Published online Jan 26, 2025. doi: 10.4330/wjc.v17.i1.99933
Revised: December 7, 2024
Accepted: January 7, 2025
Published online: January 26, 2025
Processing time: 171 Days and 0.2 Hours
Atrial fibrillation (AF)/atrial flutter (AFL) is the most common sustained cardiac arrhythmia. The known risk factors for developing AF/AFL include age, struc
Core Tip: This research discusses atrial fibrillation and flutter-related mortality in an older population, of the United States, using the nationwide Centers for Disease Control and Prevention database. The paper further demonstrates the trend analysis of demographic factors from 1999 to 2020.
- Citation: Sukaina M, Waheed M, Rehman S, Hasibuzzaman MA, Meghani R. Demographic trends in mortality with older population due to atrial fibrillation and flutter from 1999-2020. World J Cardiol 2025; 17(1): 99933
- URL: https://www.wjgnet.com/1949-8462/full/v17/i1/99933.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i1.99933
Atrial fibrillation (AF)/atrial flutter (AFL) is the most common sustained cardiac arrhythmia. The known risk factors for developing AF/AFL include age, structural heart disease, hypertension, diabetes mellitus, or hyperthyroidism[1-3]. Cardiovascular disease including cardiomyopathy, hypertension, pulmonary disease, and left ventricular hypertrophy, are the risk factors for AF[4]. A multi-cohort study by Kivimäki et al[4] found long working hours to be a potential con
One of the recognized causes of the vulnerability of AF/AFL is inflammation. The lifetime risk of AF is about one in three in men and women aged 55 and older[8-10]. An important term ‘lone AF’ (LAF) refers to those patients that do not demonstrate any cardiac abnormalities in diagnostics such as echocardiography, stress tests, or laboratory including thyroid functioning or panel[1]. The occurrence of LAF in middle-aged men is higher in sports endurance individuals, where the exercise is considered for 3 hours/week for a 2-years[1]. Prolonged sports increase the risk to three times in the prevalence of LAF, meanwhile five times in the incidence of vagal LAF[1].
AF/AFL is becoming a leading cause of disability, approximately 3–5 million people suffer from AF/AFL, and it is predicted that by 2050 it will affect more than 8 million people worldwide[11].
However, up-to-date data for the strength of the association between AF/AFL and mortality is not adequate. This study aims to attribute the trends in AF/flutter-related mortalities over the past two decades 1999-2020 concerning race and sex and disparity among them.
AF belongs to the subclass cardiovascular disease which has already been identified as an epidemic and leading cause of death globally[12]. AF is highly prevalent and poses a lifetime risk of 1 in 3 to 5 in greater than 45 years old individuals[12], and poses a 2-fold risk for myocardial infarction, and a 5-fold risk of stroke and heart failure[12].
Global prevalence in 1990 was 28.27 million[13], 33.5 million in 2010[12,14] by 2017 there were 37.57 million prevalent cases and 3.05 million incidents globally, 287241 deaths[15], which rose to 59.67 million in 2019[16,13]. Men have more prevalence of AF than women, while the mortality rate in women is higher as compared to men[14,15], with a rise of 18.8% prevalence in men compared to 1990, and an 18.9% increase in prevalent cases in women in 2010 concerning 1990[14]. AF prevalence rose in middle-income countries to 146.6% in 2019 compared with 1990, followed by a 145.2% rise in lower-middle-income countries, a 120.7% increase in lower-income countries, and a 67.8% increase in higher-income countries[13]. It is projected that the prevalence will rise to 6-12[17], to 15.9 million in the United States by 2050[12], and to 17.9 million in Europe by 2060[12,17].
Whites pose a higher risk of AF than blacks, Asians, or Hispanics, it has increased from 1 in 4 whites in the nineties to 1 in 3 after a decade[12], 1 in 5 in China, a 20-fold increase in 11 years, whereas AF related stroke increased 13-fold[18]. The incidence and prevalence rate increased by 33% and 31% respectively in 2017 compared with 1997, and the AF-related burden is expected to increase to greater than 60% by 2050[19]. The annual United States cost in the treatment of AF/AFL is $6.65 billion and is estimated to increase 3-fold by 2050[20].
pulmonary vein ectopy may be a trigger in the episodic paroxysmal AF[1].
Autonomic cardiac innervation may play a significant role in the trigger of paroxysmal AF and AFL. Normal atria are impacted by vagal influences at macro reentries, while diseased atria are impacted by adrenergic influences at micro reentries[1].
The pathophysiological factors contributing to the AF/AFL can be considered as follows.
Atrial fibrosis: Nattel[21] discusses in detail the cellular and molecular mechanism that impacts the pathophysiology and resistance to the therapy in AF, such as blood type-III collagen higher concentration of procollagen N-terminal proteinase after cardioversion is correlated with AF recurrence. Nattel[21] identifies and argues that replacement (reparative) fibrosis causes more irreversible contributing factors for causing AF as compared to reactive (interstitial) fibrosis; although the latter increases the collagen between cardiac muscle. AF may be caused by fibrosis that may be caused by several factors including connective tissue growth factor, angiotensin-II infusion[22], platelet-derived growth factor, transforming growth factor-β, extracellular matrix proteins such as fibronectin, tenascin-C, thrombospondin-1, Ca2+ entry, dysregulation of Ca2+, inward rectifier K+ channels, micro-ribonucleic acid involvement in atrial fibrotic signaling and AF promotion[21], and increase in three collagen markers—plasma procollagen type-I, collagen type I, and tissue inhibitor of matrix metalloproteinase-1[1]. Myeloperoxidase, an oxidizing agent secreted from infiltrating leukocytes also plays a significant role in the development of AF[22].
Myocardial injury: Specific increase in cardiac biomarkers including cardiac troponin T and I, as a contributing factor[1].
Inflammation: Harada et al[22] discussed the role of inflammation in the pathogenesis and pathophysiology of AF. Some of the factors contributing to inflammation are oxidative stress, apoptosis, fibrosis, endothelial dysfunction, platelet activation, and coagulation cascade activation that may lead to thrombogenesis, thoracotomy-induced pericarditis, cerebrovascular event, increased serum levels of tumor necrosis factor-α, myocardial infarction, high interleukin-6 serum levels, and inflammatory bowel disease[22].
Genetics: Roberts and Gollob discuss the role and mechanism of genes as a crucial risk factor in the AF/AFL, and readers are suggested to refer to the article for further understanding, as a person having a first-degree relative having lone AF poses 7-8 fold risk of developing AF. Various genes, chromosomes, and hormones may have a significant role in developing AF, which includes; Ser140Gly mutation in KCNQ1 gene contained in chromosome 11p15.5, mutation of Ser140Gly KCNQ1 with KCNE1, KCNE2 Arg27Cys mutation, KCNJ2 that also causes long QT syndrome type 7, also referred to as Andersen-Tawil Syndrome, Val93Ile mutation within KCNJ2, and KCNE5 mutation with Leu65Phe[20]. Asn1986 Lys mutation within SCN5A; that also causes other arrhythmic disorders including the Brugada syndrome, congenital long QT syndrome type 3, and sick sinus syndrome, c.932delC mutation within connexin protein 40 and 43, SCN5A Met1875Thr mutation, Lys1493Arg mutation, NPPA rs5063; this also can cause stroke, NPPA rs5065, chromosome 4q25 especially rs2200733, SNPs rs7193343 and rs2106261 within the ZFHX3 gene on chromosome 16q22, and rs13376333, localizes to chromosome 1q21 and is intronic to KCNN3, have genetics influence on AF/AFL[20].
The nationwide cohort data was analyzed from the database, Centers for Disease Control and Prevention (CDC WONDER) (Wide-Ranging Online Data for Epidemiologic Research). Two separate sets of analyses were conducted to perform trends in Afib/flutter-related mortality in the older population age 65+ stratified by sex and race from 1999-2020. The data as mentioned earlier was collected using the form “CDC WONDER underlying cause of death from 1999-2020”. The demographic parameters include age, gender, race, and residence consensus regions across the United States stra
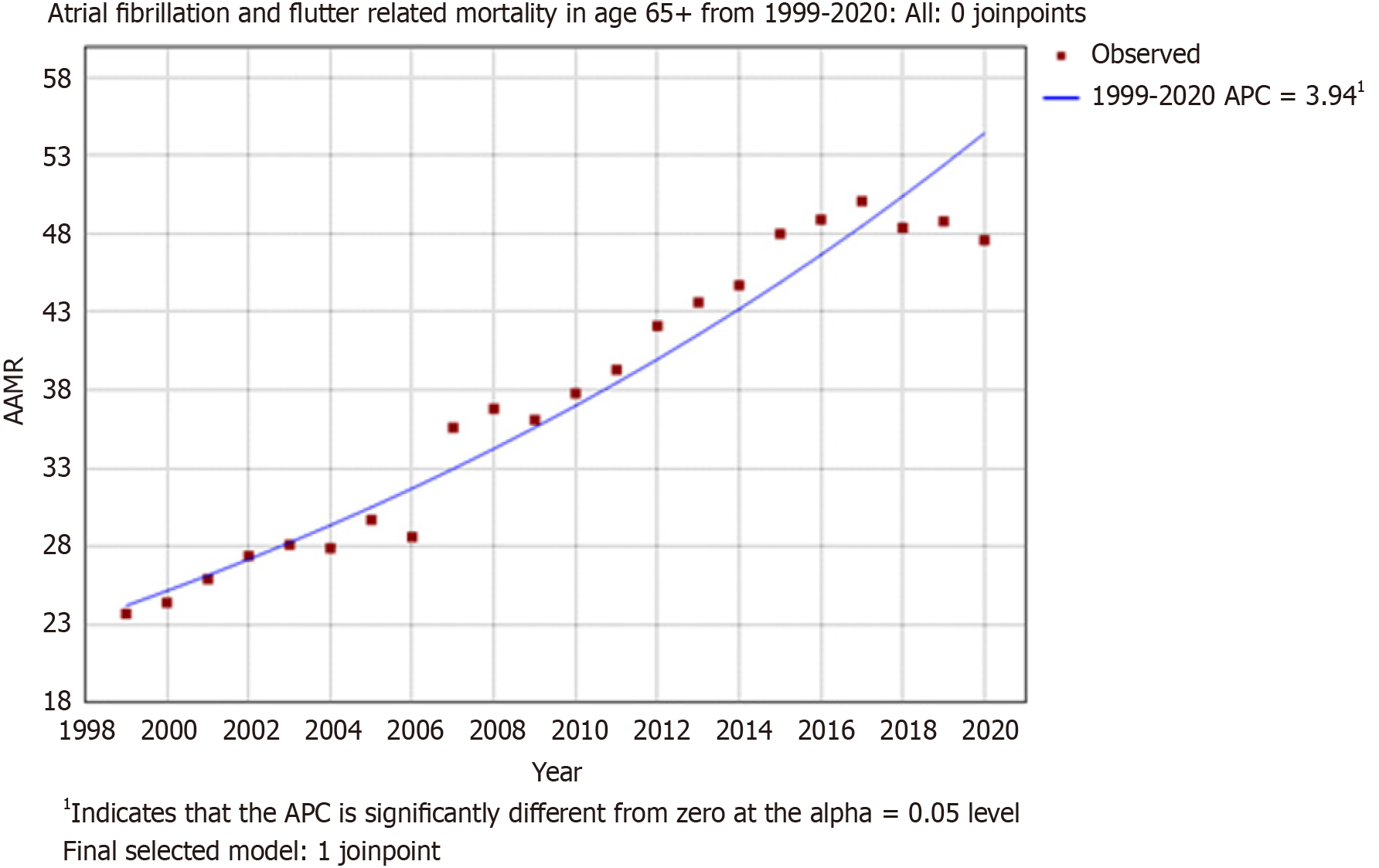
The overall trends in mortality indicate a significant rise in mortality due to AF/AFL in older adults aged 65 years and above from 1999-2020. With an AAMR in 1999, of 23.7 (95%CI: 23.2-24.2) to an AAMR of 47.6 (95%CI: 47.1-48.2) in 2020. Furthermore, a significant rise in AAPC 3.7139 (lower CI: 3.2257, upper CI: 4.2043), t-statistic = 16.1234, P value ≤ 0.000001) (Figure 1).
The results demonstrate increased mortality associated with females, overall AAMR is 39 (95%CI: 38.8-39.1). Compared with the male population overall AAMR is 37.5 (95%CI: 37.3-37.7). When stratified by race/ethnicity concerning sex, white females were more susceptible to mortality overall AAMR is 41.3 (95%CI: 41.1-41.5), when compared with black/African American females overall AAMR is 22.5 (95%CI: 22.0-22.9). A similar pattern of mortality is illustrated with white males having increased mortality rates overall AAMR is 39.3 (95%CI: 39.1-39.6) than black/African American males overall AAMR is 24.4 (95%CI: 23.8-25.0).
The trend analysis demonstrates that in females belonging to white ethnicity a significant increment in mortality from 1999-2020 in the older age group with an AAMR from 25.0 (95%CI: 24.3-25.7) to 50.1 (95%CI: 49.3-51.0) (AAPC 3.4770, lower CI: 2.6256, upper CI: 4.3354, t-statistic = 8.1086, P value ≤ 0.000001). A similar pattern of increased mortality over the past two decades was observed in females belonging to black/African American ethnicity, with an exponential rise in change in AAMR from 14.3 (95%CI: 12.5-16.0) to 28.2 (95%CI: 26.3-30.2) (AAPC 3.3389, lower CI: 1.4084, upper CI: 5.3061, t-statistic = 3.4135, P value = 0.000641). Moreover, identical observed increased mortality trends are concomitant with males belonging to the black/African American ethnicity with a rise in AAMR from 17.6 (95%CI: 14.8-20.4) to 33.8 (95%CI: 31.0-36.6) (AAPC 3.6121, lower CI: 1.3756, upper CI: 5.898, t-statistic = 8.1086, P value = 0.001437). Similarly, white males, with more AAMR from 23.6 (95%CI: 22.7-24.6) to 51.5 (95%CI: 50.5-52.6) (AAPC 3.7438, lower CI: 2.8745, upper CI: 4.6204, t-statistic = 8.5611, P value ≤ 0.000001). Figure 2 further displays trends in mortality.
To the best of our knowledge, this is the first study that estimates the trends and mortality due to AF/AFL from 1999-2020 in older adults in the United States. In this 21-year analysis of mortality data, we found a constant increase in mor
When divided by gender, our study demonstrates that the mortality rate due to AF/AFL in females is 3.8% higher than in males. Our results are consistent with the study of Dai et al[15] which suggests that more females than males died of AF/AFL in 2017. An important facet that can contribute to this gap is that women are found to be less treated by electrical cardioversion and catheter-ablation as a rhythm control strategy despite a higher incidence rate of symptomatic episodes of AF/AFL[23]. Another study by Jiao et al[24] has shown concomitant results to our study that revealed a higher mor
When stratified by race/ethnicity with regards to sex, white females have a higher incidence of mortality as compared to black/African American females with a difference in AAMR of 45.5%, in a similar fashion white males have a higher rate of mortality than black/African American males with a 37.9% difference in AAMR. White ethnicity despite gender had a significant increase in mortality. As per the study of Psaty et al[25] the incidence of AF was found to be lower in black males as compared to males of other ethnicities.
When comparing changes in AAMR individually, our current findings highlight that subsequent AF-related mortality among older white females was about 49.9% as well as black/African American females throughout 1999-2020 had an almost 50.7% increase in mortality rate. Whereas our study manifests an identical increase of 52% in the mortality rate of black/African American males and like manner, a significant rise i.e. 45.8% in the mortality rate of males belonging to white ethnicity.
The two predominant risk factors of AF/AFL-related deaths are high body mass index (BMI) and high systolic blood pressure, while the recent rise in the mortality rate due to AF/AFL is partly driven by the rising incidence of cardio
The treatment for AF/AFL includes class 1c drugs; flecainide, encainide, may slow AF[1] however it may lead to AFL[26]. Therefore, a combination of class 1c with calcium channel blockers is a considered pharmacological treatment[1].
Catheter ablation is a relatively safer and more effective treatment in the management of AF, in approximately 80% of their subjects after 1.3 procedures, with approximately 70% of their subjects did not require further antiarrhythmic drugs during intermediate follow-up[27]. Calvo et al[1] also emphasized the efficacy of circumferential pulmonary vein ablation (CPVA) in the treatment of AF. However, Sawhney et al[28] discussed that CPVA along with additional left atrial linear ablation results in AFL, when compared to segmental pulmonary vein isolation in patients with paroxysmal AF. Regardless, the APAF study conducted by Pappone et al[29] found CPVA to be safer and require less hospitalization with lesser recurrent AF as compared to antiarrhythmic drug therapy. Pappone et al[29] discussed previously conducted randomized control trials and pilot studies that also showed the superior effectiveness of ablation as compared with the drug regimen, with safer intervention rather than side effects associated with the drugs. However, sleep apnea has been found as a cause of recurrent AF post-catheter ablation[30].
AFL can be treated by cavotricuspid isthmus ablation and has a success rate of between 90%-98%, while the recurrence rate is minimal about 3%-11%[1].
Recently, liraglutide, a glucagon-like peptide 1 receptor, agonist used in the management of Diabetes Mellitus-type 2 has shown to decrease the risk of all-cause mortality in AF/AFL patients[31].
Amongst the diagnostics for evaluation or finding for AF/AFL, the very first screening for any patient complaining of chest pain, bradycardia, palpitations, shortness of breath, and tachycardia is the 12-lead electrocardiogram, which may be followed by prolonged HOLTER-monitoring throughout 24 hours to 72 hours.
Echocardiography provides the first in-line and in-office diagnostics for the AF/AFL. Kim and Youn[32] discussed that transthoracic echocardiography provides the rapid and non-invasive anatomical structure of the heart, while transesophageal echocardiography which involves an invasive method into the esophagus provides a better anatomical structure of the heart with accurate location of thrombus in the atria, and provides thromboembolic risk[19]. A relatively newer approach is intracardiac echocardiography provides real-time imaging of the heart, therefore guiding percutaneous interventions, which is useful in radiofrequency catheter ablation and left atrial appendage[32].
Three-dimensional electroanatomic mapping, which provides a real-time heart construct has provided better outcomes in the success of ablation for AF compared to conventional mapping techniques including reduced fluoroscopy time and lower radiation dose[33].
Other diagnostics include computed tomography and magnetic resonance imaging that may provide the location of tissue scar.
The advancement in diagnostics and efficacy of treatment, and access to healthcare facilities such as government-provided Medicare health insurance for the 65-plus population in the United States has resulted in timely diagnosis and intervention in the AF. However, other confounding risk factors such as obesity, prior myocardial infarction, inflammation, hypertension, birth weight, diabetes mellitus, hyperthyroidism, and hormone replacement therapy in menopausal women increase the risk of the occurrence or recurrent occurrence of AF.
Another recent development, although it had not affected the study’s data set is Medicare has outsourced its plans to private insurance companies such as United Healthcare, Humana, Aetna, or Cigna, which has segregated the recipients in low-cost HMO plans that require referrals from their primary care physician (PCP), or preferred provider proximal policy optimization plans that does not require referrals from their PCP. These commercial insurance companies, although they are catering to Meto care and Medicare Advantage patients, do require prior authorization to conduct certain advanced imaging. The process can sometimes be tedious and insurance denies the imaging, hence, access to advanced imaging is not provided, even in serious scenarios.
One of the foremost limitations of the study is that the data it is dependent upon is from CDC WONDER that relies upon International Classification of Diseases (ICD) 10 diagnosis codes. The ICD 10 for AF and AFL is sub-classified into different scenarios and diagnosis. Therefore, the accurate diagnosis and instances cannot be established. Furthermore, another limitation for the study is related to ICD 10 that does not consider into account the confounding factors such as AF with hypertension, or AF with Diabetes Mellitus. The ICD 10 data for coding and billing purpose does not categorized according to age, and gender. Similarly, patient’s BMI, related pass medical history; such as about the recurrent AF/AFL, race and ethnicity, education and other elements of demographics and socio history is not considered into the CDC WONDER data. Another limitation that cannot be omitted is a human coding error, overlooking, or malpractice, regar
In conclusion, our results show that mortality due to AF/AFL in older adults despite gender or ethnicity has overall increased from 1999 to 2020 in the United States. Females in general have a higher mortality rate than males, nonetheless, when mortality rates are compared according to the ethnicities, white females have a significantly higher mortality rate than black/African American females, likewise the mortality rate of white males is greater than black/African American males. However, based on individual study, the mortality rates had been constantly rising in each trend throughout 1999-2020, thus older age has come out to be the factor that has overcome the disparity.
| 1. | Calvo N, Brugada J, Sitges M, Mont L. Atrial fibrillation and atrial flutter in athletes. Br J Sports Med. 2012;46 Suppl 1:i37-i43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Hu D, Barajas-Martinez H, Zhang ZH, Duan HY, Zhao QY, Bao MW, Du YM, Burashnikov A, Monasky MM, Pappone C, Huang CX, Antzelevitch C, Jiang H. Advances in basic and translational research in atrial fibrillation. Philos Trans R Soc Lond B Biol Sci. 2023;378:20220174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Shaihov-Teper O, Ram E, Ballan N, Brzezinski RY, Naftali-Shani N, Masoud R, Ziv T, Lewis N, Schary Y, Levin-Kotler LP, Volvovitch D, Zuroff EM, Amunts S, Regev-Rudzki N, Sternik L, Raanani E, Gepstein L, Leor J. Extracellular Vesicles From Epicardial Fat Facilitate Atrial Fibrillation. Circulation. 2021;143:2475-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 4. | Kivimäki M, Nyberg ST, Batty GD, Kawachi I, Jokela M, Alfredsson L, Bjorner JB, Borritz M, Burr H, Dragano N, Fransson EI, Heikkilä K, Knutsson A, Koskenvuo M, Kumari M, Madsen IEH, Nielsen ML, Nordin M, Oksanen T, Pejtersen JH, Pentti J, Rugulies R, Salo P, Shipley MJ, Suominen S, Theorell T, Vahtera J, Westerholm P, Westerlund H, Steptoe A, Singh-Manoux A, Hamer M, Ferrie JE, Virtanen M, Tabak AG; IPD-Work consortium. Long working hours as a risk factor for atrial fibrillation: a multi-cohort study. Eur Heart J. 2017;38:2621-2628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Tsai WC, Haung YB, Kuo HF, Tang WH, Hsu PC, Su HM, Lin TH, Chu CS, Jhuo SJ, Lee KT, Sheu SH, Chen CY, Wu MT, Lai WT. Hormone replacement therapy and risk of atrial fibrillation in Taiwanese menopause women: A nationwide cohort study. Sci Rep. 2016;6:24132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Conen D, Tedrow UB, Cook NR, Buring JE, Albert CM. Birth weight is a significant risk factor for incident atrial fibrillation. Circulation. 2010;122:764-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 317] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 8. | Nesheiwat Z, Goyal A, Jagtap M, Shammas A. Atrial Fibrillation (Nursing). 2023 Apr 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 9. | Peters SAE, Woodward M. Established and novel risk factors for atrial fibrillation in women compared with men. Heart. 2019;105:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Vinter N, Huang Q, Fenger-Grøn M, Frost L, Benjamin EJ, Trinquart L. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham Heart Study): community based cohort study. BMJ. 2020;370:m2724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Spinoni EG, Mennuni M, Rognoni A, Grisafi L, Colombo C, Lio V, Renda G, Foglietta M, Petrilli I, D'Ardes D, Sainaghi PP, Aimaretti G, Bellan M, Castello L, Avanzi GC, Corte FD, Krengli M, Pirisi M, Malerba M, Capponi A, Gallina S, Pierdomenico SD, Cipollone F, Patti G; COVID-UPO Clinical Team†. Contribution of Atrial Fibrillation to In-Hospital Mortality in Patients With COVID-19. Circ Arrhythm Electrophysiol. 2021;14:e009375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Linz D, Gawalko M, Betz K, Hendriks JM, Lip GYH, Vinter N, Guo Y, Johnsen S. Atrial fibrillation: epidemiology, screening and digital health. Lancet Reg Health Eur. 2024;37:100786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 108] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 13. | Ohlrogge AH, Brederecke J, Schnabel RB. Global Burden of Atrial Fibrillation and Flutter by National Income: Results From the Global Burden of Disease 2019 Database. J Am Heart Assoc. 2023;12:e030438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2658] [Cited by in RCA: 3334] [Article Influence: 277.8] [Reference Citation Analysis (0)] |
| 15. | Dai H, Zhang Q, Much AA, Maor E, Segev A, Beinart R, Adawi S, Lu Y, Bragazzi NL, Wu J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990-2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2021;7:574-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 16. | Ma Q, Zhu J, Zheng P, Zhang J, Xia X, Zhao Y, Cheng Q, Zhang N. Global burden of atrial fibrillation/flutter: Trends from 1990 to 2019 and projections until 2044. Heliyon. 2024;10:e24052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 17. | Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746-2751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 990] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 18. | Guo Y, Tian Y, Wang H, Si Q, Wang Y, Lip GYH. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 19. | Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021;16:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 20. | Roberts JD, Gollob MH. A contemporary review on the genetic basis of atrial fibrillation. Methodist Debakey Cardiovasc J. 2014;10:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Nattel S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin Electrophysiol. 2017;3:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 22. | Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 356] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 23. | Volgman AS, Benjamin EJ, Curtis AB, Fang MC, Lindley KJ, Naccarelli GV, Pepine CJ, Quesada O, Vaseghi M, Waldo AL, Wenger NK, Russo AM; American College of Cardiology Committee on Cardiovascular Disease in Women. Women and atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32:2793-2807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Jiao M, Liu C, Liu Y, Wang Y, Gao Q, Ma A. Estimates of the global, regional, and national burden of atrial fibrillation in older adults from 1990 to 2019: insights from the Global Burden of Disease study 2019. Front Public Health. 2023;11:1137230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 25. | Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 1014] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 26. | Kawabata M, Hirao K, Horikawa T, Suzuki K, Motokawa K, Suzuki F, Azegami K, Hiejima K. Syncope in patients with atrial flutter during treatment with class Ic antiarrhythmic drugs. J Electrocardiol. 2001;34:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1446] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 28. | Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, Gulletta S, Paglino G, Mazzone P, Sora N, Greiss I, Santagostino A, LiVolsi L, Pappone N, Radinovic A, Manguso F, Santinelli V. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48:2340-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Kwon Y, Koene RJ, Johnson AR, Lin GM, Ferguson JD. Sleep, sleep apnea and atrial fibrillation: Questions and answers. Sleep Med Rev. 2018;39:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Haloot J, Mahmoud M, Badin A. Liraglutide mortality effect on atrial fibrillation patients. Authorea. 2021;. [DOI] [Full Text] |
| 32. | Kim TS, Youn HJ. Role of echocardiography in atrial fibrillation. J Cardiovasc Ultrasound. 2011;19:51-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Bhakta D, Miller JM. Principles of electroanatomic mapping. Indian Pacing Electrophysiol J. 2008;8:32-50. [PubMed] |