quantitative angiography, which helps confirm the presence of epicardial coronary artery spasm. Additionally, angina symptoms and electrocardiogram (ECG) changes provide important diagnostic clues. If no significant coronary artery spasm is observed but angina symptoms and ischemic ST-T changes are present, CMD is typically diagnosed. In such cases, nitroglycerin or nicorandil should be administered immediately to relieve coronary microvascular spasm. Non-invasive diagnostic techniques have advanced significantly in recent years, expanding the applicability of CMD assessments[45,46]. PET is regarded as the "gold standard" for non-invasive CMD evaluation, capable of accurately quantifying myocardial blood flow reserve, making it particularly useful for postoperative dynamic monitoring[47]. However, its high cost and equipment requirements limit its widespread clinical use. CMR, with its excellent resolution and radiation-free advantage, has emerged as a leading non-invasive tool for CMD detection[48]. Utilizing dynamic perfusion imaging and late gadolinium enhancement techniques, CMR can identify myocardial perfusion deficits and assess tissue fibrosis and microvascular damage[49]. Additionally, coronary computed tomography angiography (CCTA) has emerged as a promising non-invasive tool for evaluating CMD. While CCTA is primarily used to assess large-vessel coronary artery disease, recent advancements in computed tomography-derived fractional flow reserve (CT-FFR) and myocardial perfusion imaging have expanded its application in diagnosing CMD. CT-FFR integrates CCTA's anatomical data with computational fluid dynamics, offering insights into both large-vessel and microvascular function. The NXT trial demonstrated that CT-FFR outperformed CCTA in diagnosing coronary ischemia, achieving a diagnostic accuracy of 80%-85% for detecting microvascular dysfunction, thereby establishing its role as a valuable non-invasive tool for CMD assessment[50]. However, its sensitivity remains limited by its inability to directly visualize microvascular function and its reliance on indirect hemodynamic parameters. Therefore, while CCTA and CT-FFR offer valuable non-invasive diagnostic options, they are often used in conjunction with other imaging modalities, such as PET and CMR, to provide a more comprehensive assessment of microvascular function.
With advancements in diagnostic technology, multiparametric diagnostic models are becoming the trend for CMD evaluation[51,52]. These approaches integrate the strengths of both invasive and non-invasive techniques to provide a more comprehensive understanding of pathophysiological changes. For example, combining CFR and IMR allows simultaneous assessment of both macrovascular and microvascular functions[53], while the combined use of PET and CMR enables dynamic monitoring of postoperative myocardial perfusion changes and quantification of therapeutic effects[54]. Furthermore, AI and machine learning are increasingly being applied to analyze imaging and hemodynamic data in CMD, leveraging big data models to enhance diagnostic efficiency and accuracy. For example, AI algorithms can integrate data from multiple imaging modalities to provide a more comprehensive assessment of microvascular function[55]. Additionally, biomarker research remains a key area in CMD diagnostics. Identifying biomarkers that can accurately reflect microvascular functional changes will enable early screening and dynamic monitoring of CMD. For instance, recent studies have identified circulating microRNAs as potential biomarkers for CMD, offering a non-invasive and cost-effective diagnostic tool[56].
Despite advancements in diagnostic technologies, the early diagnosis and classification of CMD still face significant challenges[57]. On one hand, invasive diagnostic methods carry inherent risks, such as vascular injury and procedural complications, limiting their utility in long-term postoperative monitoring[58]. On the other hand, non-invasive techniques like PET and CMR offer advantages such as no radiation exposure and high resolution, but their high costs and limited availability hinder widespread clinical adoption[59]. Future developments in diagnostic technologies should prioritize the following directions: (1) Optimizing the cost-effectiveness and accessibility of existing techniques to enhance their clinical applicability; (2) Developing simple diagnostic tools based on biomarkers to support screening of high-risk populations; and (3) Advancing the standardization and process optimization of multi-modality diagnostic approaches to lay a solid foundation for precise CMD diagnosis and individualized management.
In summary, CMD diagnostic techniques are advancing toward greater precision, multidimensionality, and non-invasiveness. By integrating invasive and non-invasive methods, supported by AI and novel biomarkers, the diagnostic efficiency and accuracy for CMD are expected to improve further. These advancements will provide critical support for optimizing treatment strategies and long-term management of PCI patients.
MANAGEMENT STRATEGIES FOR CMD: A MULTI-LEVEL APPROACH
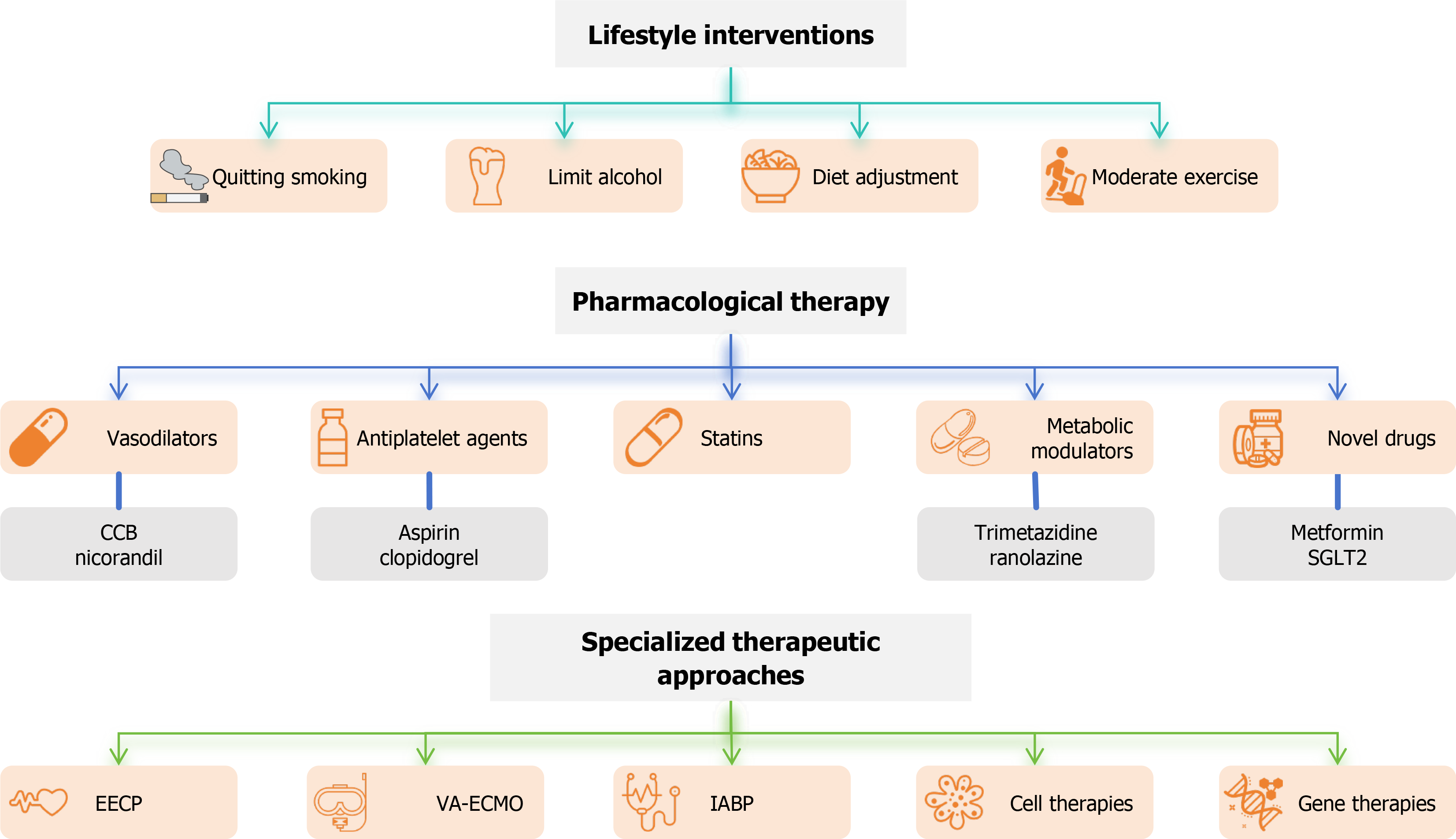
The management of CMD aims to improve myocardial perfusion, alleviate symptoms, and reduce adverse events by integrating lifestyle interventions, pharmacological treatments, and specialized therapeutic approaches (Figure 2). A comprehensive approach to CMD management involves combining pharmacological therapies with lifestyle interventions. For example, statins and antiplatelet agents can be used alongside dietary modifications and exercise to improve endothelial function and reduce cardiovascular risk. Biomarkers are also crucial for the diagnosis and management of CMD[60]. Endothelial-derived biomarkers, such as NO and soluble endothelial selectin (sE-selectin), provide insights into endothelial function[61]. Inflammatory biomarkers, such as C-reactive protein and ILs, along with oxidative stress markers like malondialdehyde and superoxide dismutase, offer valuable information on potential pathophysiological processes. Additionally, metabolic biomarkers, such as the insulin resistance index and lipid profiles, assist in monitoring metabolic risk factors associated with CMD[62]. Given the complex pathophysiology of CMD after PCI and the significant impact of MetS components on its progression, individualized, multi-level intervention strategies are critical. Prevention strategies are equally crucial in reducing the incidence and progression of CMD. Preventing CMD should focus on the early identification of key risk factors such as hypertension, diabetes, and hyperlipidemia. Regular screening and continuous monitoring of endothelial function, particularly through non-invasive imaging techniques and biomarkers, can help detect early signs of CMD. Follow-up care should adjust treatment plans based on changes in the patient's risk profile and ensure adherence to lifestyle interventions. Personalized care plays a pivotal role in improving treatment outcomes and quality of life for CMD patients. Early detection of microvascular dysfunction allows for timely intervention, while personalized treatment plans tailored to the patient's specific needs and risk factors can significantly enhance treatment effectiveness. Continuous monitoring helps dynamically adjust the treatment strategy, optimizing both short-term and long-term outcomes and substantially improving the patient's quality of life.
Figure 2 Coronary microvascular dysfunction management flowchart.
CCB: Calcium channel blocker; EECP: Enhanced external counterpulsation; IABP: Intra-aortic balloon pump; SGLT2: Sodium-dependent glucose transporters 2; VA-ECMO: Veno-arterial extracorporeal membrane oxygenation.
Lifestyle interventions: The foundation of CMD management
Lifestyle interventions serve as the cornerstone of CMD management[63]. Dietary modifications and exercise not only reduce cardiovascular risk but also directly mitigate microvascular dysfunction by improving metabolic status and inflammation[64]. Mediterranean diets, low-sugar, low-fat diets, and regular moderate-to-high-intensity aerobic exercise have been shown to significantly enhance CFR and insulin sensitivity, thereby alleviating myocardial ischemia symptoms[65]. Furthermore, for patients with concurrent obesity, hypertension, or glucose abnormalities, weight management, psychological interventions, and long-term behavioral follow-ups can significantly enhance treatment outcomes[66].
Pharmacological therapy: A key component of CMD management
Management strategies for CMD focus on addressing the key pathophysiological factors, including endothelial dysfunction, oxidative stress, and inflammation. Pharmacological therapies targeting endothelial dysfunction, such as NO donors and endothelin receptor antagonists, have shown promise in improving microvascular function. Pharmacological therapies such as statins, ACE inhibitors, and antioxidants can help restore endothelial function, reduce oxidative stress, and modulate inflammatory pathways[67]. Targeting these molecular pathways is crucial for improving microvascular function and preventing disease progression.
Vasodilators form the cornerstone of pharmacological management for CMD, primarily targeting microvascular spasm and increased microcirculatory resistance. Calcium channel blockers (CCBs) effectively alleviate angina symptoms by dilating the coronary arteries. The combined L-type and T-type calcium channel blocker, efonidipine, has been shown to reduce the frequency of angina episodes in patients with coronary slow-flow, while long-acting dihydropyridine CCBs, such as amlodipine or benidipine, have demonstrated efficacy in alleviating symptoms of primary stable microvascular angina. Studies indicate that benidipine, amlodipine, nifedipine, and diltiazem are the primary CCBs for preventing vasospastic angina episodes, with benidipine showing particular effectiveness in improving outcomes, including reductions in total events, cardiovascular events, and strokes[68]. Nicorandil, a potassium channel opener with nitrate-like properties, holds notable promise in CMD management. In addition to dilating coronary microvasculature, nicorandil exerts cardioprotective effects by reducing oxidative stress and inflammation. Research has demonstrated its significant efficacy in treating primary stable microvascular angina, effectively lowering the incidence of adverse cardiovascular events. Clinical studies have shown that nicorandil markedly improves microvascular function in patients with stable coronary artery disease. For example, among STEMI patients undergoing primary PCI, administration of nicorandil significantly reduced IMR[69].
Antiplatelet agents, such as aspirin and clopidogrel, play a crucial role in the management of CMD by reducing platelet aggregation and preventing microvascular thrombosis. Aspirin, a cyclooxygenase-1 inhibitor, reduces the synthesis of thromboxane A2, a potent vasoconstrictor and platelet aggregator, thereby improving microvascular perfusion. Clopidogrel, a P2Y12 receptor inhibitor, prevents adenosine diphosphate-induced platelet activation, further reducing the risk of microvascular thrombosis. Studies have shown that the combination of aspirin and clopidogrel can significantly improve microvascular function in patients with CMD, particularly in those with acute coronary syndromes or following PCI[70]. For example, a study by Xu et al[71] demonstrated that ticagrelor, a more potent P2Y12 inhibitor, improved coronary microvascular function in patients with non-ST-segment elevation acute coronary syndrome compared to clopidogrel. However, the use of antiplatelet agents must be carefully balanced against the risk of bleeding, particularly in patients with comorbidities or those undergoing long-term therapy.
Statins stabilize atherosclerotic plaques and prevent plaque rupture and acute cardiovascular events by reducing the formation of lipid cores within plaques, inhibiting inflammatory responses, decreasing the accumulation of macrophages and foam cells, promoting fibrous cap thickening, and lowering platelet reactivity. Studies have shown that statins improve endothelial function by increasing the synthesis and release of NO, which maintains vasodilation and normal blood flow, while simultaneously reducing the production of ET-1, thereby enhancing microcirculatory blood flow. For instance, an 8-week atorvastatin treatment significantly improved CFR, highlighting its role in enhancing endothelial function[72]. Additionally, statins have shown benefits in exercise-induced ischemia and brachial artery flow-mediated dilation in patients with cardiac syndrome X[73]. However, some patients may experience side effects, necessitating the combination of statins with other medications or enhanced care interventions. Emerging lipid-lowering therapies, such as PCSK9 inhibitors, have demonstrated substantial efficacy in reducing lipid levels, though their effects on coronary microcirculation require further validation through large-scale studies.
Metabolic modulators, such as trimetazidine and ranolazine, optimize myocardial metabolism by shifting energy production from fatty acid oxidation to glucose utilization, thereby reducing oxygen consumption and alleviating ischemic symptoms. Trimetazidine has been shown to improve microvascular function by enhancing endothelial NO synthase activity and reducing oxidative stress. A study by Rogacka et al[74] demonstrated that trimetazidine significantly prolonged exercise duration in patients with cardiac syndrome X and increased exercise tolerance in patients with INOCA accompanied by microvascular dysfunction, while also reducing the duration of ST-segment depression on ECGs. Similarly, ranolazine has been shown to improve coronary microcirculation, significantly reducing angina symptoms and enhancing exercise tolerance in patients with primary stable microvascular angina. A randomized, double-blind trial found that patients treated with ranolazine had superior Seattle Angina Questionnaire scores and myocardial perfusion reserve index (MPRI) compared to those in the placebo group[75]. The improvement was particularly pronounced in patients with lower baseline MPRI, highlighting its potential benefits for microvascular dysfunction management.
Recent pharmacological advancements have introduced novel drugs targeting specific pathways in the pathogenesis of CMD. Metformin, a widely used antidiabetic medication, has demonstrated significant cardiovascular benefits. Studies have shown that metformin improves acetylcholine-mediated endothelial-dependent microvascular function, reduces body weight, alleviates insulin resistance, and decreases ST-segment depression on ECGs, thereby alleviating angina and myocardial ischemia. Its effects are particularly pronounced in non-diabetic women with normal coronary arteries[76]. Additionally, sodium-glucose co-transporter 2 inhibitors have shown promising effects in the treatment of CMD by improving vascular endothelial function, inhibiting smooth muscle cell proliferation, and reducing oxidative stress and inflammation. These mechanisms collectively contribute to improved coronary microvascular function and reduced CMD-related symptoms. Furthermore, autologous CD34+ cells have demonstrated great potential in cardiovascular disease treatment. These cells exert anti-inflammatory effects and promote vascular repair and regeneration through microcirculation regeneration and paracrine mechanisms, significantly enhancing myocardial tissue perfusion and coronary microvascular function. Clinical studies have shown that ischemic and non-obstructive coronary artery disease patients treated with CD34+ cells experienced significant improvements in CFR within six months of therapy, along with reduced angina symptoms and improved quality of life[77].
Specialized therapeutic approaches
In terms of specialized treatments, enhanced external counterpulsation (EECP) has been widely applied to patients who do not respond to lifestyle adjustments and pharmacological therapies. Kronhaus and Lawson[78] demonstrated that by mechanically increasing myocardial blood supply, EECP significantly improved localized ischemia and endothelial function in patients with X syndrome. For patients with severe obesity or significant metabolic abnormalities, metabolic surgery represents an innovative intervention, offering not only substantial weight loss but also reversing metabolic disorders and enhancing microvascular function. Aminian et al[79] found that metabolic surgery not only reduces body weight and improves coronary microvascular function but also effectively controls blood pressure, blood glucose levels, and reduces the incidence of cardiovascular diseases. For CMD patients with hyperlipidemia who do not respond well to pharmacological treatment, lipoprotein apheresis offers another therapeutic option. By lowering OxLDL levels, it significantly improves microcirculatory resistance and blood flow regulation. A study by Wu et al[80] reported that lipoprotein apheresis effectively improves coronary microvascular function, thereby enhancing myocardial perfusion and restoring endothelial-dependent vasodilation.
Extracorporeal circulatory support systems, such as veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and intra-aortic balloon pump (IABP), are primarily used in patients with cardiogenic shock to enhance coronary blood flow and provide hemodynamic support. While these systems are not routinely used for the management of CMD, there is emerging evidence that they may have a role in ameliorating microvascular dysfunction in specific patient populations. For instance, VA-ECMO has been shown to improve coronary perfusion pressure and reduce microvascular resistance in patients with cardiogenic shock, potentially alleviating microvascular dysfunction in the acute setting[81]. Similarly, IABP can enhance diastolic coronary blood flow, thereby improving myocardial perfusion and reducing microvascular ischemia[82]. However, the use of these devices in CMD management is still investigational, and further studies are needed to determine their efficacy and safety in this context. Innovative treatment strategies, including stem cell therapies and gene therapies, are being explored as potential interventions for CMD. Gene therapy aims to deliver genetic material to repair endothelial dysfunction or modulate oxidative stress pathways, while stem cell therapies may offer the potential to regenerate damaged vascular tissues and restore normal microvascular function[83]. Additionally, traditional Chinese medicine (TCM), with its anti-inflammatory and anti-oxidative properties, has shown promise in improving microvascular function and alleviating CMD symptoms, though further research is needed to validate its clinical efficacy.
Effective management of CMD requires a comprehensive treatment strategy that combines specialized therapies, lifestyle interventions, and pharmacological treatments. For example, EECP and metabolic surgery can be integrated with lifestyle modifications, such as dietary adjustments and increased physical activity, as well as pharmacological interventions, providing a multi-layered treatment approach to optimize patient outcomes. Additionally, emphasizing the importance of multidisciplinary collaboration in CMD management is crucial. This requires the integration of expertise from cardiologists, endocrinologists, nutritionists, and physical therapists to jointly develop personalized treatment plans that address the complex pathological features of CMD. Such interdisciplinary collaboration not only helps resolve the immediate issues of CMD but also effectively reduces the risk of recurrence and complications. Meanwhile, the application of big data and AI in CMD research and patient stratification holds great promise. By analyzing large clinical datasets, AI can identify potential biomarkers, predict disease progression, and assist in developing personalized treatment plans, offering new breakthroughs in precision medicine for CMD.
Case studies provide real-world evidence on the impact of CMD and the effectiveness of interventions, highlighting the central role of microcirculatory evaluation and treatment in cardiovascular disease management. Simion et al[84] and Marpuri et al[85] focused on MINOCA, emphasizing its complex etiology and the pivotal role of microvascular mechanisms, such as microvascular spasm, plaque erosion, and inflammatory responses. They proposed utilizing coronary functional tests (e.g., acetylcholine provocation tests) and imaging techniques (e.g., CMR and OCT) to clarify the underlying causes and develop individualized treatment strategies based on agents like CCBs. Succar et al[86] highlighted the long-term impact of microvascular dysfunction following acute myocardial infarction, noting that even with successful revascularization, microcirculatory damage can increase the risk of myocardial remodeling and arrhythmias. This underscores the importance of ongoing monitoring with myocardial perfusion imaging techniques. Gao et al[87] reported a case of PCIS following PCI, and through a literature review, they pointed out that immune-inflammatory responses after myocardial injury could lead to pericarditis or pericardial effusion. They emphasized the need to differentiate these from microvascular abnormalities and highlighted the role of anti-inflammatory therapy in management. The FLOW-CTO study by García-Guimarães et al[88] revealed that in some patients undergoing interventions for chronic total occlusion (CTO) lesions, absolute coronary blood flow did not improve post-procedure. This finding suggested that microvascular dysfunction might limit the clinical benefits of revascularization, stressing the necessity of optimizing interventional strategies. These studies collectively demonstrate the critical role of microvascular dysfunction in various cardiovascular conditions, including MINOCA, post-infarction complications, CTO intervention outcomes, and PCIS. They drive a shift in clinical practice from a traditional focus on large-vessel treatment to integrated strategies incorporating microcirculatory assessment and intervention for precise cardiovascular disease management.
THE RELATIONSHIP BETWEEN CMD AND METS: A COMPLEX INTERPLAY
CMD and MetS are intricately linked through shared pathological mechanisms and reciprocal interactions, forming a vicious cycle that significantly exacerbates cardiovascular risk in post-PCI patients. The core components of MetS-obesity, hyperglycemia, hypertension, and dyslipidemia-are not only major risk factors for CMD but also key drivers of its development and progression.
The interaction between CMD and MetS involves multiple molecular pathways. Hyperglycemia leads to the formation of AGEs and activation of insulin resistance-related signaling pathways, such as the PI3K/AKT pathway. This results in elevated levels of free fatty acids in circulation, further exacerbating endothelial dysfunction[89]. Simultaneously, the nuclear factor kappa B pathway in inflammation promotes vascular inflammation, while oxidative stress impairs the availability of endothelial NO, damaging vascular relaxation function and contributing to microvascular dysfunction[90]. Additionally, adiponectin secreted by perivascular adipose tissue due to obesity plays a role in both endothelial dysfunction and inflammation. These pathways interact, ultimately leading to endothelial dysfunction and microvascular remodeling. Studies suggest that microvascular dysfunction often precedes macrovascular abnormalities during the early stages of metabolic dysregulation, highlighting the heightened sensitivity of the microcirculation to metabolic disturbances. Hypertension imposes long-term mechanical stress on the vasculature, altering both structure and function in the microvasculature. Smooth muscle cell proliferation, extracellular matrix deposition, and lumen narrowing are hallmarks of microvascular remodeling caused by hypertension. Furthermore, heightened sympathetic nervous system activation, often observed in hypertensive patients, increases myocardial oxygen demand and induces coronary microvascular spasms, compounding microvascular dysfunction.
CMD and MetS form a mutually reinforcing vicious cycle. Through mechanisms such as inflammation, oxidative stress, and insulin resistance, MetS induces microvascular dysfunction, while CMD further exacerbates myocardial ischemia, triggering additional inflammatory responses and oxidative stress[91]. This imbalance in microvascular function accelerates the progression of metabolic abnormalities, creating a feedback loop that significantly increases cardiovascular risk in post-PCI patients.
ADVANCING THE UNDERSTANDING AND MANAGEMENT OF CMD: FUTURE DIRECTIONS
Current advancements in Post-PCI CMD research include the development of more sensitive diagnostic tools, such as advanced imaging techniques and novel biomarkers. Additionally, personalized therapeutic approaches, including targeted pharmacotherapy and lifestyle interventions, are gaining traction. Future research will likely focus on understanding the genetic basis of CMD, exploring innovative treatment modalities such as gene editing, and leveraging AI to predict disease progression and optimize patient management. Additionally, the future of CMD research requires a balanced emphasis on basic and clinical science, focusing on elucidating mechanisms, optimizing diagnostics, developing precision therapies, and advancing prevention strategies. A multidimensional, multidisciplinary approach is essential for achieving comprehensive management of this complex condition. By continuing to deepen our understanding of CMD and integrating emerging technologies and innovative strategies, we can provide more effective solutions for the long-term management of PCI patients, ultimately improving patient outcomes and quality of life.
Deepening the understanding of pathological mechanisms
While substantial research has been conducted on CMD's core pathological mechanisms, there remain notable gaps[92]. Future basic research should delve into the molecular networks underlying microvascular dysfunction, with particular emphasis on the interplay between endothelial dysfunction, microvascular remodeling, and reperfusion injury. Furthermore, the impact of MetS on CMD warrants detailed exploration. Key areas include the mechanisms of obesity-related adipokines, oxidative stress pathways and AGEs triggered by hyperglycemia, microvascular pressure adaptations in hypertension, and endothelial damage caused by dyslipidemia-induced pro-inflammatory responses. Advancing the understanding of these mechanisms may uncover novel diagnostic and therapeutic targets.
Emerging fields such as non-coding RNAs, exosomes, and the gut microbiome hold critical insights into CMD development. Non-coding RNAs, including microRNAs and long non-coding RNAs, play a pivotal role in regulating endothelial function, vascular inflammation, and smooth muscle remodeling. For example, miR-126 has been shown to promote endothelial repair by enhancing NO production and suppressing pro-inflammatory pathways[93]. Targeting ncRNAs through synthetic mimics or inhibitors represents a promising therapeutic strategy to modulate key CMD mechanisms, such as endothelial dysfunction and oxidative stress. Exosomes, small extracellular vesicles, facilitate intercellular communication by transporting bioactive molecules, including miRNAs, lipids, and proteins. Studies have demonstrated that exosomes derived from healthy endothelial cells can repair damaged endothelium in CMD by delivering anti-inflammatory and pro-angiogenic factors[94]. Leveraging exosome-based therapies could revolutionize the treatment of CMD, providing a vehicle for targeted delivery of therapeutic molecules. The gut microbiome has emerged as a key player in cardiovascular health, influencing systemic inflammation, insulin resistance, and vascular function. Dysbiosis, characterized by an imbalance of gut microbiota, is linked to increased levels of trimethylamine-N-oxide, a metabolite associated with CMD and cardiovascular events[95]. Interventions aimed at modulating the gut microbiome, such as probiotics, dietary changes, or fecal microbiota transplantation, may offer novel avenues to address CMD.
Translating these findings into clinical applications is crucial for advancing CMD management. Future research should focus on developing biomarkers based on non-coding RNAs and exosomes for early CMD detection, as well as exploring microbiome-based therapies to improve microvascular function. By bridging the gap between basic research and clinical practice, we can develop more effective diagnostic and therapeutic strategies for CMD.
Optimizing diagnostic approaches
Although advancements in invasive and non-invasive techniques have provided tools for CMD detection, significant gaps remain in sensitivity, specificity, and clinical accessibility[96]. Future efforts should focus on developing integrative diagnostic techniques that combine multi-modal imaging (e.g., PET, CMR) with functional assessments (e.g., CFR, IMR), leveraging AI to enhance diagnostic precision and efficiency.
Biomarker research also remains a key area in CMD diagnostics. Identifying biomarkers that can accurately reflect microvascular functional changes will enable early screening and dynamic monitoring of CMD. Such advancements will bridge current gaps, making CMD diagnostics more accessible, efficient, and clinically actionable.
Developing precision treatment strategies
The development of precision treatment strategies will be a focal point for future CMD research. Current CMD treatments largely rely on empirical, broad-spectrum interventions, which lack the specificity required for individual patients[97]. Future studies should prioritize targeted interventions for metabolic abnormalities and develop multi-target therapies that combine anti-inflammatory, antioxidant, and endothelial-protective effects to improve microvascular function.
Innovative treatment methods, such as stem cell therapies and gene therapies targeting reperfusion injury, hold the potential to revolutionize CMD management. Meanwhile, non-pharmacological interventions, including EECP, metabolic surgeries, and lipoprotein apheresis, require further investigation to better understand their mechanisms and broaden their clinical applications.
Promoting multidisciplinary collaboration and holistic management
CMD is not solely a cardiovascular challenge but also involves metabolic, inflammatory, and psychological dimensions[98]. Future efforts should emphasize multidisciplinary collaboration, integrating expertise from cardiology, metabolism, psychology, and public health to establish comprehensive treatment frameworks and long-term follow-up systems tailored to individual patient characteristics.
The integration of big data and AI technologies will further enhance CMD research efficiency. By analyzing large-scale cohort data, researchers can accurately identify high-risk CMD patients and optimize diagnostic and treatment protocols. These advancements will pave the way for more effective patient stratification and individualized management strategies.
Strengthening preventive strategies
Prevention remains a critical yet underexplored aspect of CMD management. Intervening at the early stages of metabolic abnormalities could significantly delay CMD onset and progression[99]. The development of precision prevention models, incorporating behavioral interventions, metabolic modulation, and inflammation control, has the potential to reduce CMD incidence in post-PCI patients fundamentally. Such strategies would ultimately improve long-term patient outcomes.
CONCLUSION
CMD is one of the primary causes of persistent myocardial ischemia in patients following PCI. Its complex pathophysiological mechanisms and multidimensional clinical manifestations pose significant challenges to diagnosis and management. This article provides a comprehensive review of CMD's pathophysiology, diagnostic techniques, and management strategies, with an in-depth discussion of the impact of MetS on CMD development and progression.
The core pathological mechanisms of CMD include endothelial dysfunction, microvascular remodeling, reperfusion injury, and metabolic abnormalities. The major components of MetS-obesity, hyperglycemia, hypertension, and dyslipidemia-exacerbate microcirculatory dysfunction through inflammatory responses, oxidative stress, and insulin resistance. Advances in CMD diagnostic techniques have broadened the tools available for early detection, ranging from traditional invasive assessments (e.g., CFR and microcirculatory resistance index) to emerging non-invasive imaging technologies (e.g., PET and CMR). However, current diagnostic methods still face limitations in sensitivity, cost-effectiveness, and accessibility, highlighting the need for further optimization. In terms of management, CMD treatment strategies emphasize a combination of healthy lifestyle interventions and pharmacological therapies, complemented by specialized therapeutic approaches such as EECP and metabolic surgery to enable multi-level interventions. Precision therapies targeting metabolic abnormalities represent a crucial direction for future CMD management. Additionally, TCM, with its multi-target effects, shows potential value in CMD management, though further high-quality clinical studies are required to validate its efficacy.
Future research should focus on elucidating the interplay between CMD and MetS, optimizing diagnostic tools, and developing individualized, multi-targeted precision treatment plans. Strengthening multidisciplinary collaboration and implementing holistic management practices are equally important. By deepening our understanding of pathophysiology and advancing diagnostic and therapeutic approaches, significant breakthroughs in CMD management are expected, offering new hope for improving the long-term prognosis of PCI patients.
In summary, CMD remains a significant challenge in the management of PCI patients, and its diagnosis and treatment are rapidly evolving. Integrating the latest research findings with clinical practice, and emphasizing precision interventions and comprehensive management of metabolic abnormalities, will be central to advancing the field of CMD in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade B, Grade D
Novelty: Grade A, Grade B, Grade B, Grade B
Creativity or Innovation: Grade B, Grade B, Grade B, Grade C
Scientific Significance: Grade A, Grade B, Grade B, Grade B
P-Reviewer: Khurram MF; Kumar D; Paparoupa M S-Editor: Luo ML L-Editor: A P-Editor: Zhao S