Published online Feb 26, 2025. doi: 10.4330/wjc.v17.i2.101588
Revised: December 25, 2024
Accepted: January 21, 2025
Published online: February 26, 2025
Processing time: 158 Days and 14.7 Hours
Coronary heart disease (CHD) and pulmonary embolism (PE) are thrombotic diseases. Patients with CHD and PE are common in clinical practice. However, the clinical diagnosis of PE is challenging due to overlapping primary symptoms, such as chest tightness and dyspnea. This confluence frequently leads to the misdiagnosis of PE, thus precipitating treatment delays and compromising patient outcomes. Herein, we report the case of a patient with both diseases who under
A 51-year-old man with a history of hypertension for 2 years visited a local hospital because of paroxysmal chest tightness for 1 d and was diagnosed with CHD. However, he refused hospitalization. He visited our hospital for the treatment of recurring symptoms. A comprehensive examination after admission revealed elevated D-dimer levels, and computed tomography pulmonary angio
D-dimer is useful in screening for PE, whereas computed tomography pulmonary angiography is important for diagnosis. For patients with CHD and PE, coronary artery bypass grafting combined with anticoagulant and antiplatelet therapy is feasible.
Core Tip: Coronary heart disease and pulmonary embolism are common cardiovascular diseases encountered in clinical practice. Their primary symptoms, such as chest tightness and dyspnea, are similar and lack specificity. Therefore, when these two diseases coexist in clinical practice, they are frequently misdiagnosed or missed. Here, we report the case of a patient who presented with paroxysmal chest tightness lasting 2 d. He was diagnosed with coronary heart disease combined with pulmonary embolism. The patient underwent coronary artery bypass grafting and received anticoagulant and antiplatelet drugs, resulting in a favorable prognosis.
- Citation: Xu JQ, Jiang MX, Wang F, Yang KQ, Xu YJ, Wang YJ, Dong SJ. Coronary heart disease with pulmonary embolism: A case report. World J Cardiol 2025; 17(2): 101588
- URL: https://www.wjgnet.com/1949-8462/full/v17/i2/101588.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i2.101588
Cardiovascular disease is one of the leading causes of death worldwide, with atherosclerotic coronary heart disease (CHD) being the most prevalent and accounting for two-thirds of global cardiovascular disease deaths annually[1-3]. CHD is a formidable cardiac pathology characterized by gradual narrowing or complete occlusion of the coronary arteries owing to atherosclerotic plaque formation. This process results in myocardial ischemia and hypoxic necrosis, collectively termed ischemic heart disease, which is primarily divided into chronic myocardial ischemia syndrome and acute coronary syndrome[4]. Currently, several treatment methods exist for CHD, including drug intervention and surgery. For patients with severe CHD involving multiple vessels and significant left main stem lesions and those with good general condition who can tolerate open chest surgery, coronary artery bypass grafting (CABG) is the preferred treatment. Postoperative treatment should include combined anticoagulants, antiplatelet agents, β-receptor antagonists, and statins to improve prognosis[5,6].
Pulmonary embolism (PE) is a general term for a group of diseases caused by various emboli blocking the pulmonary artery or its branches, triggering a series of pathophysiological sequelae. Among these, pulmonary thromboembolism is the predominant subtype. Pulmonary thromboembolism mainly originates from deep venous thrombosis, which is a distinct manifestation of the same disease in different parts of the body and at different stages. Therefore, these conditions are collectively referred to as venous thromboembolism (VTE). The morbidity and mortality rates of PE are high, making it the third most common cardiovascular and cerebrovascular disease, closely following CHD and stroke[7]. Both CHD and PE have many similarities in clinical symptoms and electrocardiogram (ECG) findings. Therefore, they are easily misdiagnosed clinically. Chest discomfort constitutes the predominant clinical presentation in emergencies. A single-center retrospective study showed that 10%-20% of patients admitted to the emergency department with complaints of chest discomfort were initially diagnosed with acute coronary syndrome. These patients then underwent examinations such as cardiac enzymes and coronary angiography (CAG), which may occasionally lead to missed or misdiagnosed PE. The application of the Wells score is helpful for the initial suspicion of acute PE (APE). Plasma D-dimer level is of great significance for excluding APE, and the combination of the two can improve the specificity of APE diagnosis[8,9].
A 51-year-old man presented to the hospital with paroxysmal chest tightness that had persisted for 2 d.
The onset of symptoms occurred spontaneously approximately 2 d prior and was characterized by transient chest tightness lasting approximately 30 sec, spontaneously resolving without intervention.
The patient had a history of hypertension for 2 years. No treatment was administered at home after the above symptoms appeared, and he went to a local hospital 1 d ago, where comprehensive examination revealed the following: Troponin T levels (14.14 pg/mL), triglyceride levels (1.81 mmol/L), cholesterol (5.87 mmol/L), high density lipoprotein cholesterol (1.55 mmol/L), and low density lipoprotein cholesterol (3.59 mmol/L). ECG revealed sinus rhythm and ST-T changes. Chest computed tomography revealed bilateral pulmonary inflammation, coronary sclerosis, and intrahepatic calcification. Although the doctor recommended hospitalization, the patient refused hospitalization for personal reasons. However, the recurrence of these symptoms prompted subsequent admission to the cardiology department of our hospital for further treatment.
The patient denied a family history of congenital heart disease, heart failure, or respiratory illnesses.
Physical examination results on admission were as follows: Temperature (36.2°C), pulse rate (75 beats/min), respiration rate (18 breaths/min), and blood pressure (18.4/11.3 kPa). The patient’s consciousness and spirit levels were normal. Bilateral lung auscultation revealed clear respiratory sounds, without notable dry or wet rales. Cardiac auscultation revealed a heart rate of 75 beats/min with a regular rhythm, with no obvious pathological murmurs in any valve auscultation area. The abdomen was flat and soft, with no tenderness or rebound pain, and no signs of edema were observed in either lower limb.
Biochemical profiling revealed the following: Total cholesterol (6.20 mmol/L), high density lipoprotein cholesterol (1.68 mmol/L), low density lipoprotein cholesterol (4.27 mmol/L), blood homocysteine (16.60 μmol/L), creatine kinase isoenzyme (5.07 ng/mL), lactate dehydrogenase (283.80 U/L), and D-dimer (0.90 mg/L). Coagulation parameters, brain natriuretic peptide levels, and thyroid function test results showed no discernible abnormalities.
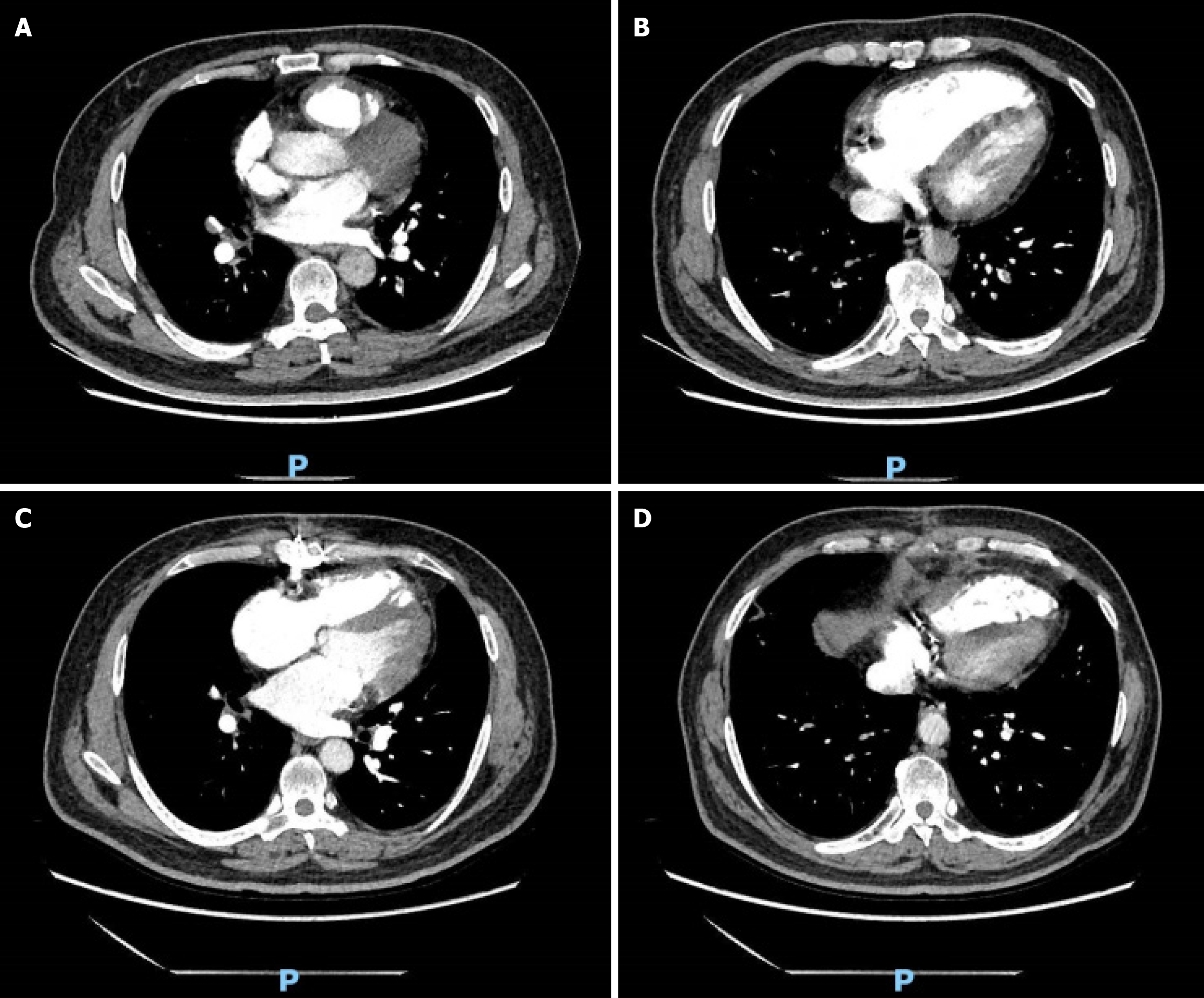
ECG revealed a sinus rhythm with ST-T changes. Echocardiography revealed right heart enlargement, partial hypokinesis of the left ventricular wall, right heart hypofunction, mild tricuspid regurgitation, moderate pulmonary hypertension, low left ventricular diastolic function, and an ejection fraction of 60%. PE could not be ruled out based on the above examination results. Therefore, computed tomography pulmonary angiography (CTPA) was performed to further elucidate the diagnosis, suggesting pulmonary hypertension, multiple PEs, and infectious lesions in both lungs (Figure 1A and B). CAG was performed, and the results indicated that the blood supply to the coronary artery was right-dominant, and severe calcification was observed along the left and right coronary artery tracing areas. Specifically, the findings included 80% stenosis of the left main coronary artery distal segment, 90% stenosis at the origin of the left anterior descending (LAD) artery with complete proximal occlusion, and thrombolysis in myocardial infarction (TIMI) grade 0 for coronary blood flow. Additionally, 60%-70% stenosis was observed at the left circumflex artery origin, accompanied by TIMI grade 3. The right coronary artery demonstrated no significant stenosis, with TIMI grade 3.
Based on a comprehensive assessment, the final diagnoses were unstable angina pectoris, coronary atherosclerotic heart disease, PE, pulmonary hypertension (moderate), hypertension grade 1 (at very high risk), hyperlipidemia, and pulmonary infection.
The cardiologist attempted to implant a stent in the LAD artery but ultimately failed. Consequently, the patient was referred to the cardiac surgery department for further consultation and intervention. Upon comprehensive assessment, the patient’s diagnosis was confirmed, interventional therapy was deemed unfeasible, and conservative medical treatment was ineffective. Therefore, CABG was recommended to improve the heart blood supply. We performed CABG on the LAD and obtuse margin using the left internal mammary artery and great saphenous vein. The patient recovered well postoperatively. Anticoagulation and antiplatelet therapy with warfarin and aspirin were initiated early postoperatively, along with secondary preventive drugs for CHD, such as rosuvastatin calcium, rabeprazole sodium, metoprolol tartrate, and isosorbide mononitrate.
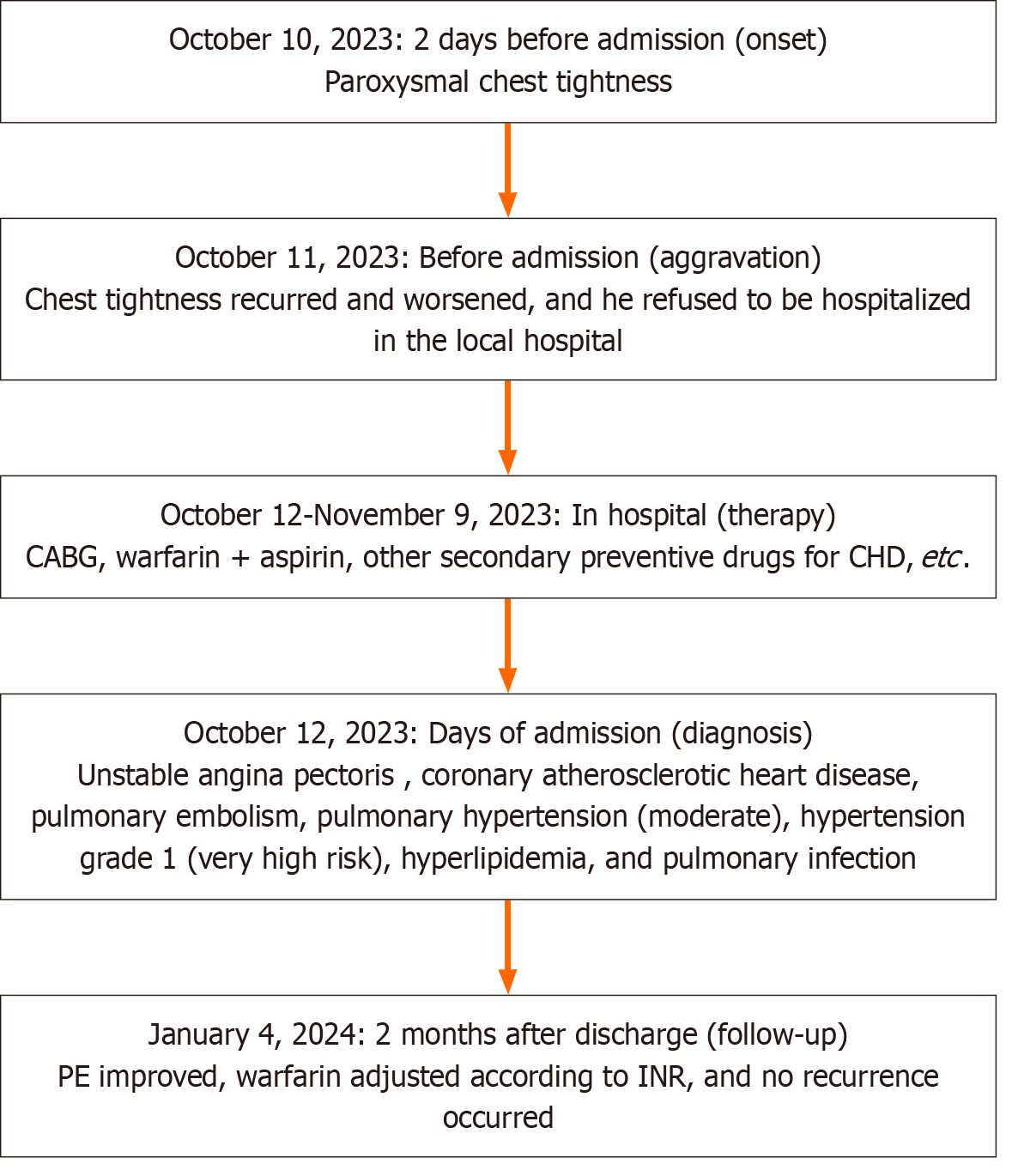
Prior to discharge, echocardiography indicated mild pulmonary hypertension, and CTPA demonstrated that the filling defect of both pulmonary arteries was reduced compared to the preoperative state, indicating a considerable improvement in the patient’s condition. Subsequent outpatient management entailed oral continuation of the above-mentioned drugs alongside ezetimibe and ambrisentan tablets, with regular monitoring of the international standardized ratio. At the time of writing, the patient had been under follow-up for over 2 mo and was recovering well (Figure 1C and D). A case summary is presented in Figure 2.
The clinical symptoms of PE are diverse and lack specificity, with studies showing that approximately 5%-10% of patients present with chest pain and dyspnea. Patients with PE can present with dyspnea, syncope, and cyanosis. The so-called “triad of acute pulmonary embolism”, namely, dyspnea, chest pain, and hemoptysis, is infrequently encountered[10]. Consequently, nonspecific clinical symptoms accompanied by ECG changes often complicate diagnosis, leading to misdiagnosis, particularly in CHD. In the present case, the patient initially exhibited an episode of chest tightness, which was relieved after rest. This is a typical manifestation of unstable angina pectoris associated with CHD. After admission, a thorough examination revealed that the D-dimer level was elevated (0.90 mg/L), and ECG showed right heart dysfunction, mild tricuspid regurgitation, moderate pulmonary hypertension, and impaired left ventricular diastolic function. Consequently, PE was suspected and subsequently confirmed via CTPA, which revealed multiple PEs in both lungs. Therefore, when patients present with chest pain or tightness after activity accompanied by abnormal ECG findings, in addition to CHD, angina pectoris, and myocardial infarction, PE must also be considered as a differential diagnosis. Patients with suspected PE should undergo arterial blood gas analysis to assess hypoxemia[11]. Furthermore, D-dimer levels should be checked promptly, as it serves as a pivotal diagnostic adjunct. The sensitivity of D-dimer for PE diagnosis is high, ranging from 92% to 100%, but its specificity is low, at only 40%-43%. Therefore, a negative D-dimer level can be used as an exclusion index for the diagnosis of PE. If the D-dimer level is less than 500 μg/L, PE can be ruled out. In contrast, if the D-dimer level is elevated, CTPA can be used to further confirm the diagnosis[12,13]. Owing to its noninvasive nature, high sensitivity, and specificity, CTPA has replaced conventional gold standard pulmonary arteriography and has become the preferred diagnostic method[14].
Both cardiovascular events and PE are underpinned by high vascular inflammation and blood hypercoagulability, which serve as the basis for embolic events. Common risk factors, such as smoking, obesity, hypertension and hyperlipidemia, can exacerbate vascular inflammation and disrupt the balance between procoagulant and anticoagulant factors in the bloodstream. This imbalance increases blood viscosity, thereby promoting the occurrence of embolic events[15,16]. Emboli that cause PE may originate from various anatomical sources, including the inferior vena cava, superior vena cava, and the right cardiac chamber, with a predominant origin in the deep veins of the lower extremities. Notably, in this case, colored Doppler ultrasonography of the lower limb veins revealed no obvious abnormalities, whereas colored Doppler ultrasonography of the lower limb arteries indicated the presence of calcification spots without concurrent thrombosis. Moreover, this patient underwent CABG using the left internal mammary artery and the great saphenous vein as bridging vessels. Although CABG can significantly improve quality of life for patients with CHD, surgery also has inherent risks. These risks include vascular trauma and prolonged postoperative immobilization, which predispose them to venous thrombosis. Du et al[17] conducted a clinical study that included 8956 patients undergoing CABG, among whom the incidence of postoperative VTE was 1.75%, PE was 0.61%, deep venous thrombosis was 1.28%. A total of 0.15% of patients experienced both conditions within 30 days after surgery. Furthermore, post-CABG patients may experience dyspnea and pleural effusion owing to sternal pain and pleural exudates, which may mask the clinical manifestations of PE and lead to misdiagnosis. Therefore, patients undergoing CABG should undergo early exercise, mechanical prophylaxis with graduated compression stockings and intermittent pneumatic compression devices, or pharmacological prophylaxis with subcutaneous heparin injection to prevent VTE[18].
Clinical reports of individual cases of CHD combined with PE have been rare in recent years[19,20]. The diagnosis and treatment of patients with a combination of the two diseases are often complicated. The key to ensuring an accurate diagnosis is to improve the clinicians’ understanding of the clinical manifestations and pathophysiology of PE, enhance the awareness of the differential diagnosis of CHD and PE, pay attention to its risk factors, and rationally use laboratory or imaging examinations. If a patient is suspected to have PE, arterial blood phase analysis and D-dimer testing should be performed as soon as possible. Second, early echocardiography, which is a crucial tool for risk stratification and identification of other fatal diseases such as aortic dissection, should be conducted. For definitive diagnosis, radionuclide ventilation/perfusion scanning or CTPA should be performed. In addition, CAG is recommended as soon as possible to determine the treatment plan for patients with typical CHD symptoms.
Based on this case, we summarize the following experiences in the treatment of CHD with PE. First, the treatment of patients with CHD complicated by PE should emphasize these two aspects simultaneously. On the one hand, anticoagulant therapy, such as low-molecular-weight heparin or warfarin, should be administered. On the other hand, antiplatelet therapy, such as aspirin or clopidogrel, should also be administered. Anticoagulants reduce the risk of thrombotic events in the arteries. Moreover, antiplatelet drugs and low-dose anticoagulants have also been recommended by the European Heart Association for secondary prevention in individuals with chronic coronary syndrome[21]. Second, individualized treatment strategies should be implemented after a comprehensive assessment of the patient’s overall condition. If PE presents acutely, thrombolysis or anticoagulation therapy should be administered first. Conversely, if CHD is more severe, percutaneous coronary intervention or CABG should be performed first. Moreover, patients should be followed up and monitored for changes in the patient’s condition to improve the prognosis.
This case has significance in guiding the treatment of patients with CHD and PE. CABG combined with anticoagulation and antiplatelet therapies is feasible for such patients. Clinically, D-dimer screening should be performed. If PE is suspected, CTPA should be performed as soon as possible to confirm the diagnosis and determine a personalized treatment strategy.
| 1. | Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J Am Coll Cardiol. 2022;80:2361-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 955] [Reference Citation Analysis (0)] |
| 2. | Perak AM, Ning H, Khan SS, Bundy JD, Allen NB, Lewis CE, Jacobs DR Jr, Van Horn LV, Lloyd-Jones DM. Associations of Late Adolescent or Young Adult Cardiovascular Health With Premature Cardiovascular Disease and Mortality. J Am Coll Cardiol. 2020;76:2695-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 3. | Khan MA, Hashim MJ, Mustafa H, Baniyas MY, Al Suwaidi SKBM, AlKatheeri R, Alblooshi FMK, Almatrooshi MEAH, Alzaabi MEH, Al Darmaki RS, Lootah SNAH. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus. 2020;12:e9349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 4. | Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234:16812-16823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 593] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 5. | Sulava EF, Johnson JC. Management of Coronary Artery Disease. Surg Clin North Am. 2022;102:449-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Schwinger RHG. [Secondary prevention for coronary heart disease]. MMW Fortschr Med. 2023;165:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wenger N, Sebastian T, Engelberger RP, Kucher N, Spirk D. Pulmonary embolism and deep vein thrombosis: Similar but different. Thromb Res. 2021;206:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Wang J, Wu XY, Liang Y, Guo W. Predictive value of the Wells score combined with D-dimer level in identifying acute pulmonary embolism in patients with coronary heart disease with chest pain. Chin Med J (Engl). 2020;133:2253-2255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Duffett L, Castellucci LA, Forgie MA. Pulmonary embolism: update on management and controversies. BMJ. 2020;370:m2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Freund Y, Cohen-Aubart F, Bloom B. Acute Pulmonary Embolism: A Review. JAMA. 2022;328:1336-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 11. | Pulmonary Embolism and Pulmonary Vascular Disease Group of the Chinese Medical Association Respiratory Medicine Branch; Pulmonary Embolism and Pulmonary Vascular Disease Working Committee of the Chinese Medical Association Respiratory Physician Branch; National Pulmonary Embolism and Pulmonary Vascular Disease Prevention and Control Collaboration Group. [Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism]. Zhonghua Yixue Zazhi. 2018;98:1060-1087. [DOI] [Full Text] |
| 12. | Patel H, Sun H, Hussain AN, Vakde T. Advances in the Diagnosis of Venous Thromboembolism: A Literature Review. Diagnostics (Basel). 2020;10:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Giannitsis E, Mills NL, Mueller C. D-Dimer in suspected pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2023;12:721-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Essien EO, Rali P, Mathai SC. Pulmonary Embolism. Med Clin North Am. 2019;103:549-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 15. | Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 727] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 16. | Gregson J, Kaptoge S, Bolton T, Pennells L, Willeit P, Burgess S, Bell S, Sweeting M, Rimm EB, Kabrhel C, Zöller B, Assmann G, Gudnason V, Folsom AR, Arndt V, Fletcher A, Norman PE, Nordestgaard BG, Kitamura A, Mahmoodi BK, Whincup PH, Knuiman M, Salomaa V, Meisinger C, Koenig W, Kavousi M, Völzke H, Cooper JA, Ninomiya T, Casiglia E, Rodriguez B, Ben-Shlomo Y, Després JP, Simons L, Barrett-Connor E, Björkelund C, Notdurfter M, Kromhout D, Price J, Sutherland SE, Sundström J, Kauhanen J, Gallacher J, Beulens JWJ, Dankner R, Cooper C, Giampaoli S, Deen JF, Gómez de la Cámara A, Kuller LH, Rosengren A, Svensson PJ, Nagel D, Crespo CJ, Brenner H, Albertorio-Diaz JR, Atkins R, Brunner EJ, Shipley M, Njølstad I, Lawlor DA, van der Schouw YT, Selmer RM, Trevisan M, Verschuren WMM, Greenland P, Wassertheil-Smoller S, Lowe GDO, Wood AM, Butterworth AS, Thompson SG, Danesh J, Di Angelantonio E, Meade T; Emerging Risk Factors Collaboration. Cardiovascular Risk Factors Associated With Venous Thromboembolism. JAMA Cardiol. 2019;4:163-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 17. | Du W, Zhao X, Nunno A, Li Y, Gu Y. Risk factors for venous thromboembolism in individuals undergoing coronary artery bypass grafting. J Vasc Surg Venous Lymphat Disord. 2020;8:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Eisenga J, Hocking J, Kluis A, DiMaio JM, Shih E, Schaffer J, Moore DO, Ryan W, Hutcheson K; Heart Hospital Consortium on Deep Venous Thrombosis. A comprehensive deep venous thrombosis prophylaxis regimen in isolated coronary artery bypass grafting. JTCVS Open. 2024;17:145-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Zelfani S, Manai H, Laabidi S, Wahabi A, Akeri S, Daghfous M. Pulmonary embolism mimicking acute myocardial infarction: a case report and review of literature. Pan Afr Med J. 2019;33:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Jiang MH, Wang XH, Zhang LF, Zhou X, Huang X, Cao XB. [Clinical analysis of two cases of coronary heart disease combined with pulmonary embolism]. Zhonghua Laonian Duo Qiguan Jibing Zazhi. 2017;16:298-299. [DOI] [Full Text] |
| 21. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 4539] [Article Influence: 907.8] [Reference Citation Analysis (0)] |